Dear Editor,
We read with interest the recent paper by Cantini et al., describing the safety and clinical impact of baricitinib therapy in Coronavirus disease 2019 (COVID-19).1 COVID-19, caused by 2019 novel coronavirus (2019-nCoV), is increasing rapidly in an epidemic scale and has spread in over 200 countries, causing more than three million confirmed cases and two hundred thousand deaths as of May 5th, 2020. But currently, there is no vaccine against 2019-nCoV or effective treatment for COVID-19.2
The typical symptoms of COVID-19 are fever, cough and dyspnea, and the leading cause of mortality is acute respiratory distress syndrome (ARDS). As an immunopathologic event, ARDS is characterized by cytokine storm, which is an excessive systemic inflammatory response triggered by the release of proinflammatory cytokines.3 Therefore, diminishing the cytokine storm may be an important part of treatment in patients with severe COVID-19.4 Mesenchymal stem cells (MSCs) have been shown to possess powerful immunomodulatory properties and beneficial effects for preventing or reducing the cytokine storm.5 Hence, MSCs therapy may be a promising option for the treatment of severe COVID-19. On this basis, we conducted a retrospective study to evaluate the treatment efficacy and side effects of MSCs therapy on severe COVID-19.
All hospitalized patients met the following criteria were consecutively recruited from February 20th, 2020 to March 30th, 2020: (1) definite diagnosis of severe COVID-19; (2) age≥18 years; (3) receiving MSCs therapy. All patients have signed written informed consent in line with the Declaration of Helsinki.
The diagnosis of severe COVID-19 was made according to the Guideline for Diagnosis and Treatment for COVID-19 of National Health Commission of China (version 5.0).6 The detailed diagnostic criteria were one of the conditions 2 to 4 plus condition 1: (1) confirmation by real-time RT-PCR assay; (2) respiratory distress, RR≥30 beats/min; (3) oxygen saturation level≤93% in resting state; (4) arterial partial pressure of oxygen (PaO2)/fraction of inspiration O2 (FiO2) ≤300 mmHg (1 mmHg=0.133 kPa).
Clinical grade MSCs were given at a dose of 1 × 106 mononuclear cells per kilogram of weight. Promethazine hydrochloride (intramuscular injection, 25 mg) was used before the injection of MSCs to prevent allergies. For patients received two or three times MSCs therapy, the interval of injection was 5 days. Laboratory tests were conducted 2 to 3 h before the injection and 48 to 72 h after the injection.
Data were presented as mean±SD for continues variables with normal distribution, and median and interquartile range (IQRs) otherwise. Independent continuous variables were compared using the Student t-test or the Mann-Whitney test. Paired continuous variables were compared using the paired t-test or the Wilcoxon signed-rank test. Categorical variables were compared using the Chi-square test or the Fisher exact test (if any expected value <5). All of the analyses were conducted as 2-sided tests and p<0.05 was considered statistically significant.
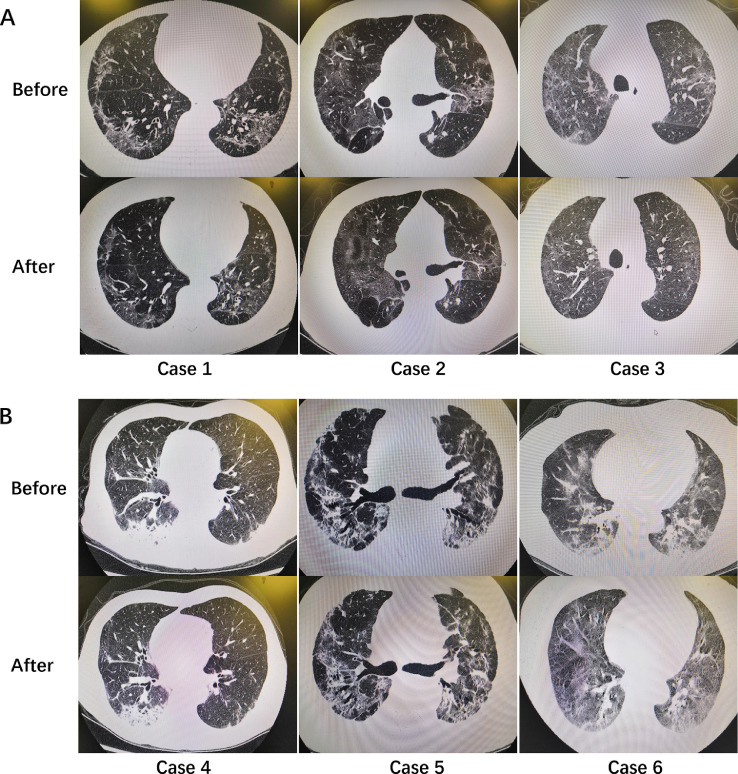
Totally, 25 patients were enrolled according to the criteria. Among them, 20 cases (80%) were male and 5 cases (20%) were female. The median age was 70 (IQR: 59,71) years. Seven cases received MSCs therapy for one time, 7 cases received for two times and 11 cases received for three times. After MSCs therapy, 16 cases (64%) gained apparently CT scan improvement and all cases gained clinical improvement (Fig. 1 ). No fatalities occurred during hospitalization. However, 3 cases experienced treatment related side effects, specifically liver dysfunction, heart failure and allergic rash.
Fig. 1.
Chest CT scans of severe COVID-19 cases before and after MSCs therapy. A, cases with apparently CT scan improvement; B, cases without apparently CT scan improvement.
The laboratory findings before and after MSCs therapy were shown in Table 1 . Inflammation indexes, including white blood cells (WBC) counts, C-reaction protein (CRP), procalcitonin (PCT) and interleukin-6 (IL-6) did not change significantly after MSCs therapy. Similarly, significant changes of IgG and IgM were not found either. However, the serum levels of lactate (LAC), cardiac troponin T (cTnT) and creatine kinase-MB (CK-MB) elevated significantly after MSCs therapy (p < 0.05).
Table 1.
Laboratory findings before and after MSCs therapy.
| Variables | The 1th time |
The 2th time |
The 3th time |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | |
| WBC (*109/L) | 6.3 ± 1.8 | 6.5 ± 2.0 | 0.475 | 7.1 ± 2.6 | 6.5 ± 1.6 | 0.315 | 6.0 ± 0.5 | 6.2 ± 0.7 | 0.186 |
| CRP (mg/L) | 1.8(0.5,8.9) | 0.9(0.5,6.9) | 0.287 | 0.6(0.5,6.8) | 0.9(0.5,3.7) | 0.678 | 0.8(0.5,1.1) | 1.0(0.5,3.4) | 0.484 |
| PCT (ng/ml) | 0.07(0.05,0.1) | 0.07(0.05,0.09) | 0.113 | 0.08±0.05 | 0.08(0.06,0.1) | 0.221 | 0.07±0.02 | 0.07±0.02 | 0.108 |
| IL-6 (pg/ml) | 5.5(2.6,10.9) | 5.2(2.8,9.1) | 0.775 | 8.1 ± 6.1 | 6.9(3.9,15.0) | 0.296 | 8.6 ± 6.0 | 7.1(3.0,13.6) | 0.721 |
| LAC (mmol/L) | 1.8 ± 0.7 | 2.9 ± 1.2 | 0.030 | 2.1 ± 1.2 | 3.5 ± 1.5 | 0.000 | 3.0 ± 0.4 | 3.4 ± 1.2 | 0.782 |
| ALT (U/L) | 27.3(19.5,50.6) | 30.0 ± 15.1 | 0.085 | 35.0 ± 17.1 | 31.7 ± 20.7 | 0.472 | 34.0 ± 16.4 | 32.4 ± 15.2 | 0.139 |
| Cr (μmol/L) | 54.6 ± 11.8 | 56.1 ± 12.0 | 0.293 | 55.8 ± 17.8 | 55.5 ± 19.5 | 0.867 | 65.6 ± 21.5 | 65.5 ± 18.2 | 0.923 |
| cTnT (ng/ml) | 12.7 ± 6.8 | 18.3 ± 13.4 | 0.029 | 9.6 ± 6.5 | 10.2 ± 9.2 | 0.686 | 2.3 ± 3.0 | 5.2 ± 2.1 | 0.132 |
| CK-MB(μmol/L) | 1.1(0.6,1.8) | 0.9(0.8,1.9) | 0.861 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.031 | 0.6 ± 0.2 | 0.3 ± 0.3 | 0.135 |
| IgM (s/co) | 0.8(0.5,1.7) | 0.6(0.4,0.7) | 0.343 | 0.8 ± 0.8 | 0.5(0.1,3.8) | 0.715 | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.424 |
| IgG (s/co) | 20.7(13.5,80.8) | 32.4 ± 20.7 | 0.214 | 33.5 ± 28.3 | 27.2 ± 26.0 | 0.123 | 18.8 ± 15,3 | 13.2 ± 7.6 | 0.461 |
Values are presented as mean ± SD or median (P25, P75). WBC, White blood cells; CRP, C-reaction protein; PCT, procalcitonin; IL-6, interleukin-6; LAC, lactate; ALT, alanine aminotransferase; Cr, creatinine; cTnT, cardiac troponin T; CK-MB, creatine kinase-MB.
There are two main mechanisms of MSCs therapy for COVID-19. Firstly, MSCs could lodge in the pulmonary vascular bed after injection, release anti-inflammatory mediators and reduce the cytokine storm caused by viral infection.7 Secondly, MSCs could secrete angiopoietin-1 and keratinocyte growth factor, which are pivotal in the restoration of alveolar capillary barriers disrupted by COVID-19.8
In our series, all the patients with severe COVID-19 survived and entered recovery after MSCs therapy, and only 3 patients experienced treatment side effects. This result indicated that MSCs therapy might be an effective therapeutic for severe COVID-19. However, none of the inflammation indexes changed significantly after MSCs therapy. The reason is unclear, may be related to three factors. Firstly, inflammation indexes, such as WBC counts and CRP were totally normal before MSCs therapy in most cases, which means that cytokine storm was mild to moderate and not serious in these cases. Secondly, relative studies have shown that MSCs will be cleared within 24 to 48 h after injection.9 Nevertheless, in our study, laboratory tests were conducted 48 to 72 h after injection. As a result, we might miss the optimal time to track the changes of inflammation indexes. Thirdly, the inflammation indexes tested and analyzed in this study were limited, and whether other cytokines like IL-2 and IL-7 would decrease after MSCs therapy is unknown.
Additionally, we found that the serum levels of LAC, cTnT and CK-MB were elevated significantly after MSCs therapy. The reason is unclear, but remind us that the use of MSCs therapy should be extremely cautious in patients with metabolic acidosis or coronary heart disease. Moreover, the infusion speed of MSCs must be slow enough. In this study, we injected MSCs saline solution at a speed of ∼20 drops per minute, but there was still a patient experiencing heart failure while on treatment.
The major limitations of this study were small series, retrospective and no placebo. Therefore, additional prospective studies involving large cohort of patients are needed in order to confirm and supplement the present findings.
In conclusion, we suggested that MSCs therapy might be a promising option for the treatment of severe COVID-19, but should be used cautiously, especially in patients with metabolic acidosis or coronary heart disease.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Acknowledgements
The authors wish to thank all the clinicians for their hard work and sacrifices.
Ethical standards
As a retrospective study and data analysis was conducted anonymously, written informed consent was not required in this study.
References
- 1.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infection. 2020 doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. 105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infection. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J., Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L., Li T.S. [Interpretation of "guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (Trial Version 5)"] Zhonghua Yi Xue Za Zhi. 2020;100(11):805–807. doi: 10.3760/cma.j.cn112137-20200205-00199. [DOI] [PubMed] [Google Scholar]
- 7.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R.M., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Resp J. 2020 doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 9.Armitage J., Tan D.B.A., Troedson R., Young P., Lam K.V., Shaw K., Sturm M., Weiss D.J., Moodley Y.P. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur Respir J. 2018;51(3) doi: 10.1183/13993003.02369-2017. [DOI] [PubMed] [Google Scholar]