Abstract
The effects of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) on the risk of COVID-19 infection and disease progression are yet to be investigated. The relationship between ACEI/ARB use and COVID-19 infection was systematically reviewed. To identify relevant studies that met predetermined inclusion criteria, unrestricted searches of the PubMed, Embase, and Cochrane Library databases were conducted. The search strategy included clinical date published until May 9, 2020. Twelve articles involving more than 19,000 COVID-19 cases were included. To estimate overall risk, random-effects models were adopted. Our results showed that ACEI/ARB exposure was not associated with a higher risk of COVID-19 infection (OR = 0.99; 95 % CI, 0–1.04; P = 0.672). Among those with COVID-19 infection, ACEI/ARB exposure was also not associated with a higher risk of having severe infection (OR = 0.98; 95 % CI, 0.87–1.09; P = 0.69) or mortality (OR = 0.73, 95 %CI, 0.5–1.07; P = 0.111). However, ACEI/ARB exposure was associated with a lower risk of mortality compared to those on non-ACEI/ARB antihypertensive drugs (OR = 0.48, 95 % CI, 0.29−0.81; P = 0.006). In conclusion, current evidence did not confirm the concern that ACEI/ARB exposure is harmful in patientswith COVID-19 infection. This study supports the current guidelines that discourage discontinuation of ACEIs or ARBs in COVID-19 patients and the setting of the COVID-19 pandemic.
Keywords: Antihypertensive, Hypertension, Systematic review, Meta-analysis
1. Introduction
Since the first confirmed case in Wuhan in 2019 [1], the Coronavirus Disease 2019 (COVID-19) has rapidly spread on a global scale. More than three million confirmed infections have been reported according to the World Health Organisation (WHO) [2]. To date, there are no specific or effective therapies approved for treatment of this fatal disease [3]. In many countries, public health services have been overwhelmed by the rapid rise in new COVID-19 cases. This has resulted in a high severity and mortality [4,5]. Therefore, it is important that clinicians identify the risk factors or acquiring COVID-19 infection, pay attention to the risk factors associated with critical disease and death, and take appropriate interventions. By focusing on these aspects, infections may be prevented, the efficacy of treatment may be enhanced, and the risk of death may be reduced.
The latest systematic review investigating COVID-10 patients reported that hypertension and cardiovascular disease (CVD) were associated with a higher risk of severity and mortality in patients infected with COVID-19 [6]. However, patients with these comorbidities are likely to be treated with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), which could upregulate the expression of ACE2 receptor in animal studies [7,8]. Since ACE2 is the functional receptor for coronavirus entry into cells [9], there is concern regarding an increased risk of COVID-19 infection among those who use ACEI/ARB treatment [10]. Conversely, there is evidence that exogenous ACE2 supplementation can reduce inflammation and increase oxygenation in animal models of acute respiratory distress syndrome (ARDS) [11]. Epidemiological studies have also shown that ACEI/ARB use may reduce the risk of pneumonia in the general population [12,13]. These observations raise the question as to whether ACEI/ARB exposure is associated with risk or progression of COVID-19. To provide more accurate evidence, we conducted a meta-analysis. Thus, the aims of this work were as follows: (1) to determine whether ACEI/ARB use is associated with an increase in likelihood of viral infectivity; (2) to investigate whether there are differences in severity and mortality between ACRI/ARB users and non- ACRI/ARB users; (3) to evaluate, in particular, whether ACEI/ARB exposure was associated with a lower risk of mortality when compared to non-ACEI/ARB antihypertensive drug exposure. Our findings provide vital guidance for current clinical work on the prevention and treatment of COVID-19 infection.
2. Materials and methods
2.1. Literature search
To ensure high-quality evidence, we followed the Preferred Reporting Items of Systematic Reviews and Meta-analysis (PRISMA) statement. A comprehensive search of the PubMed, Embase, and Cochrane Library databases was performed to identify all relevant articles published between Jan 1, 2020 and May 9, 2020. The search terms were as follows: ‘Corona Virus Disease-2019 OR 2019 novel coronavirus OR SARS-CoV-2 OR COVID-19 OR 2019-nCoV’ AND ‘antihypertensive agent OR hypertension OR angiotensin-converting enzyme inhibitors OR angiotensin receptor blockers OR ACEI OR ARB’. Additionally, a manual search of the retrieved articles, related review articles, and meta-analyses was conducted to identify other relevant articles.
2.2. Inclusion criteria
Observational studies that met all the following criteria were included: (1) study design: case-control, case-crossover, self-controlled case series (SCCS) or cohort study; (2) antihypertensive treatment: ACEI/ARB use versus non-ACEI/ARB use; (3) outcomes: the incidence of COVID-19, critical cases, or death; (4) adequate data were used to extract the risk estimates if the adjusted data were not provided in the publication. Editorials, correspondences, conference abstracts and commentary articles were excluded in our study. When information was incomplete in the publication, attempts were made to contact the study investigators to obtain missing information.
2.3. Data extraction and quality assessment
Two investigators independently extracted all data from publications in a double-blinded manner. Any disagreements were resolved by a third investigator. The following information was extracted: name of first author, publication year, research type, study location, age, gender, number of participants, confounder adjustments, and study quality. If more than one estimate of effect was provided, the most-adjusted estimate was used for analysis. The Newcastle Ottawa Scale (NOS) was used to evaluate the methodological quality of the included publications [14]. The NOS features eight criteria and yields scores ranging from 0 (high risk of bias) to 9 (low risk of bias). Studies with NOS scores of >7 were regarded as high quality.
2.4. Data synthesis and analysis
All meta-analytical calculations were performed with STATA software (version 14.0, Stata Corp LP, College Station, Texas). To provide a quantitative estimate of the association between ACEI/ARB use and severity or mortality risk in COVID-19 patients, the odds ratios (ORs) (most adjusted, if available) and the corresponding 95 % CIs were extracted from published articles. When the ORs were not given, tabular data were used to calculate the corresponding OR. Statistical heterogeneity of the included studies was calculated by using the χ2 test and the I2 statistic. An I2 >50 % or a P < 0.05 for the Q-statistic indicated substantial heterogeneity [15]. A random-effects model of the DerSimonian-Laird model was used to compare variance between studies [16]. Egger’s regression test was employed to evaluate the publication bias [17]. Publication bias was not formally assessed because each meta-analysis included fewer than 10 studies. A P < 0.05 was considered statistically significant [18].
3. Results
3.1. Search results
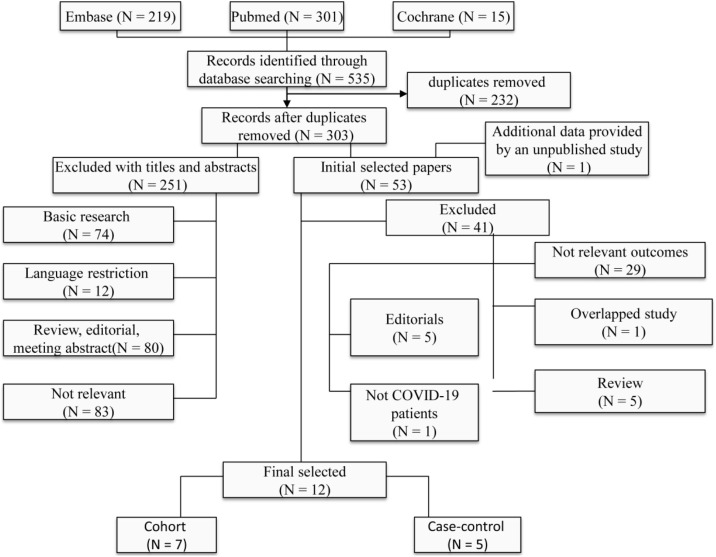
The search strategy identified 535 studies, of which 232 articles were duplicates. A total of 251 articles were excluded after reading the title and abstract. The remaining 53 articles were evaluated for this meta-analysis. One of the articles [19] was published as a poster in our hospital and the author was contacted for additional information. In total, 12 articles [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]] were eligible for this meta-analysis. The twelve articles of seven cohort studies and five case-control studies. Together, the included articles evaluated more than 19,000 COVID-19 patients . The list of excluded studies and the reasons for exclusion are listed in Fig. 1 .
Fig. 1.
Flow chart showing the meta-analysis studies selection.
3.2. Patient characteristics
The patient characteristics of the included studies are shown in Table 1 . Four studies [22,26,28,30] were performed in Italy and USA, and one study [29] included the data from 11 countries in Asia, Europe, and North America. All other studies [[19], [20], [21],[23], [24], [25],27] came from China, mainly in Wuhan. All of the selected studies were published in 2020 with different sample patient sizes that ranged from 42 to 8910 patients. The overall average age of the subjects was greater than 60 years. Clinical outcomes were defined as COVID-19 infection in three studies, severity in severn studies, and mortality in eight studies. The results of the quality assessment of the included studies are presented in Supplementary Table S1 and S2.
Table 1.
Characteristics of the Included Studies.
| Author | Country (city) | Study design | Study period | Age | Male | Measurement of ACEI/ARB use | ACEI/ARB* | Non- ACEI/ARB* | Outcome | Confounder adjustment | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feng et al 2020 | China (Wuhan, Shanghai, Anhui) | Multi-center retrospective case-control | Jan 1 to Feb 15 2020 | 53 (40−64) | 57 % | Medical record review | 33 | 80 | Severity | No | 5 |
| Li Juyi et al 2020 | China (Wuhan) | Retrospective, single-center case series | Jan 15 to Mar 15 2020 | 66 (59−73) | 52 % | Medical record review | 115 | 247 | Severity and mortality | No | 4 |
| Mancia et al 2020 | Italy (Lombardy region) | Population-based case-control | Feb 21 to Mar 11 2020 | 68 ± 13 | NA | Databases of health care use | ACEI 1502 ARB 1394 | NA | Infection and Severity | No | 9 |
| Meng et al 2020 | China (Shenzheng) | Single-center retrospective cohort | Jan 11 to Feb 23 2020 | 64 (56−69) | 57 % | Medical record review | 17 | 25 | Severity and mortality | No | 4 |
| Reynolds et al 2020 | USA (New York) | Population-based cohort | Mar 1 to Apr 15 2020 | 64 (54–75) | 50 % | Pharmacy fill records | 1091 | 986 | Infection and Mortality | No | 9 |
| Tedeschi et al 2020 | Italy (Bologna) | Prospective cohort | Feb 1 to Apr 4 2020 | 76 (67−83) | 72 % | Medical record review | 165 | 136 | Mortality | Age, gender, presence of CV comorbidities and COPD | 8 |
| Yang et al 2020 | China (Wuhan) | Single-center retrospective cohort | Jan 5 to Feb 22 2020 | 66 (57−75) | 49 % | Medical record review | 43 | 83 | Severity and mortality | No | 5 |
| Zhang et al 2020 | China (Wuhan) | Retrospective, multi-center cohort study | Dec 31 2019 to Feb 20 2020 | 64 (55−69) | 53 % | Medical record review | 188 | 940 | Mortality | Age, gender, comorbidities and in-hospital medications | 9 |
| Mehra et al 2020 | Asia, Europe, and North America | Retrospective, multi-center study case-control | Dec 20 2019 to Mar 12 2020 | 49 ± 16 | 60% | Medical record review | ACEI 770 ARB 556 | non-ACEI 8140 non-ARB 8354 | Mortality | Age, race, coexisting conditions and medications | 8 |
| Yu et al 2020 | China (Zhejiang and Jiangsu) | Retrospective, multi-center cohort study | Jan 17 to Feb 19 2020 | 60 (52−68) | 53 % | Medical record review | 103 | 173 | Mortality | Sex, age, smoking, symptom, diabetes, cardiovascular diseases, chronic liver disease, and other comorbidity | 9 |
| Mehta et al | USA (Ohio and Florida) | Retrospective cohort | Mar 8 to Apr 12 2020 | 49 ± 21 | 40% | Electronic medical records | ACEI 116 ARB 98 | non-ACEI 1619 non-ARB 1637 | Infection and Mortality | PS matched | 9 |
| Li xiaochen et al | China (Wuhan) | Retrospective case-control | Jan 26 to Feb 5 2020 | 60 (48−69) | 50.90 % | Medical record review | 42 | 503 | Severity | No | 4 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CV, cadiovascular; COPD, chronic obstructive pulmonary disease; NA, not available; PS, propensity score. * Number of COVID-19 case.
3.3. Meta-analysis
3.3.1. ACEI/ARB use and risk of COVID-19 infection
Three studies reported the susceptibility to COVID-19 infection in patients on ACEI/ARB treatment. The combined OR of COVID-19 infection risk was 0.99 (95 % CI, 0.95–1.04), and no heterogeneity was observed among the studies (I2 = 0%, P = 0.504; Table 2 ). When the studies were grouped by drug type, the risk of COVID-19 infection was not significantly increased among individuals exposed to ACEI (OR = 0.98, 95 %CI, 0.92–1.04) or ARB (OR = 1.01, 95 % CI, 0.95–1.07, P = 0.044).
Table 2.
Meta-analysis for studies included in the analysis.
| Outcomes | Number of studies | Number of estimates | Pooled OR (95 % CI), I2 statistics (%), P-value for the heterogeneity Q test | Model used |
|---|---|---|---|---|
| COVID-19 infection | 3 | 4 | 0.99 (0.95–1.04); I2 = 0%, P = 0.504 | Random effects |
| ACEI | 3 | 3 | 0.98 (0.92–1.04); I2 = 0%, P = 0.542 | Random effects |
| ARB | 3 | 3 | 1.01 (0.95–1.07); I2 = 8.9%, P = 0.334 | Random effects |
| COVID-19 Mortality | 8 | 9 | 0.73 (0.5–1.07); I2 = 70.7%, P = 0.11 | Random effects |
| Type of data | Random effects | |||
| Unadjusted | 4 | 4 | 0.91 (0.51–1.61); I2 = 33.4%, P = 0.212 | Random effects |
| Adjusted | 4 | 5 | 0.66 (0.38–1.12); I2 = 82.2%, P < 0.001 | Random effects |
| Study location | Random effects | |||
| China | 5 | 5 | 0.65 (0.46−0.91); I2 = 0%, P = 0.529 | Random effects |
| Other countries | 3 | 4 | 0.88 (0.48–1.62); I2 = 86.1%, P < 0.001 | Random effects |
| Patient with indication | 6 | 7 | 0.62 (0.38–1.02); I2 = 74.8%, P = 0.001 | Random effects |
| ACEI/ARB vs non-ACEI/ARB antihypertensive drug | 4 | 4 | 0.48 (0.29−0.81); I2 = 0%, P = 0.3796 | Random effects |
| COVID-19 Severity | 7 | 8 | 0.98 (0.87–1.09); I2 = 42.8%, P = 0.093 | Random effects |
| Patient with indication | 5 | 6 | 0.95 (0.83–1.1); I2 = 57.6%, P = 0.038 | Random effects |
3.3.2. ACEI/ARB use and risk of mortality in COVID-19 patients
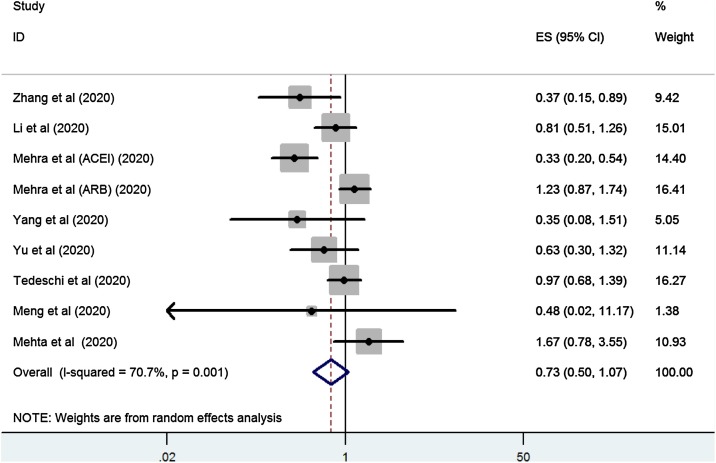
The overall analysis included nine studies. Together, 1631 COVID-19 cases with ACEI/ARB exposure and 11620 COVID-19 cases without ACEI/ARB esposure were included. The risk associated with ACEI/ARB use and increased mortalitywas estimated. Overall, the risk of mortality in ACEI/ARB-exposed was similar to non-ACEI/ARB exposed COVID-19 patients (OR = 0.73, 95 % CI, 0.5–1.07, P = 0.11). However, the studies had high (I2 = 70.7 %; P = 0.001; Fig. 2 ). A subgroup analyses of the data that included estimates showed that there was no significant increase in the mortality risk of patients with ACEI/ARB exposure regardless of unadjusted (OR = 0.66, 95 % CI, 0.38–1.12, P = 0.121) or adjusted estimates (OR = 0.91, 95 % CI, 0.51–1.61, P = 0.87). When studies were grouped by study location, there was a significantly lower mortality risk in studies from China (OR = 0.65, 95 % CI, 0.46−0.91, P = 0.013). There were no significant increase in mortality risk in studies form other countries (OR = 0.88, 95 % CI, 0.48–1.62, P = 0.689).
Fig. 2.
Forest plot of ACEI/ARB exposure and risk of mortality in COVID-19 patients.
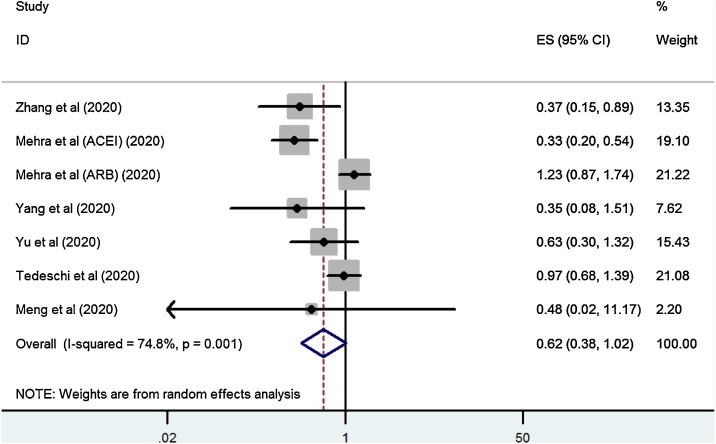
When our analysis was limited to the studies that only included patients on ACEI/ARBs for antihypertensive indications, a significantly lower risk of mortality was observed among those who used ACEI/ARB (OR = 0.62, 95 % CI, 0.38–1.02, P = 0.059; Fig. 3 ). After excluding studies that enrolled patients with hypertension not on antihypertensive treatment, a meta-analysis of four studies also found that ACEI/ARB exposure was associated with a lower risk of mortality compared to those on non-ACEI/ARB antihypertensive drugs (OR = 0.48, 95 % CI, 0.29−0.81, P = 0.006; I2 = 0%).
Fig. 3.
Forest plot of ACEI/ARB exposure and risk of mortality in COVID-19 patients with antihypertensive indication.
3.3.3. ACEI/ARB use and influence on COVID-19 severity
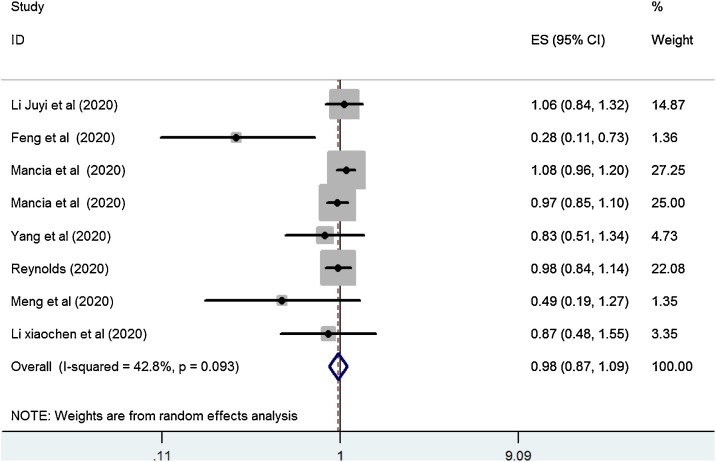
Seven studies that included 3070 COVID-19 cases with ACEI/ARB exposure and 3830 COVID-19 cases unexposed to ACEI/ARB reported on COVID-19 severity in relation to ACEI/ARB exposure. The combined OR of COVID-19 severity was 0.98 (95 % CI, 0.87–1.09, P = 0.69; Fig. 4 ). Moderate heterogeneity was observed among the studies (I2 = 42.8 %; P = 0.093). When our analysis was limited to the studies that only included patients on ACEI/ARB for antihypertensive indications, ACEI/ARB use was not associated with a higher risk of COVID-19 severity (OR = 0.95, 95 % CI, 0.83–1.1, P = 0.521; I2 = 57.6 %).
Fig. 4.
Forest plot of ACEI/ARB exposure and risk of severity of COVID-19.
4. Discussion
This review included 12 articles that encompassed more than 19,000 COVID-19 cases. The findings suggest that ACEI/ARB use did not increase the risk of a positive COVID-19 test, or increase the risk of more severe infections, and did not increase mortality risk among patients with COVID-19. However, patient exposure to ACEI/ARBs for the trestment of hypertension was associated with a lower risk of mortality. This has potentially important implications in clinical practice.
Several systematic reviews [6,31,32] have demonstrated that individuals with underlying illness such as hypertension and CVD are susceptible to COVID-19 infection. However, ACEI/ARB treatment is widely used among these patients. This raises concerns about potential advantages or disadvantages of ACEI/ARB use COVID-19 infection[33]. SARS-CoV-2 binds to the extracellular domain of the transmembrane ACE2 receptor to entry host cells [9]. While ACEI and ARB have been shown to upregulate ACE2 expression in animal studies [7,8], it is reasonable to hypothesize that hypertensive patients taking these drugs may have a higher risk of COVID-19 infection. Contrary to this hypothesis, our analysis did not provide evidence that patients should stop or substitute the ACEI or ARB medications previously prescribed. This may be result for two reasons. First, the effects of ACEI/ARB on the level of human plasma ACE2 are inconsistent [34]Second, none of the studies evaluated the effects of ACEI/ARBs on lung-specific expression of ACE2 [35]. To be relevant in SARS-CoV-2 infection, the effects on ACE2 would need to be present on respiratory epithelium. Second, beta blockers are identified as preventing ACE2 activity [36], which could underestimate the risk of COVID-19 associated ACEI/ARBs.
Another important issue is the use of ACEI/ARBs on the clinical outcomes of COVID-19 patients. Upon binding to ACE2, SARS-CoV subsequently reduces ACE2 expression in host cells. This results in activation of RAS, which in turn causes severe acute lung injury and exacerbates the progression of pneumonia [11]. According to this theory, a RAS inhibitor may improve the clinical outcomes of patients with COVID-19. In our overall analysis, ACEI/ARB exposure was not associated with a lower risk of COVID-19 severity or mortality. This may be explained by the drug indication. The latest systematic review [6] identified that COVID-19 patients with hypertension and CVD faced a greater risk of progressing to more critical or mortal illness. Several studies [20,22,25,30] included patients without hypertension and CVD in the non-ACEI/ARB group, which may underestimate the protective effect of ACEI/ARB use in COVID-19 patients. Further analysis of studies that only included patients using an ACEI/ARB indicated for hypertension found that patients on ACEI/ARBs had a lower risk of mortality. This finding is further reinforced by the relatively lower but significant risk observed in patients with hypertension.
To our knowledge, this systematic review and meta-analysis is the first to evaluate the role of ACEI/ARBs as an antihypertensive regimen in patients with COVID-19. The key strength of our meta-analysis lies in its large sample size and comprehensive search. The accumulating evidence and large sample size enhanced the statistical power of this study to provide more precise and reliable risk estimates. In the included studies, more than 19,000 COVID cases were included in the analysis of severity and mortality. Furthermore, we conducted additional analyses to control for confounding factors by indication.
Nonetheless, this review has some major limitations. The most important limitation of our meta-analysis is the residual number of unknown confounders. Previous studies have reported that sex, age, smoking, and diabetes greatly affect the prognosis of COVID-19 infection. However, these potential confounders are considered in most of the included studies. Future research should report analyses stratified by possible risk factors that fully adjust for potential confounders to rule out alternative explanations. Another limitation is that there was only a small number of eligible high-quality studies was small, which may have influenced the accuracy of the results. Third, the measurement of ACEI/ARB exposure was through medical record review, which is less reliable than other methods. This may have influenced the findings. Fourth, although only mild heterogeneity was observed in the analysis of COVID-19 severity, the existence of clinical heterogeneity is expected to lead to a degree of statistical heterogeneity in the results. The definitions of COVID-19 severity and outcomes were inconsistent among the included studies. Finally, the results of this review are susceptible to selection bias given that most of the patients in the study population were in a hospital.
In conclusion, the results of the meta-analysis suggest that use of ACEI/ARB in patients with COVID-19 does not increase the risk of COVID-19 infection, severity, or mortality. However, a lower risk of mortality was observed among those patients who were taking ACEI/ARB for the treatment of hypertension. The findings suggest that ACEI/ARB treatment should be continued in COVID-19 patients who are taking these medications for antihypertensive treatment.
Authors’ contributions
H.Y.J. and Z.X. conceived the study and revised the manuscript critically for important intellectual content. Z.X., and J.Y. made substantial contributions to its design, acquisition, analysis and interpretation of data. L.Y.P. participated in the design, acquisition, analysis and interpretation of data. All authors read and approved the final manuscript.
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China (Grant No. LY20H090012).
Declaration of Competing Interest
The authors declare that they have no competing interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.104927.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7(11) doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong H., Wang Y., Zhang Z.L., Liu Y.X., Le K.J., Cui M., Yu Y.T., Gu Z.C., Gao Y., Lin H.W. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol. Res. 2020;104872 doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai C.C., Wang C.Y., Wang Y.H., Hsueh S.C., Ko W.C., Hsueh P.R. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int. J. Antimicrob. Agents. 2020;55(105946) doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., Wu Y., Sun L., Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Virol. 2020;127(104371) doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. Apr 23. pii: S0163-4453(20)30234-6. doi: 10.1016/j.jinf.2020.04.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igase M., Kohara K., Nagai T., Miki T., Ferrario C.M. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade. Hypertension Res. 2008;31:553–559. doi: 10.1291/hypres.31.553. [DOI] [PubMed] [Google Scholar]
- 8.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 9.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gracia-Ramos A.E. Is the ACE2 overexpression a risk factor for COVID-19 infection? Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.03.011. Apr 4. pii: S0188-4409(20)30378-7. doi: 10.1016/j.arcmed.2020.03.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinoharaand Y., Origasa H. Post-stroke pneumonia prevention by angiotensin-converting enzyme inhibitors: results of a meta-analysis of five studies in Asians. Adv. Ther. 2012;29:900–912. doi: 10.1007/s12325-012-0049-1. [DOI] [PubMed] [Google Scholar]
- 13.Liu C.L., Shau W.Y., Chang C.H., Wu C.S., Lai M.S. Pneumonia risk and use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. J. Epidemiol. 2013;23:344–350. doi: 10.2188/jea.JE20120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins . 2014. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available at: www.cochrane-handbook.org [6 December 2014] [Google Scholar]
- 15.Higginsand J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau J., Ioannidis J.P., Terrin N., Schmid C.H., Olkin I. The case of the misleading funnel plot. Bmj. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J., Shi X., Ma J., Lv F., Wu J., Pan Q., Yang J., Cao H., Li L. 2020. Long Term Use of ACEI/ARB Contributes to the Outcomes of COVID-19 Patients with Hypertension: A Multicenter Retrospective Study. [Google Scholar]
- 20.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., Xiong W., Yang D., Chen R., Lu F., Lu Y., Liu X., Chen Y., Li X., Li Y., Summah H.D., Lin H., Yan J., Zhou M., Lu H., Qu J. COVID-19 with different severity: a multi-center study of clinical features. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. Apr 10. doi: 10.1164/rccm.202002-0445OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L., Xia M., Chen M.M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.X., Chen J., She Z.G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.J., Wang X., Touyz R.M., Xia J., Zhang B.H., Huang X., Yuan Y., Rohit L., Liu P.P., Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317242. Apr 17. doi: 10.1161/CIRCRESAHA.120.317134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedeschi S., Giannella M., Bartoletti M., Trapani F., Tadolini M., Borghi C., Viale P. Clinical impact of renin-angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa492. Apr 29. doi: 10.1161/HYPERTENSIONAHA.120.15143. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G., Tan Z., Zhou L., Yang M., Peng L., Liu J., Cai J., Yang R., Han J., Huang Y., He S. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension (Dallas, Tex : 1979) 2020 doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 25.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. Apr 12. pii: S0091-6749(20)30495-4. doi: 10.1016/j.jaci.2020.04.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., Carmona-Rubio A.E., Jacob M., Procop G.W., Harrington S., Milinovich A., Svensson L.G., Jehi L., Young J.B., Chung M.K. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. May 5. doi: 10.1001/jamacardio.2020.1855. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1624. Apr 23. doi: 10.1001/jamacardio.2020.1624. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-Aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006923. May 1. doi: 10.1056/NEJMoa2006923. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007621. May 1. doi: 10.1056/NEJMoa2007621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. Renin-angiotensin-Aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2008975. May 1. doi: 10.1056/NEJMoa2008975. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linand C.W., Huang Y.Y. 2020. Does the Direct Renin Inhibitor Have a Role to Play in Attenuating Severity of the Outbreak Coronavirus Disease 2019 (COVID-19) p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-Aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Moreira Mda C., Heringer-Walther S., Schultheiss H.P., Siems W.E., Wessel N., Walther T. Beta blockers prevent correlation of plasma ACE2 activity with echocardiographic parameters in patients with idiopathic dilated cardiomyopathy. J. Cardiovasc. Pharmacol. 2015;65:8–12. doi: 10.1097/FJC.0000000000000156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.