Highlights
-
•
Sheep and goats were domesticated around 9000 BC in the Middle East. Milk from sheep is very popular in many populations and areas. Taste and high fat content of cheeses from sheep milk make them popular. In many countries, dairy products from sheep and goats are consumed by children and people with allergies to cow milk. Other regions of the world, are not suitable for cows and goats, and sheep milk is an essential part of local daily diet.
-
•
Consumption of raw milk and raw milk products have a zoonotic risk. The main pathogens associated with sheep milk are: Brucella melitensis, Campylobacter spp., Coxiella burnetii, Leptospira spp. Listeria spp., Salmonella spp., Shiga-toxin producing Escherichia coli, tick borne encephalitis, Toxoplasma gondii, and Rift Valley fever. This risk of these pathogens can be reduced by proper flock health management, prevention of contamination during milking and adequate milk processing, transport and storage. In small scale production systems there is a higher risk, compared to industrialized production systems because of a less protocolled and controlled production process. Especially, young children, elderly people, pregnant women and immunocompromised (YOPI) persons, and those suffering from disease should be aware of the risk of consuming raw milk and raw milk products. Therefore, strict hygiene practices throughout the production process and supply chain in combination with stringent periodic control of these products should be applied. Although pasteurization influences the taste of milk, it sufficiently reduces zoonotic risks while not negatively influencing the nutritional value.
-
•
This manuscript describes major and minor pathogens that might be transmitted in milk from sheep. Also, unintended contamination and multiplication and prevention thereof are described. We summarize some major human outbreaks caused by consumption of milk from sheep and finally discuss the implications.
Keywords: Sheep, Milk, Zoonoses, Foodborne infection
Abstract
Sheep were domesticated around 9000 BC in the Middle East, and since then milk from sheep gradually became very popular, not only for drinking but also for making cheeses and other dairy products. Nowadays, these dairy products are also important for people with an allergy to cow milk, and these products are an essential part of the local daily diet in regions of the world that are not suitable for cows and goats.
Consumption of raw milk and raw milk products has a zoonotic risk, and with regard to sheep, the main pathogens associated with such dairy products are: Brucella melitensis, Campylobacter spp., Listeria spp., Salmonella spp., Shiga-toxin producing Escherichia coli, Staphylococcus aureus, tick borne encephalitis virus, and Toxoplasma gondii. Especially, young children, elderly people, pregnant women and immunocompromised (YOPI) persons, and those suffering from disease should be aware of the risk of consuming raw milk and raw milk products. This latter risk can be reduced by proper flock health management, prevention of contamination during milking, adequate milk processing, transport, and refrigerated storage. Only processes equaling pasteurization sufficiently reduce zoonotic risks from milk and milk products, but proper cooling is essential and recontamination must be prevented. Therefore, strict hygiene practices throughout the production process and supply chain especially for raw milk and raw dairy products, should be applied. Small scale production systems pose a greater risk compared to industrialized production systems because of a less protocolized and controlled production process.
This manuscript describes zoonotic risks of pathogens from sheep and their milk borne transmission. Additionally, routes of contamination, possibilities for multiplication, and prevention measures thereof are described. We summarize some major human outbreaks caused by consumption of sheep milk and products made thereof, and finally discuss their implications.
1. Introduction
Nowadays, milk from sheep and products made from sheep milk are popular in many populations and areas. Furthermore, these products are consumed by people with allergies to cow milk. In ancient days, except for nomadic life and backyard farming, milk was collected from small farms with small numbers of animals, and milk and milk products were consumed mainly by those living nearby. In the early 1900’s, urbanization level and the demand for dairy products increased, leading to industrialization of the dairy industry in more developed countries. In contrast with the dairy cattle industry, currently, milk production from sheep in many countries still takes place on a small scale and is of minor importance, volume wise (Ranadheera et al., 2018). However, sheep and goat milk have probably been used for a longer time by mankind than cow milk, as sheep and goats were domesticated from around 9000 BC in the Middle East, while cattle were domesticated more than a millennium later (Brandford Oltenacu, 2004).
In the EU, for example, sheep milk production nowadays represents only 1.8 % of the annual milk production, and production mainly (2011: 92 %) takes place in Greece, Spain, France, Italy, and Romania. Notably, two thirds of the world’s sheep milk is produced in the Mediterranean area. In 2016, Europe produced 3.0 million tons of sheep milk, mainly used for the production of dairy products (Gonzales-Barron et al., 2017). Mostly, processing takes place at farm level, in small local dairies or in regionally operating cheese factories, often using raw milk (Gonzales-Barron et al., 2017). This small scale production results in a wide range of special products made from sheep milk that are mainly regionally consumed (Ranadheera et al., 2018).
Foodborne infections are responsible for millions of human illness cases each year, and the morbidity rate is higher in less developed countries. However, in developed countries, raw milk and raw milk products are nowadays widely promoted, by certain groups in the society, as healthy and more tasteful compared to heat treated milk, thus increasing health risks in countries with otherwise high health standards.
One of the oldest signs of foodborne infections in humans possibly caused by consumption of sheep milk was found by anthropological examination of skeletons of people who fled for the vulcanic eruption of Mount Vesuvius, 79 AD. Typical bone lesions of brucellosis were found in 17.4 % of adult victims. This was consistent with the presence of cocco-like forms that morphologically and dimensionally seemed to be Brucella spp., suggested to be linked to consumption of dairy products from sheep (Capasso, 2002).
The risk of milk borne infections has decreased during industrialization, because of the introduction of thermal processing of milk, standardization of production processes, good manufacturing and hygiene practices along the food supply chain, as well as the implementation of regulations. Nevertheless, as long as pathogenic microorganisms are present in raw milk, consumption of (raw) dairy products will remain a public health concern.
Zoonoses are infections that can spread from animal to man. Most zoonotic agents from small ruminants are transmitted by direct contact or through inhalation after becoming airborne. Also transmission by vectors can occur. Therefore, people with direct and indirect contact, like farmers, veterinarians and slaughterhouse personnel are at prime risk. However, transmission of pathogens from animals to humans can also occur via consumption of milk and meat, especially when these products are consumed raw (Ganter, 2015).
Ample research has been published on the risks associated with consumption of raw milk and products made thereof. However, mainly focusing on cow’s milk, and only limited on sheep’s milk. This review aims to describe zoonotic risks of pathogens from sheep and their milk borne transmission, possible effects on human health, and risks of antimicrobial resistant bacteria. Furthermore, we describe routes of contamination, possibilities for multiplication and prevalence of these pathogens in milk from sheep and products made thereof as well as measures to control these, and summarize and discuss the critical control points in this process.
2. Risks of zoonotic pathogens from sheep and their milk borne transmission
Milk is a natural product, secreted by the mammary gland of mammals. Milk is rich in proteins, lipids, and sugars, and contains some minerals and vitamins. Presence of micro-organisms, of which some may pose a risk to human health, is normal. Most pathogenic micro-organisms that can be present in milk are associated with more milk-producing animal species, such as bovines, small ruminants, horses, donkeys and camelids, but others are more specifically associated with a single species (EFSA BIOHAZ Panel, 2015). Some pathogens need to reach high numbers to cause disease in man, which makes illness caused by properly stored milk or milk products unlikely, as proper storage will prevent growth of most of these pathogens. However, some pathogens are able to grow at refrigerator temperatures, in which case a low initial microbiological load can result in life threatening loads even after properly storage. Furthermore, some other pathogens have a low infectious dose, thus growth is not necessary to cause infection in man.
Milk can get contaminated with micro-organisms in two ways: by endogenous or by exogenous transfer. In the former, micro-organisms are excreted with the milk; in the latter, they are introduced during or after milking either from the udder skin, from the environment (faeces, dust, apparatus, human, etc.), or during processing. In both cases, thermal treatment reduces the microbiological load of milk. Hygiene, both in the stable, during milking and at further processing can prevent or at least minimize exogenous contamination of milk and products made thereof (EFSA BIOHAZ Panel, 2015; Gonzales-Barron et al., 2017). In addition, hygiene in the stable and during milking reduces the transfer of pathogens from either the environment or infected animals to non-infected animals, thus also affecting the number of animals possibly excreting pathogens in the milk.
For this review, we distinguished between major and minor pathogens, based on expert opinions. This was done by discussing the combination of human incidence and severity of symptoms, from which the first one was leading factor. Major and minor pathogenic micro-organisms transmitted with milk and dairy can be divided in different groups (Table 1 ): 1) bacteria, like Brucella melitensis, pathogenic Escherichia coli, Listeria spp., and Salmonella spp. as most frequently described in sheep milk, 2) fungi, mainly causing bovine, but also described as cause of ovine mastitis, 3) parasites, with Toxoplasma gondii as main hazard, and 4) viruses that can be transmitted from sheep milk to humans like tick borne encephalitis virus (TBEV) and Rift Valley fever virus (RVFV).
Table 1.
Micro-organisms with zoonotic potential, associated with sheep and their possibly milk borne transmission.
| Micro-organisms that may be present in sheep milk | |
| Bacteria | Brucella spp. |
| Campylobacter spp. | |
| Coxiella burnetii | |
| Leptospira spp. | |
| Listeria spp. | |
| Salmonella spp. | |
| Shiga-toxin producing Escherichia coli Staphylococcus aureus | |
| Fungi | Not known |
| Parasites | Toxoplasma gondii |
| Viruses | Rift Valley fever virus (RVFV) |
| Tick borne encephalitis virus (TBEV) | |
| Micro-organisms with minor risk of transmission via sheep milk | |
| Bacteria | Bacillus anthracis |
| Bacillus cereus | |
| Chlamydia abortus | |
| Clostridium spp. | |
| Corynebacterium spp. | |
| Erysipelothrix rhusiopathiae | |
| Helicobacter spp. | |
| Mannheimia haemolytica | |
| Mycobacterium bovis | |
| Streptococcus spp. | |
| Yersinia spp. | |
| Fungi | Several species (see Table 3) |
| Parasites | Cryptosporidium spp. |
| Giardia duodenalis | |
| Viruses | Orf virus |
| Middle East respiratory syndrome coronavirus (MERS-CoV) | |
In the following paragraphs, we describe the main zoonotic micro-organisms with relevance to public health that are possibly associated with sheep and elaborate on their threat to public health via consumption of raw sheep milk and products made thereof. Micro-organisms of supposed minor zoonotic risk via milk and dairy products are briefly described in Table 2, Table 3 .
Table 2.
Bacteria associated with sheep and with minor zoonotic potential via milk or products made thereof.
| Agent | Explanation of the possible risk for humans |
|---|---|
| Bacillus anthracis (spore forming Gram-positive rod) | Human cases are mainly associated with contact with slaughtered of dead or succumbing animals, but food infections (meat) are possible. Milk is not considered as a vehicle (AFSSA, 2008; EFSA BIOHAZ Panel, 2015). |
| Bacillus cereus (spore forming Gram-positive rod) | Contaminates milk via environment. Associated (in EU) with milk from bovine animals, donkeys and horses, not with milk from small ruminants (EFSA BIOHAZ Panel, 2015). |
| Chlamydia abortus (Gram negative) | Human cases mainly associated after direct or indirect contact with shedding sheep and goats in the periparturient period. The pathogen is not considered to be transmissible via milk (EFSA BIOHAZ Panel, 2015). |
| Clostridium spp. (spore forming anaerobic Gram-positive rod) | Several species have zoonotic potential. In milk or cheese, clostridial spores don’t germinate and cells don’t replicate. Numbers necessary to cause illness are not reached (Turchi et al., 2016; Drouin and Lafrenière, 2012). Milk is not considered as a vehicle (EFSA BIOHAZ Panel, 2015). |
| Corynebacterium spp. (Gram-positive rod) | Infection is mainly labor-related (cutaneous infection) (Peel et al., 1997). Milk is a very rare transmission route for C. pseudotuberculosis (Claeys et al., 2013). |
| Erysipelothrix rhusiopathiae (Gram-positive rod) | Humans are generally infected by direct contact with animals or animal products (Brooke and Riley, 1999). The pathogen is not considered to be transmissible via milk (EFSA BIOHAZ Panel, 2015). |
| Helicobacter spp. (Gram-negative spirochaete) | Cause gastric infections in humans. H. pylori is found in sheep milk (Verraes et al., 2014). The role as zoonotic agent is under debate. The pathogen is not considered to be transmissible via milk (EFSA BIOHAZ Panel, 2015). |
| Mycobacterium avium subsp. paratuberculosis (acid fast rod) | Suggested cause of Crohn’s disease, however not conclusive (EFSA BIOHAZ Panel, 2013a). The zoonotic risk of this pathogen remains under debate. The pathogen is not considered to be transmissible via milk (EFSA BIOHAZ Panel, 2015). |
| Mycobacterium bovis (acid fast rod) | Mainly associated with cows. Experimentally infected sheep shed in milk (Keyhani, 1970). Raw milk or raw milk cheese are possible routes of transmission. |
| Streptococcus spp. (Gram-positive coccus) | For some pathogenic species, food is a known transmission route. Infections caused by consumption of raw milk and dairy products containing S. equi subs. zooepidemicus are rare and mainly related to cow milk (Barrett, 1986; EFSA BIOHAZ Panel, 2015; Eyre et al., 2010; Steward et al., 2017). |
| Yersinia spp. (Gram-negative rod) | Although Y. enterocolitica is present in sheep in Europe (Van Engelen et al., 2014), only Y. pseudotuberculosis is considered a hazard of low risk in Europe in relation to milk from small ruminants (EFSA BIOHAZ Panel, 2015). |
Table 3.
Non-bacterial agents associated with sheep with minor zoonotic potential via milk or products made thereof.
| Agent | Explanation of the possible risk for humans | |
|---|---|---|
| Fungi | ||
| Several species (such Candida albicans, Candida krusei, Candida tropicalis, and Trichophyton verrucosum) | In cattle, various pathogenic fungi can infect the udder and be exreted in milk. Of these species, only Candida albicans is considered a possible hazard in raw milk, but transmission via milk has not been reported. No information on the risk of fungi in sheep has been found. (Streinu-Cercel, 2012; Panelli et al., 2014; EFSA BIOHAZ Panel, 2015). | |
| Parasites | ||
| Cryptosporidium spp. | Zoonotic potential differs per species. Cryptosporidium parvum, and Cryptosporidium cervine, might be relevant. Milk borne human cases are not described (FAVV, 2015). Therefore, although considered to be transmissible via milk, Cryptosporidium spp. are not considered as a hazard in sheep milk in Europe (EFSA BIOHAZ Panel, 2015). | |
| Giardia duodenalis | Immunocompromised or young individuals are at risk (Giangaspero et al., 2005). G. duodenalis consists of several assemblages which seem more or less host specific (Monis and Thompson, 2003). Milk borne human illnesses are not described. Raw milk and dairy are not considered a transmission route (EFSA BIOHAZ Panel, 2015). | |
| Viruses | ||
| Orf virus | Causes skin lesions in humans and small ruminants. Lesions on the udder of small ruminants can be present. Nevertheless, direct contact is the main route of infection. The pathogen is not considered to be transmissible via milk (EFSA BIOHAZ Panel, 2015). | |
| Middle East respiratory syndrome coronavirus (MERS-CoV) | In humans, the course of infections can vary from asymptomatic to death (Miguel et al., 2015). Dromedary camels are strongly suspected to be the source of this zoonotic disease. Inoculation of sheep and goats did not result in shedding of MERS-CoV (Adney et al., 2016). Therefore, the possible role of MERS-CoV as zoonotic agent from sheep milk seems to be limited. |
2.1. Bacteria
2.1.1. Brucella spp
Brucella spp. are Gram-negative bacteria that cause brucellosis, one of the major zoonotic infections worldwide (Pappas et al., 2006). In sheep, Brucella melitensis is the most common cause of brucellosis, but Brucella abortus can also play a minor role in small ruminants (Ganter, 2015). Brucellosis in sheep causes economic losses as a consequence of abortion, fertility problems, decreased milk production, and increased costs of treatment. B. melitensis is still endemic in West and Central Asia, Africa, and Latin America. In Europe, B. melitensis is mainly present in Mediterranean countries. In Australian flocks, B. melitensis has never been detected (FSANZ, 2009b).
In humans, Brucella spp. cause non-specific, mild clinical signs, or more severe and chronic symptoms. Four species of brucella are pathogenic to human, of which B. abortus and B. melitensis are associated with sheep. B. melitensis causes the most severe symptoms. In countries where brucellosis is not endemic, diagnosis may be delayed, resulting in a more severe course of the disease (RIVM, 2013). For humans, vaccines are not available (Ducrotoy et al., 2017), and treatment with antibiotics is difficult (El-Sayed and Awad, 2018).
Brucella spp. infected animals secrete the bacterium during and after parturition and abortion in genital fluids, and in milk, but contamination of milk may also occur from the environment (Claeys et al., 2013). B. melitensis does not grow under refrigerated conditions, but small numbers (10–100 bacteria) can cause disease in man (EFSA BIOHAZ Panel, 2015; El-Sayed and Awad, 2018). Prevalence of Brucella spp. in raw sheep milk will depend on the endemic status of a region, and can be as high as 46 % (FSANZ, 2009b). People get infected by Brucella spp. via contact with birth products of shedding animals and after consumption of contaminated animal products. Risk factors for brucellosis are working on a dairy farm, consumption of raw milk, home slaughtering of sheep, and working in a slaughterhouse (El-Metwally et al., 2011). Control of animal brucellosis, food hygiene measures and pasteurization of milk, known to adequately reduce Brucella spp. (Juffs and Deeth, 2007), are the appropriate methods to reduce the occurrence of this disease (Ducrotoy et al., 2017).
B. abortus is not considered by EFSA as a main hazard in sheep milk in the EU due to the low incidence of human cases. B. melitensis, however, is considered a main hazard due to the severity of the disease and the number of cases (EFSA BIOHAZ Panel, 2015).
2.1.2. Campylobacter spp
Campylobacter spp. are Gram-negative curved bacteria and part of normal gut flora of many animal species, including dairy livestock, such as sheep (Horrocks et al., 2009). Several Campylobacter spp. have zoonotic potential, causing both mild (gastrointestinal disease) and severe symptoms (Guillain Barré syndrome) in man (Huang et al., 2015). Campylobacter jejuni and Campylobacter coli are the most frequently reported species causing foodborne disease (Horrocks et al., 2009). The infectious dose varies between 100 and 10,000 bacteria (FAVV, 2013; Juffs and Deeth, 2007; Verraes et al., 2014). But also Campylobacter fetus subsp. fetus, which causes abortion in sheep, is zoonotic (Huang et al., 2015).
Infection of animals with Campylobacter spp. occurs vertically or horizontally through faeces, and during abortion (Horrocks et al., 2009; Huang et al., 2015). Presence of Campylobacter spp. in milk is mainly caused by (in)direct faecal contamination (van Bokhorst-van de Veen et al., 2015), and indirect with Campylobacter fetus subsp. fetus after abortion (Huang et al., 2015).
Data reported to EFSA (2011–2015) show an overall prevalence of Campylobacter spp. in any type of milk of 0.6 %. Data on sheep milk are however scarce (EFSA and ECDC, 2013, 2014; EFSA and ECDC, 2015a, b; EFSA and ECDC, 2016). In a review, Verraes et al. (2014) only mentions one study in which Campylobacter spp. was detected in sheep milk, with a prevalence of 2.2 % (N = 90 samples). No additional data were available in the EFSA opinion on risks of raw drinking milk. A meta-analysis carried out by Christidis et al. (2016) on data available in North America, Europe and Oceania, showed a weighted mean prevalence of campylobacter in raw milk of any type of animal of 1.2 %. Regional and animal differences were noticeable; the highest prevalence was observed in the United Kingdom (6.4 %), and cow milk (1.3 %) more frequently contained campylobacter than milk from other animal species. For sheep milk, a prevalence of 1.1 % was estimated, based on five studies, including a total of only 135 samples from sheep.
Campylobacter spp. do not grow below 30 °C and numbers will decline slowly in milk during storage. Raw milk consumption is frequently associated with outbreaks of campylobacteriosis. Pasteurization is adequate to reduce the numbers of Campylobacter spp. in raw milk (Juffs and Deeth, 2007; EFSA BIOHAZ Panel, 2015; Huang et al., 2015).
Campylobacter spp. may survive during manufacturing of cheese (Christidis et al., 2016), which is confirmed by data of EFSA (2011–2015) that show an overall prevalence of Campylobacter spp. in cheeses of 0.2 %. For cheeses made from sheep milk, a prevalence of 1.3 % (n = 2) was reported for C. jejuni. Cheeses were made from raw or low heat-treated milk. Reviewing data on prevalence studies of Campylobacter spp. in cheeses and butter in general in Europe, however, did not reveal any positives (FAVV, 2015; Verraes et al., 2015). In raw sheep milk, Campylobacter spp. are considered a main hazard (EFSA BIOHAZ Panel, 2015), but also products made thereof are associated with campylobacteriosis.
2.1.3. Coxiella burnetii
Q fever is a zoonotic disease caused by the intracellular Gram-negative bacterium Coxiella burnetii. This pathogen is found worldwide, with the exception of New Zealand (Pires et al., 2017). Although this bacterium can be shed by other animal species like dogs, cats, birds and ticks, ruminants are the main animal reservoir of C. burnetii, which may cause reproductive disorders. After shedding, which mainly occurs in birth products after abortion, but also after normal parturition, C. burnetii contaminated aerosols are the main route of transmission to humans. Worldwide, several airborne outbreaks of Q fever have been related to shedding of C. burnetii by small ruminants (Van den Brom et al., 2015). In the Netherlands, a large Q fever outbreak with shedding dairy sheep and dairy goats as source caused illness in more than 4000 people. Serious complications of Q fever are known and even mortality has been described (Kouijzer et al., 2018).
In addition to this airborne route, a foodborne route may exist as C. burnetii can be excreted in milk from ruminants, and shedding in milk can persist for several months, for goats longer than for sheep. Despite this, the role of food in the transmission of C. burnetii to humans is still under debate (Verraes et al., 2015). Being an intracellular bacterium, C. burnetii does not grow in milk. C. burnetii is considered to be heat resistant, but the pasteurization process for milk is designed for a 5–6 logarithmic reduction in numbers of this specific pathogen (Juffs and Deeth, 2007; Pexara et al., 2018). Some publications suggest that Q fever outbreaks can be caused by consumption of raw milk (Gale et al., 2015; Pexara et al., 2018). These authors conclude that risk of C. burnetii infection through consumption of unpasteurized milk and milk products cannot be considered negligible but is low in comparison to transmission via inhalation of contaminated aerosols. Viable C. burnetii in cheese made from unpasteurized milk are rarely detected (Gale et al., 2015), but DNA from C. burnetii has been demonstrated by PCR in unpasteurized cheeses (Galiero et al., 2016). Outbreaks of coxiellosis caused by dairy products have not been described for Europe, USA and Canada (FAVV, 2015).
Therefore, it is not clear whether infection from raw milk and raw milk products will result in clinical Q fever (EFSA AHAW Panel, 2010; EFSA BIOHAZ Panel, 2015; Pexara et al., 2018).
2.1.4. Leptospira spp
Several species of the Gram-negative genus Leptospira are zoonotic, causing leptospirosis, a disease with a wide spectrum of symptoms in humans, ranging from mild infection to severe multi-organ infection syndromes with high mortality (Fratini et al., 2016). The disease is more common in tropical regions than in a moderate climate and the source of human infection is direct or indirect contact with urine of an infected animal. Infections are mainly acquired through occupational or recreational exposures. Dairy cattle and sheep are known as maintenance hosts of some Leptospira species and livestock farming is a major occupational risk factor worldwide. Dairy farming, especially milking, has the highest risk and is associated with the serovar Hardjo, that can also be present in sheep (Levett, 2001). However, sheep seem less susceptible to leptospirosis than other animal species (Bertelloni et al., 2017). The transmission of Leptospira spp. occurs through urine or other material from infected animals, water, soil or forages. Transmission to humans may occur through contaminated milk, following either direct excretion in milk (rarely) or urinal contamination of milk (Levett, 2001; Ganter, 2015). Nevertheless, milk is not regarded as a transmission route by EFSA (EFSA BIOHAZ Panel, 2015).
2.1.5. Listeria spp
The Gram-positive genus Listeria comprises a number of species, of which Listeria monocytogenes is the most relevant for human health (Rocha et al., 2017). An infection is typically foodborne (Buchanan et al., 2017), and may pass asymptomatically, or with mild gastroenteritis, or may result in severe outcomes with sepsis, meningitis and, for pregnant women, miscarriage or stillbirth (Rocha et al., 2017). The infective dose of L. monocytogenes is assumed to be >1000 bacteria for the general population, but lower (10–100) for susceptible populations (FSANZ, 2009b). New data from EFSA suggest that this dose is generally higher, and that 90 % of the invasive cases of listeriosis are caused by food containing >2000 CFU/g (EFSA BIOHAZ Panel et al., 2018).
Listeria spp. are ubiquitously present in the environment (Buchanan et al., 2017), which (e.g. feed and contaminated equipment) is the most important contamination source of milk, but direct contamination of milk by shedding animals also occurs (Gonzales-Barron et al., 2017; Jordan et al., 2016). If so, L. monocytogenes may be shed intermittently in high numbers in milk of infected animals (FAVV, 2015; Verraes et al., 2014). Shedding may continue for a long period, also in ewes not showing signs of disease (Gonzales-Barron et al., 2017; Schoder et al., 2011). Ewes with subclinical mastitis are a more important source of contamination than the environment (Condoleo et al., 2017), and in small flocks, one single ewe can have a large impact on L. monocytogenes levels in bulk tank milk. In addition to risk factors as flock size, and feeding silage (Schoder et al., 2011), contamination prevalence may depend on the season, with a decreasing trend from start to end of season after lambing (Condoleo et al., 2017).
It is assumed in general, however, that milk is only contaminated with low numbers (0.01–10 cfu/L), which is based on data from cow milk (EFSA BIOHAZ Panel, 2015). In milk, Listeria spp. compete with the natural microbiota, and growth is therefore limited (Claeys et al., 2013). Cheese, however, and especially soft and semi-soft cheese, is an ideal medium for growth of L. monocytogenes (van Asselt et al., 2017), even more so, since this pathogen grows at low temperatures and shelf life of cheeses is relatively long. Using pasteurized milk for cheese making effectively reduces the number of L. monocytogenes present in cheese. However, this pathogen is frequently associated with recontamination events (Juffs and Deeth, 2007; Buchanan et al., 2017; van Asselt et al., 2017).
A meta-analysis of Gonzales-Barron et al. (2017) demonstrated goat milk cheeses significantly more often to contain L. monocytogenes (12.8 %; 7 studies) than sheep milk cheeses (3.6 %). Data reported to EFSA (2011–2015) show that for cheeses made from sheep milk, soft and semi-soft cheeses were most often contaminated (2.3 %), which is also the case for cow milk cheeses. Interestingly, hard (1.7 %) and fresh cheeses (2.0 %) made from sheep milk contain L. monocytogenes more frequently than cheeses made from cow milk (1.4 %). For hard cheeses made from sheep milk, 0.8 % exceeded the limit of 100 cfu/g. These data counterspeak the general opinion that hard cheeses do not pose such a great risk concerning L. monocytogenes, which is based on the fact that L. monocytogenes has a lesser ability to grow in hard cheeses (Buchanan et al., 2017; Lahou and Uyttendaele, 2017; van Asselt et al., 2017). However, in all cases, soft and semi-soft cheeses showed the highest number of samples exceeding the European Union limit of 100 cfu/g. Based on data presented, it seems fair to suggest that cheese made from sheep or goat milk pose a greater risk to the health of the consumer than cow milk cheese. Although L. monocytogenes is present in raw milk, it is not considered a main hazard in this product (EFSA BIOHAZ Panel, 2015). This pathogen is, however, a hazard in dairy products (EFSA BIOHAZ Panel et al., 2018).
2.1.6. Salmonella spp
Salmonella spp. are Gram-negative bacteria that belong to the family of Enterobacteriaceae. The genus is divided into two species, of which mainly serovars belonging to the group of Salmonella enterica subsp. enterica are associated with warm blooded animals and human disease. While Salmonella Enteritidis and Salmonella Typhimurium are serotypes that are most frequently reported to cause human foodborne illness, in essence all serotypes are pathogenic for humans (European Commission, 2003). Symptoms vary from mild (gastroenteritis) to severe (e.g. sepsis) and from acute to chronic (e.g. irritable bowel syndrome) (Steiner, 2013).
The main contamination route of milk with Salmonella spp. is via faeces or other material from the environment, although Salmonella spp. may be excreted directly in the milk by an infected animal (Claeys et al., 2013; van Asselt et al., 2017). Salmonella spp. will not grow in refrigerated milk (EFSA BIOHAZ Panel, 2015), nor in cheese. However, in cheese survival can extend for a long period (Alemdar and Aĝaoĝlu, 2010). Pasteurization of milk effectively inactivates Salmonella spp. serotypes (Juffs and Deeth, 2007).
The infectious dose depends on the serotype, but is estimated to be as low as one organism (FAVV, 2013) or between 10 and 100 (Juffs and Deeth, 2007), and thus very low concentrations in raw milk and cheese may already cause foodborne illness (van Bokhorst-van de Veen et al., 2015).
Data reported to EFSA (2011–2015) show an overall prevalence of Salmonella spp. in any type of milk (raw, pasteurized, cow, goat, sheep) of 0.1 %. Out of 47 recorded milk samples from sheep, two were positive (4.3 %). Data on prevalences of Salmonella spp. in sheep milk are scarce. The meta-analysis study of Gonzales-Barron et al. (2017) calculated an overall prevalence of 1.4 % (95 % confidence interval (CI): 0.3–6.6 %) in raw sheep milk, based on four studies.
Data of EFSA showed a prevalence of 0.2 % for Salmonella spp. in cheese in general, as well as in cheeses made from sheep milk. In their review on microbial hazards of raw milk dairy products (any dairy animal origin), Verraes et al. (2015) only found one study in which Salmonella spp. was detected in cheese. In this Portugese study, cheeses from milk of cow, goat and sheep were analysed (N = 70 samples), and the positives were (semi-)soft sheep milk cheeses (FAVV, 2015).
In raw sheep milk Salmonella spp. are considered a main hazard (EFSA BIOHAZ Panel, 2015), as is the case for products made thereof.
2.1.7. Shiga-toxin producing Escherichia coli
Escherichia coli are Gram-negative rods that belong to the family of Enterobacteriaceae. Traditionally, enteric E. coli have been divided into six pathotypes (Clements et al., 2012). Of these, isolates belonging Shiga-toxin producing E. coli (STEC) are of particular importance in the context of food safety. Illnesses associated with STEC range from mild to bloody diarrhoea, to haemorrhagic colitis and the haemolytic uraemic syndrome, and thrombocytopenia (EFSA BIOHAZ Panel, 2013b). The infective dose is low (<10 bacteria) (FSANZ, 2009b). Ruminants, in particular cattle and sheep, are considered to be the main reservoir of STEC (FSANZ, 2009b; EFSA BIOHAZ Panel, 2013b). In a Dutch study among 24 dairy sheep farms, STEC was found on all of these farms (Opsteegh et al., 2018).
Contamination of milk with pathogenic E. coli occurs through faecal material present on teats and udder, or from the environment (EFSA BIOHAZ Panel, 2015). Also E. coli O157:H7, the major STEC serotype with regard to public health, can be present in raw milk from sheep (1%) (Verraes et al., 2014). A study from Caro et al. (2011) showed even a prevalence of 18 % for E. coli O157, 8% for E. coli O111, and 6% for E. coli O26. Mean levels (based on Most Probable Number (MPN)) for E. coli O157 and O111 were 0.22 and <0.04 MPN/mL, respectively. The meta-analysis of Gonzales-Barron et al. (2017) on prevalence data of STEC in sheep milk shows an average prevalence of 4.8 % (95 % CI: 2.2–10.4 %; based on 8 studies), not different from the prevalence in goat milk but higher than in cow milk (1.8 %), as reported to EFSA (2011–2015). Data on sheep milk are hardly present in the EFSA database.
Data reported to EFSA (2011–2015) show an overall prevalence of STEC in cheeses made from sheep milk of 2.0 %. The meta-analysis of Gonzales-Barron et al. (2017) showed a difference in STEC prevalence between cheeses made from sheep milk (2.8 %; 5 studies) and goat milk (4.3 %; 13 studies). This study also showed that, although STEC was more prevalent in raw milk cheese from sheep or goat (10.0 %), STEC was also quite frequently present in pasteurized cheeses (4.7 %). As pasteurization effectively destroys pathogenic E. coli (Juffs and Deeth, 2007), this most likely is a result of processing failures or recontamination.
In raw sheep milk STEC is considered a main hazard (EFSA BIOHAZ Panel, 2015), as is the case for products made thereof.
2.1.8. Staphylococcus aureus
Staphylococcus aureus is a Gram-positive bacterium, and a main cause of mastitis in sheep (Macori et al., 2017). Spreading of an infection within a flock occurs through contact with milking equipment, contaminated bedding and milk (van Asselt et al., 2017). Milk can also get contaminated through milking equipment, the environment and milkers (Claeys et al., 2013). Monitoring of hygiene on sheep farms together with reduction of S. aureus are the main prevention measurements (Gonzales-Barron et al., 2017). S. aureus grows slowly in milk at 7 °C (EFSA BIOHAZ Panel, 2015).
S. aureus belongs to the group of coagulase-positive staphylococci, which are associated with human illness (van Bokhorst-van de Veen et al., 2015). Foodborne illness is caused by enterotoxins, known as staphylococcal enterotoxins (SET). Enterotoxins are only produced when sufficient numbers of S. aureus are present, are not produced at temperatures <10 °C (van Bokhorst-van de Veen et al., 2015), and are heat stable (van Asselt et al., 2017). Although S. aureus is a fairly heat resistant vegetative micro-organism, pasteurization is considered effective in reducing numbers to an adequately low level (Juffs and Deeth, 2007).
Prevalence of S. aureus in raw sheep milk is estimated at 39.4 % (95 % CI: 22.7–58.9 %; based on 6 studies); no studies on S. aureus in sheep cheese are available. However, in goat milk, S. aureus is detected at a similar rate (35.2 %; 95 % CI 23.2–49.3 %, based on 19 studies) as in sheep milk, and for goat milk cheese the prevalence is 16 % (based on 21 studies) (Gonzales-Barron et al., 2017). Presence of S. aureus in dairy may also come from post-pasteurization contamination, poor hygiene and/or human error (Claeys et al., 2013).
Although raw milk is likely to be spoiled before S. aureus reaches numbers sufficiently high to produce enterotoxins in milk (Verraes et al., 2014), data reported to EFSA (2011–2015) show a prevalence of SET in milk of 1.4 % (N = 1109 samples). Only four samples were sheep milk, and all were negative. Although S. aureus and SET are present in and associated with outbreaks of raw drinking milk from cows, goats, sheep, horses and donkeys, it is not considered a main hazard in this product in the EU, based on incidence and/or severity of disease (EFSA BIOHAZ Panel, 2015).
In cheese, the above mentioned numbers necessary to produce SET can be reached due to initial high numbers already present in the raw milk or due to an insufficient start of the fermentation process allowing S. aureus to grow (van Asselt et al., 2017). Preventing foodborne problems caused by S. aureus starts with preventing mastitis and limiting growth in milk bulk tanks by temperature control (<6 °C), in addition to pasteurization, rapid drop of pH, salt supplementation, proper fermentation during the cheese production process, and temperature control during further storage reduces the risk (van Bokhorst-van de Veen et al., 2015).
In Europe, a food safety criterion is set for SET in cheese. Levels of SET have to be determined when the number of coagulase-positive staphylococci in cheese exceeds the limit of 100.000 cfu/g. According to data reported to EFSA (2011–2015), SET was present in 2.5 % of the cheeses made from sheep milk. For cheeses made from cow milk and goats milk this is 0.5 % and 1.8 %, respectively.
In raw sheep milk S. aureus is not considered a main hazard (EFSA BIOHAZ Panel, 2015), but it is a hazard in products made thereof (FAVV, 2015).
2.1.9. Mannheimia haemolytica
Mannheimia haemolytica, a significant mastitis pathogen, can also be occasionally a potential zoonotic pathogen. Infection of people with this pathogen, transmitted from various animal sources, can lead, among others, to endocarditis, splenic abscessation (Takeda et al., 2003), and even fatal septicaemia (Punpanich and Srijuntongsiri, 2012). No milk borne transmission is described. The pathogen is not considered to be transmissible via milk (EFSA BIOHAZ Panel, 2015).
2.2. Fungi
In people, fungi can cause infection, particularly in those who are immunocompromised (Streinu-Cercel, 2012). In cattle, various pathogenic fungi can infect the udder and be excreted in milk, such as Candida albicans, Candida krusei, and Candida tropicalis (Dhanashekar et al., 2012). Of these species, only C. albicans is considered a possible hazard in raw milk, but as transmission via milk has not been reported, it is not considered a risk in raw milk (EFSA BIOHAZ Panel, 2015). Pathogenic fungi can survive moderate heat treatment. Total inactivation by heat can be established with a sufficient combination of temperature and duration. No information on the risk of fungi in sheep has been found. Therefore, sheep are not considered as a risk for fungal infections in humans after consumption of milk or milk products.
2.3. Parasites
2.3.1. Toxoplasma gondii
Toxoplasmosis is caused by the obligate intracellular parasite Toxoplasma gondii. In sheep and goats, T. gondii causes abortion, stillbirth, birth of weak lambs and fertility problems (Van Engelen et al., 2014). In humans, toxoplasmosis is mainly a risk when primary infection is acquired during pregnancy or in immunocompromised individuals. In other circumstances, infections are usually either asymptomatic or self-limiting (Deng et al., 2016; Belluco et al., 2017; Hussain et al., 2017). In the Netherlands and the USA, the disease burden estimates of this parasite are high (Hussain et al., 2017; Mangen et al., 2017).
Toxoplasma is a parasite with a sexual cycle in cats resulting in the production of oocysts, and an asexual cycle in a wide range of animal species including man. Cats are the key end host in the transmission cycle of this parasite and excrete oocysts in their faeces. Animals, such as sheep, get infected by ingestion of oocysts from e.g. water, grass or other feed that is contaminated with cat faeces. People, in addition, can get infected successively by ingestion of tissue cysts present in meat of infected animals or by ingestion of tachyzoites that are excreted in milk from animals suffering from acute toxoplasmosis (Deng et al., 2016). Ingestion by contaminated food is considered the main route of transmission for humans (Belluco et al., 2017; Boughattas, 2017).
Presence of (DNA of) T. gondii in milk from small ruminants has been described (Hussain et al., 2017), also in milk from sheep specifically (Camossi et al., 2011; de Santana Rocha et al., 2015; Fusco et al., 2007; Luptakova et al., 2015). Tachyzoites can survive at least several days in milk (EFSA BIOHAZ Panel, 2015). Furthermore, illness has been reported due to consumption of raw goat milk (Deng et al., 2016), and T. gondii does survive the processing of fresh cheese. However, in a systematic review on the risk of toxoplasmosis from food consumption, unpasteurized milk has been considered as unimportant (Belluco et al., 2017). This may be explained by the fact that tachyzoites are more sensitive to environmental conditions than tissue cysts and oocysts, (FSANZ, 2014). A meta-analysis on the milk-borne infection route of humans by Boughattas (2017) showed that risk factors for the milk borne infection route of T. gondii are consumption of goat milk and milk products, being immunocompromised, and living in North America, Middle East, and Latin territories. Also EFSA mentioned that most reported cases of toxoplasmosis acquired through the consumption of raw milk are from outside Europe (EFSA BIOHAZ Panel, 2015).
2.4. Viruses
2.4.1. Rift valley fever virus
Rift Valley fever (RVF) is an arboviral zoonotic disease which affects animals like cattle, sheep and goats, as well as humans. Infections caused by RVF virus (RVFV) have been reported at least since the 1930’s in Africa and the Arabian Peninsula (Nicholas et al., 2014). In humans, infections mostly remain asymptomatic, however a significant number of infected people develop severe disease which includes haemorrhage, encephalitis, visual disturbances, and even occurrence of death (Ng’ang’a et al., 2016); RVFV is considered a major pathogen.
RVFV can spread by bites of mosquitos, but also by contact with infected animals and their products or tissues. Risk factors to acquire RVF are gender, contact with birthing animals, slaughtering and skinning of animals, and possibly drinking of raw milk (Nicholas et al., 2014). Pasteurization of milk decreases the risk of RVFV transmission to humans. Trade in small ruminants and their products plays an important role in spreading diseases such as RVF (Sherman, 2011).
Although, RVFV is excreted in the milk by infected animals (Claeys et al., 2013), transmission via milk is not considered important (OIE, 2009; EFSA BIOHAZ Panel, 2015).
2.4.2. Tick-borne encephalitis virus
Tick-borne encephalitis virus (TBEV) belongs to the genus Flavivirus, and is divided into three subtypes. The subtype which is endemic in Central and Western Europe is causing relatively mild symptoms in humans compared to the Siberian and Far-Eastern subtypes (Bogovic and Strle, 2015). Although TBEV is mainly transmitted from animals to humans via tick bites, transmission also occurs via consumption of raw milk and dairy products. TBEV is excreted in the milk by infected animals (Claeys et al., 2013). Symptoms seem to occur more frequently when the virus is transmitted through ingestion via food, than after transmission by tick bites (Dobler et al., 2012). TBEV is killed during pasteurization, and TBE in humans can be prevented by vaccination (Bogovic and Strle, 2015; EFSA BIOHAZ Panel, 2015; Offerdahl et al., 2016). Foodborne TBE outbreaks in Europe mainly occur after consumption of raw milk and dairy products from goats, followed by raw milk and dairy products from cows, but also cases after consumption of raw sheep milk and cheese have been described (Dobler et al., 2012; Grešíková et al., 1975; Kriz et al., 2009; Labuda et al., 2002; Zeman et al., 2004). Only one study, conducted in Poland, describes prevalence data for TBEV in raw milk, which involved screening raw milk from cows (11.1 % of 63 samples), sheep (22.2 % of 27 samples) and goats (20.7 % of 29 samples) (Cisak et al., 2010; EFSA BIOHAZ Panel, 2015). TBEV remains stable in refrigerated milk, but is inactivated during pasteurization (Offerdahl et al., 2016). In Europe, raw milk and dairy products from small ruminants are considered a main hazard for TBEV infection (EFSA BIOHAZ Panel, 2015).
3. Antibiotic resistance
Antibiotic resistance is a growing threat to both human and animal health, primarily through an increased risk of treatment failures. Bacteria can become resistant to antibiotics by mutations in genes associated with the mechanism of action of the compound or by acquisition of foreign DNA coding for resistance determinants through horizontal gene transfer. These processes occur independently of the use of antibiotics. However, once a resistant mutant emerges, used antibiotics eliminate the susceptible population and resistant bacteria will predominate (Munita and Arias, 2015). Therefore, resistant bacteria nowadays are more frequently seen as a consequence of the use of antibiotics than in the pre-antibiotic era (Ungemach et al., 2006; De Neeling et al., 2007; Scott and Menzies, 2011).
Several studies have been published on antibiotic resistant bacteria in sheep milk, both in milk from individual sheep, and in bulk milk, and also in raw sheep milk cheese. Studies were focused on resistant zoonotic bacteria, commensal bacteria, mastitis-causing bacteria, and specific resistances, including methicillin-resistant S. aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing Gram-negative bacteria, and vancomycin-resistant enterococci (VRE) (Abdalhamed et al., 2018; Abo-Shama, 2014; Alian et al., 2012; Ariza-Miguel et al., 2014; Azara et al., 2017; Burriel, 1997; Carfora et al., 2016; Ceniti et al., 2017; Corrente et al., 2003; Giacinti et al., 2017; Jiménez et al., 2013; Lollai et al., 2016; Macori et al., 2017; Mousavi et al., 2014; Obaidat et al., 2018; Onni et al., 2011; Ortigosa et al., 2008; Sanciu et al., 2012; Solomakos et al., 2009; Spanu et al., 2012, 2014). Comparing literature data on antibiotic resistant bacteria in raw sheep milk is complicated because of several differences, e.q. in methodologies used to detect resistant bacteria, in determining susceptibility of bacterial isolates, in antibiotics in the test panel, and in interpretation criteria used to categorize isolates as susceptible or resistant. Additionally, differences between countries probably largely relate to farm management regarding hygiene and storage of milk, prevalence of resistant bacteria in sheep and farm environment, and policy of antibiotic use.
In December 2008, the Dutch government and farming industry agreed to reduce the use of antimicrobials in farm animals (LNV, 2008). At the end of 2018, a reduction of more than 63 % had been achieved compared to 2009, and almost no antibiotics that are important in treating infections in humans have been used for animals in recent years (MARAN,2018; NethMap, 2019). The aim for the remaining period ending ultimately 2020 is another reduction of more than ten per cent, making a total reduction of at least 75 % per cent in a period of twelve years. During this journey, several hurdles were taken step by step, for example, by improving stable climate, management and use of vaccinations. Together with a reduction in antibiotic use, a slight reduction in resistance appeared (NethMap, 2019).
Although the use of antibiotics in small ruminants in the Netherlands was low compared to other farm animals like pigs, poultry and cattle, and accordingly it is likely that small ruminants only play a minor role in the development of antibiotic resistance in the entire livestock industry (Santman-Berends et al., 2014), raw sheep milk may contain antibiotic resistant bacteria, including multidrug resistant bacteria. Raw milk and raw milk products can therefore act as a source for bacteria that are resistant to different groups of antimicrobials, potentially constituting a health risk for consumers.
The principles of controlling resistance development in bacteria present in sheep milk involve infection control at herd level and prudent use of antibiotics. The risk for human health can significantly be reduced by heat treatment of raw milk, and there are no indications that antibiotic resistant strains are more resistant to heat treatment than susceptible strains.
4. Human outbreaks of disease caused by sheep milk
In the EU, EFSA annually collects data on foodborne outbreaks from member states. Distinction is made between strong evidence outbreaks in which the association between causative agent, the implicated food vehicle and the patient is based on strong epidemiological or microbiological evidence, and weak evidence outbreaks in which this is not the case. In the period 2011–2016, a total number of 3736 strong evidence foodborne outbreaks were reported to EFSA. Dairy was involved in 6.2 % (231 outbreaks) of these outbreaks. Milk was the implicated food vehicle in 70 of these outbreaks, cheese in 129, and other dairy products in the remaining 32 outbreaks.
The most frequently reported causal agent of dairy based strong evidence outbreaks in the EU was Salmonella spp., followed by staphylococcal enterotoxin (SET), and Campylobacter spp. Other agents involved were pathogenic E. coli, flavivirus, Bacillus spp. (mainly Bacillus cereus), calicivirus (mainly norovirus), Listeria spp., B. melitensis, Clostridium spp. (Clostridium perfringens), Yersinia spp., and Cryptosporidium parvum. In some outbreaks, the causal agent was unknown. Most outbreaks were associated with cheese and milk. Milk borne outbreaks were mainly caused by Campylobacter spp. (56 %), flavivirus (11 %), S. aureus/SET (10 %), Salmonella spp., and STEC (both 7%), while Salmonella spp. (52 %) and S. aureus/SET (30 %) were the main reported hazards in cheese related outbreaks.
Information on the animal source was available in only 32 outbreaks. Cow milk or cheese was involved in 12 of those outbreaks, sheep milk or products made thereof in 11 cases, and goat milk or products made thereof in 10 cases. From the remaining 196 it was not known from which dairy species the milk originated. Pathogens associated with those outbreaks in which sheep milk or cheese made from sheep milk was involved were SET (7), Salmonella spp. (2), Campylobacter spp., and TBEV (both 1). In a risk assessment on raw drinking milk, EFSA mentioned that 27 strong evidence outbreaks involving raw milk were reported to EFSA in the period 2007–2012 (EFSA BIOHAZ Panel, 2015). None of these involved sheep milk or products made thereof. Based on data reported to EFSA, two outbreaks occurred in 2011 caused by SET in cheese made from sheep milk, and from 2007 to 2016, only eleven outbreaks were reported caused by consumption of sheep milk and products made thereof. One of the reasons for these few reported outbreaks could be the limited consumption of raw drinking milk from sheep in Europe (EFSA BIOHAZ Panel, 2015). The same conclusion was made by Verraes et al. (2014) who did not find any outbreak described in literature attributed to sheep milk.
The only reported outbreak to EFSA of campylobacteriosis by sheep milk and products made thereof during 2011–2015 took place in the Netherlands and was caused by Campylobacter fetus in fresh cheese, a rather uncommon species (Koppenaal, 2017).
In their risk assessment study, EFSA BIOHAZ Panel (2015) conducted an additional search on raw drinking milk associated outbreaks caused by TBEV which did not reveal any outbreaks related to sheep milk; mainly (raw) goat milk is mentioned as implicated food vehicle (Kriz et al., 2009). It should be noted that TBEV is endemic in many European countries and most human infections are caused by tick bites.
In Europe, milk borne outbreaks of Brucella spp. are not common anymore as many countries are free from brucellosis (OIE, 2009), and only a few outbreaks of human brucellosis caused by B. melitensis have been described in which sheep milk or milk products were involved (Karagiannis et al., 2012). It is assumed that in European countries where brucellosis is endemic, the foodborne risk of brucellosis is mostly related to consumption of raw milk from goats and sheep and products made thereof (Verraes et al., 2015). In an epidemiological study in a specific Greek endemic region during 2003–2005, it was shown that raw milk or cheese from goats or sheep was the route of infection in only 8.5 % of the cases; animal contact being the major route of infection (Minas et al., 2007).
An overview of reported outbreaks in the United States of America (USA) is available via the online National Outbreak Reporting System of the Centers for Disease Control and Prevention (CDC, 2017). During 1998–2016, 19,991 foodborne outbreaks were reported, and only 190 were caused by milk (1.0 %), and 98 by cheese (0.5 %). Milk borne outbreaks were mainly caused by Campylobacter spp., STEC, and Salmonella spp. Other causal agents were Cryptosporidium parvum, L. monocytogenes, Yersinia enterocolitica, and S. aureus, other (single) bacteria, multiple bacteria, unknown or chemical. Similar to milk borne outbreaks, most cheese related outbreaks were caused by Campylobacter spp., followed by Salmonella spp., L. monocytogenes, norovirus, STEC, Brucella spp., S. aureus, Shigella spp., and other/unknown. Remarkable is the high contribution of Campylobacter spp. in cheese related outbreaks, as compared to the EU.
Information on the type of dairy animal is hardly available, and if so, none of the outbreaks involved sheep milk or cheeses made thereof.
In a non-exhaustive list of outbreaks in Europe, the United States of America and Canada, due to consumption of dairy products produced by Verraes et al. (2015), only two of the 64 outbreaks were associated with sheep milk dairy products, both caused by S. aureus enterotoxins. An overview of global outbreaks attributed to cheese (1973–2006) produced by FSANZ (2009b) listing 84 outbreaks, included only four outbreaks caused by sheep milk cheese (2 S. aureus, 1 Campylobacter spp., 1 Shigella spp.) and 16 by goat milk cheese. The sheep milk cheese outbreaks all occurred in Europe, those involving goat milk cheeses in all regions (Europe, USA, Canada and other). It must be noted, however, that in Australia, raw milk cheeses were not produced (FSANZ, 2009a and 2009b).
In New Zealand, 21 outbreaks were linked to the consumption of raw milk in the period from 2006 to 2013 (MPI, 2013). As the consumption of sheep milk is negligible in New Zealand, this type of product was not taken into consideration in the microbiological risk assessment associated with the consumption of raw milk, and no data are available on sheep milk (MPI, 2013).
5. Preventing animal infection, product contamination and bacterial growth
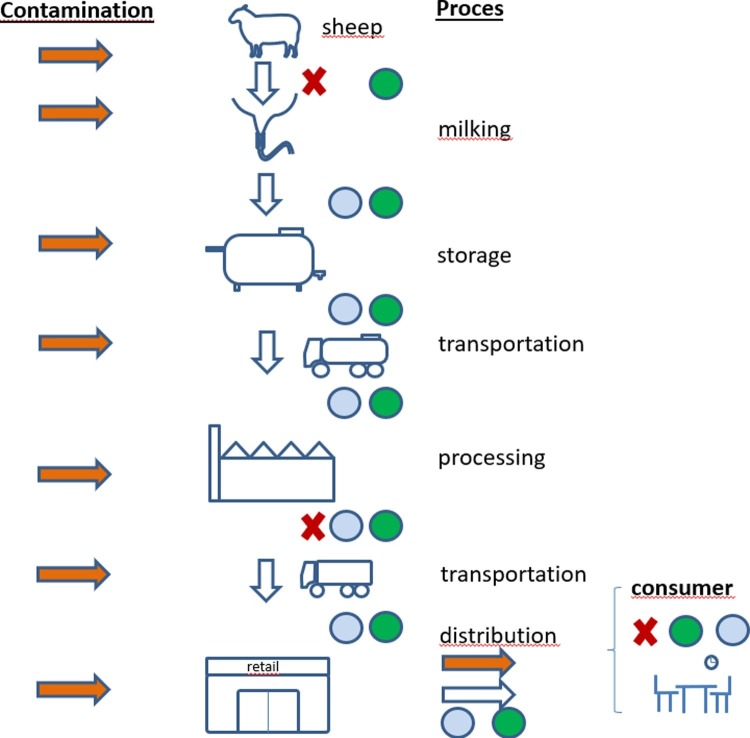
Milk and dairy products made from milk are complex natural products (Raynal-Ljutovac et al., 2008), and contamination can occur at different stages of production, processing and storage (Fig. 1 ). This means that handling these products from farm to fork should be carried out with care and awareness of the risks (Leedom, 2006).
Fig. 1.
Schematic overview of the dairy supply chain from farm to fork (consumer), showing possible entry points of pathogens (contamination: orange arrows) and control measures (blue dots: low temperature; green dots: hygiene; red crosses: eradication programs and pathogen reduction treatments of milk (e.g. pasteurization)). In cases where processing and retail take place on the dairy sheep farm, almost the same is applicable (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Several pathogens pose a risk to human health in relation to consumption of raw sheep milk or sheep milk products. Hygiene is important to prevent contamination, either when sheep are infected or when contamination occurs during processing of milk (Willis et al., 2018), but hygiene alone will not be enough to control pathogens. Eradication programs, for example for B. melitensis (Blasco, 2010), improve not only herd health but will also prevent zoonosis in humans (Fouskis et al., 2018).
After eradication of diseases from a herd or a country, monitoring of the disease status should be encouraged as part of the quality control system. Monitoring can be done in several ways, either by blood or bulk milk testing, and should be performed on a regular base. Proper identification of animals and registration of animal movements, preferably in a central database (Santman-Berends et al., 2016), is needed in combination with quarantine and testing of animals before they enter the flock as standard procedure on dairy sheep farms.
Eradication programs in combination with monitoring health status works for some pathogens like B. melitensis. However, for many pathogens, eradication programs are not available yet (Ganter, 2015). Therefore, hygiene in the barn, during milking and milk processing is the most effective route to prevent or reduce contamination of milk. For example for Salmonella spp. and Campylobacter spp., spreading through faeces in the bedding (Hutchison et al., 2005), resulting in dirty teats, or during milking can result in contamination of the milk; for these pathogens clean bedding and application of a high hygiene standard during milking are important.
Besides the above mentioned ways of contaminating milk, some pathogens like S. aureus and L. monocytogenes are excreted in milk and can infect other sheep (Gelasakis et al., 2015). Contamination of milk could occur during milking but also via the bedding in situations where ewes are leaking milk. Prevention of milk-milk contamination can be done by milking infected animals in separate groups, providing clean bedding, giving attention to hygiene during milking or by culling infected animals while vaccinating naïve animals.
Silage can play an important role in Listeria spp. (Wagner et al., 2005) and Clostridium spp. infections. One of the ways to prevent contamination of milk is to prevent the presence of soil into silage, and proper conservation and storage of silage (Driehuis et al., 2018).
As many of the pathogens in milk can cause mastitis in sheep, mastitis should be considered as a zoonotic risk (Abebe et al., 2016; Botelho et al., 2018). Therefore, mastitis prevention measures are to a large extent also measures to prevent zoonotic infections through consumption of milk with pathogens.
The composition of milk makes it a suitable product for survival and growth of a wide range of pathogens during milking, processing and storage. In general, cooling reduces the proliferation rate of bacteria but some of them, like Listeria spp., are still able to multiply under refrigerator conditions. After milking, excellent temperature control throughout the whole food supply chain and at home should be assured in order to minimize growth of pathogenic bacteria and production of toxins (Porcellato et al., 2018).
To make milk safe for human consumption, heat treatment is the golden standard. Other techniques are either not allowed or not used. However, recently cold-pressed (pascalized) milk became available on the Australian market (NSW Food Authority, 2016; NVWA BuRO, 2017). The world-wide standard for pasteurization, according to the International Dairy Federation, is either a ‘low-temperature-long-time’ treatment (at least 63 °C for 30 min; batch pasteurization) or a ‘high-temperature-short-time’ treatment (at least 15 s at 72 °C), or treatments with an equivalent effect (Juffs and Deeth, 2007). These treatments effectively reduce the level of pathogenic bacteria present in milk. Sterilization of milk needs an ultra-high temperature treatment (UHT), a short time at least at 135 °C, which ensures a microbiologically stable product when stored at 30 °C for 15 days or for 7 days at 55 °C (in closed containers).
Furthermore, thermization is used, a sub-pasteurization process, conducted at temperatures ranging between 57 and 68 °C lasting for a short time (10–20 seconds). It is applied to extend the storage life of raw milk before normal pasteurization, by controlling the psychrotrophic bacteria. Thermization results in a 3–4 log reduction of the commensal microbiota of milk and is not designed to inactivate all vegetative pathogens present. In addition, it is used in the production process of certain types of cheese, to reduce the bacterial load, but without disadvantages for cheese ripening and whey protein degradation (Claeys et al., 2013; Juffs and Deeth, 2007).
6. Considerations
Milk from sheep is very popular in certain populations and areas. Taste and high fat content of cheeses from sheep milk make it popular. Nowadays, these dairy products are also important for people with an allergy to cow milk, and these products are an essential part of the local daily diet in regions of the world that are not suitable for cows and goats.
Healthy animals are the starting point for the production of safe and healthy food, but it should be kept in mind that several pathogens can be shed by animals not showing any signs of illness. Also in many cases of high somatic cell counts found in milk, no clinical signs of disease are seen. This indicates the need for a control plan for subclinical mastitis.
Raw milk does contain micro-organisms, and some of them pose a health risk to humans. Factors that impact the level of contamination of raw milk include animal-related factors like general animal health, herd size, age and production status, environment-related factors like housing, faeces, feed, soil, and water, and factors related to milking and operation of milking equipment (FSANZ, 2009b). Production and processing of milk from sheep is often performed under less stringent hygiene and quality control regulations compared to that from cattle. Several factors are described as possible reasons for this difference, like lower production per head, milking system, flock management, and wide geographic spread of production (Klinger and Rosenthal, 1997). Furthermore, it is suggested by Gonzales-Barron et al. (2017) that for goat milk cheeses, which are mainly produced in regions of the Mediterranean and Middle East, the higher levels of pathogens associated with this type of cheeses, compared to milk and milk products from sheep, may arise from the more artisanal production system. The same may be true for sheep milk cheeses compared to cow milk cheeses. Overall, these authors showed that pathogens are more prevalent in cheese made from goat milk compared to that from sheep (9,6 %–2,5 %) ( Gonzales-Barron et al., 2017). This is however not reflected in the data reported to EFSA (2011–2015) that show a pathogen prevalence in cheeses made from cow, goat and sheep milk of 0.9 %, 1.1 %, and 1.3 %, respectively. However, prevalence of pathogens in milk is higher for sheep (7.7 %) and goat (3.7 %) than for cow (1.0 %). In the risk assessment carried out by Juffs and Deeth (2007) for the Australian situation, contamination of raw milk from sheep was considered to occur more frequently than contamination of cow and goat milk with regard to presence of STEC and L. monocytogenes, and less frequently with regard to C. jejuni and Salmonella spp. S. aureus was considered to be more prevalent in goat milk than in either cow or sheep milk.
As milk from small ruminants may be used more often for on farm production of cheese, compared to cow milk, the prevalence of pathogens will strongly depend on the health status of the herd and the food hygiene practices of the farmer. Stringent hygiene measures during milking are essential to prevent cross contamination between animals during milking. Furthermore, good hygienic practices throughout the entire production process are important to safeguard the health of dairy consumers. Presence of S. aureus in cheese from both raw and pasteurized milk has been described as an indicator of low hygiene during milking and processing (Gonzales-Barron et al., 2017).
Heat treatment of milk results in a reduction of the number of micro-organisms. The thermal death point differs for different micro-organisms. Duration as well as temperature influence survival rates of pathogenic micro-organisms during heat treatment. With increasing temperature, duration can be decreased to have the same results (Dhanashekar et al., 2012). Thermization treatment will not only have a positive effect by controlling spoilage micro-organisms and to some extend pathogenic micro-organisms, it also reduces lactic acid bacteria numbers and the biodiversity of raw milk bacteria in general. This inactivation of lactic acid bacteria might enhance the growth ability of potentially pathogenic enterococci (Samelis et al., 2009).
It is important to realize that in outbreaks caused by L. monocytogenes, it is quite difficult to identify the source, as the incubation period of listeriosis is fairly long, and can be up to many weeks. Thus, although L. monocytogenes is considered a main hazard in dairy products, the number of strong evidence outbreaks is limited.
Additionally, contamination of milk with veterinary drug residues can affect human health. Therefore, milk and milk products must be free from residues.
Several micro-organisms causing disease in small ruminants have zoonotic potential, especially many of those causing abortion (Van den Brom et al., 2012; Van Engelen et al., 2014). Some of them are not known as causing foodborne disease as is for example the case with Chlamydia abortus, which is able to infect humans after direct or indirect contact. Therefore, persons who work with small ruminants should be aware of these zoonoses as well.
Consumption of raw milk and products made thereof will always pose a risk. Therefore, stringent hygiene measures in combination with periodic control of these products should be applied.
7. Conclusion
Consumption of raw sheep milk and raw milk products made thereof can pose a zoonotic risk, although the registered number of confirmed cases is low. This risk can be further reduced by proper flock health management, prevention of contamination during milking and adequate milk processing, transport and storage. In the dairy industry, much knowledge is present about these risks, and how to control them. In small scale production systems of sheep milk and dairy products, a higher risk remains compared to industrialized production because of a less protocolled and controlled production process. Especially the so called YOPIs - young children, elderly people, pregnant women, and immunocompromised persons - and those suffering from disease should be aware of the risk of consuming raw sheep milk and raw sheep milk products. Pasteurization, and similar or stronger processes, adequately reduces zoonotic risks of sheep milk and products made thereof, but preventing recontamination and proper cooling throughout the supply chain and at home are essential to keep these products safe.
Declaration of Competing Interest
All authors declare not to have a conflict of interest.
Acknowledgement
We would like to thank Sander Prins for his contribution.
References
- Abdalhamed A.M., Zeedan G.S.G., Zeina H.A.A.A. Isolation and identification of bacteria causing mastitis in small ruminants and their susceptibility to antibiotics, honey, essential oils, and plant extracts. Vet. World. 2018;11(March 3):355–362. doi: 10.14202/vetworld.2018.355-362. 2018 Epub 2018 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abebe R., Hatiya H., Abera M., Megersa B., Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016;12(1):270. doi: 10.1186/s12917-016-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Shama U.H. Prevalence and antimicrobial susceptibility of Staphylococcus aureus isolated from cattle, buffalo, sheep and goat’s raws milk in Sohag Governorate. Egypt. Assiut. Vet. Med. J. 2014;60:63–72. [Google Scholar]
- Adney D.R., Brown V.R., Porter S.M., Bielefeldt-Ohmann H., Hartwig A.E., Bowen R.A. Inoculation of goats, sheep, and horses with MERS-CoV does not result in productive viral shedding. Viruses. 2016;8(8) doi: 10.3390/v8080230. 2016 Aug 19 pii: E230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AFSSA . Bacillus anthracis. Agence Française de Sécurité Sanitaire des Aliments, Maisons-Alfort (FR); 2008. Note of the French food safety agency (Afssa) on treatments applicable to milk from animals in the event of clinical suspicion and after confirmation of infection by the anthrax bacillus; p. 4. Request no. 2008-SA-0243. [Google Scholar]
- Alemdar S., Aĝaoĝlu S. Survival of Salmonella typhimurium during the ripening of herby cheese (otlu peynir) J. Food Saf. 2010;30(3):526–536. [Google Scholar]
- Alian F., Rahimi E., Shakerian A., Momtaz H., Riahi M., Momeni M. Antimicrobial resistance of Staphylococcus aureus isolated from bovine, sheep and Goat raw milk. Glob. Vet. 2012;8(2):111–114. 2012. [Google Scholar]
- Ariza-Miguel J., Hernández M., Fernández-Natal I., Rodríguez-Lázaro D. Methicillin-resistant Staphylococcus aureus harbouring mecC in livestock in Spain. J. Clin. Microbiol. 2014;52(11):4067–4069. doi: 10.1128/JCM.01815-14. E2014 Nov pub 2014 Sep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azara E., Piras M.G., Parisi A., Tola S. Antimicrobial susceptibility and genotyping of Staphylococcus aureus isolates collected between 1986 and 2015 from ovine mastitis. Vet. Microbiol. 2017;205:53–56. doi: 10.1016/j.vetmic.2017.05.006. 2017 Jun Epub 2017 May 10. [DOI] [PubMed] [Google Scholar]
- Barrett N.J. Communicable disease associated with milk and dairy products in England and Wales: 1983-1984. J. Infect. 1986;12(3):265–272. doi: 10.1016/s0163-4453(86)94320-3. [DOI] [PubMed] [Google Scholar]
- Belluco S., Simonato G., Mancin M., Pietrobelli M., Ricci A. Toxoplasma gondii infection and food consumption: a systematic review and meta-analysis of case-controlled studies. Crit. Rev. Food Sci. Nutr. 2017:1–12. doi: 10.1080/10408398.2017.1352563. [DOI] [PubMed] [Google Scholar]
- Bertelloni F., Turchi B., Cerri D., Pinzauti P., Fratini F. Leptospira spp. And Brucella ovis seroprevalence in sheep: preliminary results of one year surveillance program. J. Hell. Vet. Med. Soc. 2017;68(4):2585–3724. [Google Scholar]
- Blasco J.M. Control and eradication strategies for Brucella melitensis infection in sheep and goats. Prilozi. 2010;31(1):145–165. [PubMed] [Google Scholar]
- Bogovic P., Strle F. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J. Clin. Cases. 2015;3(5):430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho A.C.N., Ferreira A.F.M., Fracalanzza S.E.L., Teixeira L.M., Pinto T.C.A. A perspective on the potential zoonotic role of Streptococcus agalactiae: searching for a missing link in alternative transmission routes. Front. Microbiol. 2018;9:608. doi: 10.3389/fmicb.2018.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughattas S. Toxoplasma infection and milk consumption: meta-analysis of assumptions and evidences. Crit. Rev. Food Sci. Nutr. 2017;57(13):2924–2933. doi: 10.1080/10408398.2015.1084993. [DOI] [PubMed] [Google Scholar]
- Brandford Oltenacu E.A. Domstication of animals. In: Pond W.G., editor. Encyclopedia of Animal Science. Taylor and Francis; 2004. pp. 294–296. [Google Scholar]
- Brooke C.J., Riley T.V. Erysipelothrix rhusiopathiae: bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J. Med. Microbiol. 1999;48(9):789–799. doi: 10.1099/00222615-48-9-789. [DOI] [PubMed] [Google Scholar]
- Buchanan R.L., Gorris L.G.M., Hayman M.M., Jackson T.C., Whiting R.C. A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control. 2017;75:1–13. [Google Scholar]
- Burriel A.R. Resistance of coagulase-negative staphylococci isolated from sheep to various antimicrobial agents. Res. Vet. Sci. 1997;63(2):189–190. doi: 10.1016/s0034-5288(97)90016-3. 1997 Sep-Oct. [DOI] [PubMed] [Google Scholar]
- Camossi L.G., Greca-Junior H., Correa A.P., Richini-Pereira V.B., Silva R.C., Da Silva A.V. Detection of Toxoplasma gondii DNA in the milk of naturally infected ewes. Vet. Parasitol. 2011;177(3–4):256–261. doi: 10.1016/j.vetpar.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Capasso L. Bacteria in two-millennia-old cheese, and related epizoonoses in Roman populations. J. Infect. 2002;45(2):122–127. doi: 10.1053/jinf.2002.0996. [DOI] [PubMed] [Google Scholar]
- Carfora V., Giacinti G., Sagrafoli D., Marri N., Giangolini G., Alba Methicillin-resistant and methicillin-susceptible Staphylococcus aureus in dairy sheep and in-contact humans: an intra-farm study. J. Dairy Sci. 2016;99(6):4251–4258. doi: 10.3168/jds.2016-10912. 2016 Jun Epub 2016 Apr 6. [DOI] [PubMed] [Google Scholar]
- Caro I., Mateo J., Rúa J., del Rosario García-Armesto M. Occurrence of Escherichia coli O157, O111 and O26 in raw ewe’s milk and performance of two enrichment broths and two plating media used for its assessment. Int. J. Food Microbiol. 2011;146(1):84–87. doi: 10.1016/j.ijfoodmicro.2010.11.014. [DOI] [PubMed] [Google Scholar]
- CDC . 2017. National Outbreak Reporting System (NORS)https://wwwn.cdc.gov/norsdashboard/ (27-11-2017) Available: [Google Scholar]
- Ceniti C., Britti D., Santoro A.M.L., Musarella R., Ciambrone L., Casalinuovo F., Costanzo N. Phenotypic antimicrobial resistance profile of isolates causing clinical mastitis in dairy animals. Ital. J. Food Saf. 2017;6(2):6612. doi: 10.4081/ijfs.2017.6612. 2017 May 3 eCollection 2017 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christidis T., Pintar K.D.M., Butler A.J., Nesbitt A., Thomas M.K., Marshall B. Campylobacter spp. prevalence and levels in raw milk: a systematic review and meta-analysis. J. Food Prot. 2016;79(10):1775–1783. doi: 10.4315/0362-028X.JFP-15-480. [DOI] [PubMed] [Google Scholar]
- Cisak E., Wójcik-Fatla A., Zajac V., Sroka J., Buczek A., Dutkiewicz J. Prevalence of tick-borne encephalitis virus (TBEV) in samples of raw milk taken randomly from cows, goats and sheep in Eastern Poland. Ann. Agric. Environ. Med. 2010;17(2):283–286. [PubMed] [Google Scholar]
- Claeys W.L., Cardoen S., Daube G., De Block J., Dewettinck K., Dierick K. Raw or heated cow milk consumption: review of risks and benefits. Food Control. 2013;31(1):251–262. [Google Scholar]
- Clements A., Young J.C., Constantinou N., Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes. 2012;3:71–87. doi: 10.4161/gmic.19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condoleo R., Mezher Z., Marozzi S., Guzzon A., Fischetti R., Senese M. Risk assessment of human listeriosis from semisoft cheeses made from raw sheep’s milk in Lazio and Tuscany (Italy) Risk Anal. 2017;37(4):661–676. doi: 10.1111/risa.12649. [DOI] [PubMed] [Google Scholar]
- Corrente M., Greco G., Madio A., Ventriglia G. Methicillin resistance in staphylococci isolated from subclinical mastitis in sheep. New Microbiol. 2003;26(1):39–45. 2003 Jan. [PubMed] [Google Scholar]
- De Neeling A.J., Van den Broek M.J.M., Spalburg E.C., Van Santen-Verheuvel M.G., Dam-Deisz W.D.C. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet. Microbiol. 2007;122:366–372. doi: 10.1016/j.vetmic.2007.01.027. [DOI] [PubMed] [Google Scholar]
- de Santana Rocha D., de Sousa Moura R.L., Maciel B.M., Guimaraes L.A., O’Dwyer H.N., Munhoz A.D. Detection of Toxoplasma gondii DNA in naturally infected sheep’s milk. Genet. Med. Res. 2015;14(3):8658–8662. doi: 10.4238/2015.July.31.14. [DOI] [PubMed] [Google Scholar]
- Deng H., Dam-Deisz C., Luttikholt S., Maas M., Nielen M., Swart A. Risk factors related to Toxoplasma gondii seroprevalence in indoor-housed Dutch dairy goats. Prev. Vet. Med. 2016;124:45–51. doi: 10.1016/j.prevetmed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Dhanashekar R., Akkinepalli S., Nellutla A. Milk-borne infections. An analysis of their potential effect on the milk industry. Germs. 2012;2(3):101–109. doi: 10.11599/germs.2012.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobler G., Gniel D., Petermann R., Pfeffer M. Epidemiology and distribution of tick-borne encephalitis. Wiener Med. Wochenschr. 2012;162(11):230–238. doi: 10.1007/s10354-012-0100-5. [DOI] [PubMed] [Google Scholar]
- Driehuis F., Wilkinson J.M., Jiang Y., Ogunade I., Adesogan A.T. Silage review: animal and human health risks from silage. J. Dairy Sci. 2018;101(5):4093–4110. doi: 10.3168/jds.2017-13836. [DOI] [PubMed] [Google Scholar]
- Drouin P., Lafrenière C. Clostridial spores in animal feeds and milk. In: Chaiyabutr Narongsak., editor. Milk Production – An up-to-Date Overview of Animal Nutrition, Management and Health. 2012. p. 375. [Google Scholar]
- Ducrotoy M., Bertu W.J., Matope G., Cadmus S., Conde-Álvarez R., Gusi A.M. Brucellosis in Sub-Saharan Africa: current challenges for management, diagnosis and control. Acta Trop. 2017;165:179–193. doi: 10.1016/j.actatropica.2015.10.023. [DOI] [PubMed] [Google Scholar]
- EFSA, ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2011. EFSA J. 2013;11(4):3129. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2012. EFSA J. 2014;12(2):3547. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015;13(12):4329. doi: 10.2903/j.efsa.2018.5500. [191 pp.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015;13(1):3991. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA J. 2016;14(12):4634. doi: 10.2903/j.efsa.2017.5077. [231 pp.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel Scientific opinion on q fever. EFSA J. 2010;8(5):1595. [Google Scholar]
- EFSA BIOHAZ Panel Scientific Opinion on the public health hazards to be covered by inspection of meat (bovine animals) EFSA J. 2013;11(6):3266. doi: 10.2903/j.efsa.2013.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel Scientific Opinion on VTEC‐seropathotype and scientific criteria regarding pathogenicity assessment. EFSA J. 2013;11(4):3138. [Google Scholar]
- EFSA BIOHAZ Panel Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015;13(1):3940. [95 pp.] [Google Scholar]
- EFSA BIOHAZ Panel, Ricci A., Allende A., Bolton D., Chemaly M., Davies R., Fernández Escámez. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018;16:e05134. doi: 10.2903/j.efsa.2018.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Metwally M.T., Elwan M.A., El-Bahnasawy M.M., Khalil H.H., Sabah A.A., Morsy A.T. Zoonotic brucellosis: an underestimated or misdiagnosed disease in Egypt. J. Egypt. Soc. Parasitol. 2011;41(1):35–46. [PubMed] [Google Scholar]
- El-Sayed A., Awad W. Brucellosis: evolution and expected comeback. Int. J. Vet. Sci. Med. 2018 doi: 10.1016/j.ijvsm.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . 2003. Opinion of the Scientific Committee on Veterinary Measures Relating to Public Health on Salmonellae in Foodstuffs; p. 65. [Google Scholar]
- Eyre D.W., Kenkre J.S., Bowler I.C.J.W., McBride S.J. Streptococcus equi subspecies zooepidemicus meningitis—a case report and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29(12):1459–1463. doi: 10.1007/s10096-010-1037-5. [DOI] [PubMed] [Google Scholar]
- FAVV . Evaluatie van de risico’s en baten van de consumptie van rauwe melk van andere diersoorten dan koeien (dossier Sci Com 2012/12: eigen initiatief) 2013. Advies 11-2013 van 22 maart 2013 van het Wetenschappelijk Comité. Brussel, pp. 88. [Google Scholar]
- FAVV . Evaluatie van de microbiologische risico’s van de consumptie van zuivelproducten op basis van rauwe melk (dossier Sci Com 2014/06: eigen initiatief) 2015. Advies 02-2015 van 27 februari 2015 van het Wetenschappelijk Comité. Brussel, pp. 42. [Google Scholar]
- Fouskis I., Sandalakis V., Christidou A., Tsatsaris A., Tzanakis N., Tselentis Y., Psaroulaki A. Trans R the epidemiology of Brucellosis in Greece, 2007-2012: a’ one Health’ approach. Soc Trop Med Hyg. 2018;112(3):124–135. doi: 10.1093/trstmh/try031. [DOI] [PubMed] [Google Scholar]
- Fratini F., Turchi B., Ferrone M., Galiero A., Nuvoloni R., Torracca B. Is Leptospira able to survive in raw milk? Study on the inactivation at different storage times and temperatures. Folia Microbiol. (Praha) 2016;61(5):413–416. doi: 10.1007/s12223-016-0452-0. [DOI] [PubMed] [Google Scholar]
- FSANZ . Food Standards Australia; New Zealand: 2009. Risk Assessment of Raw Milk Cheese; p. 304. [Google Scholar]
- FSANZ . Food Standards Australia; New Zealand: 2009. Microbiological Risk Assessment of Raw Cow Milk; p. 119. [Google Scholar]
- FSANZ . 2014. Toxoplasma gondii.https://www.foodstandards.gov.au/publications/Documents/Toxoplasma%20gondii%20-%20jan%202014.pdf (January 2014) Available: [Google Scholar]
- Fusco G., Rinaldi L., Guarino A., Proroga Y.T.R., Pesce A., Giuseppina D.M. Toxoplasma gondii in sheep from the Campania region (Italy) Vet. Parasitol. 2007;149(3):271–274. doi: 10.1016/j.vetpar.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Gale P., Kelly L., Mearns R., Duggan J., Snary E.L. Q fever through consumption of unpasteurised milk and milk products – a risk profile and exposure assessment. J. Appl. Microbiol. 2015;118(5):1083–1095. doi: 10.1111/jam.12778. [DOI] [PubMed] [Google Scholar]
- Galiero A., Fratini F., Cammà C., Di Domenico M., Curini V., Baronti I. Occurrence of Coxiella burnetii in goat and ewe unpasteurized cheeses: screening and genotyping. Int. J. Food Microbiol. 2016;237:47–54. doi: 10.1016/j.ijfoodmicro.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Ganter M. Zoonotic risks from small ruminants. Vet. Microbiol. 2015;181(1–2):53–65. doi: 10.1016/j.vetmic.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Gelasakis A.I., Mavrogianni V.S., Petridis I.G., Vasileiou N.G., Fthenakis G.C. Mastitis in sheep--The last 10 years and the future of research. Vet. Microbiol. 2015;181(1–2):136–146. doi: 10.1016/j.vetmic.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Giacinti G., Carfora V., Caprioli A., Sagrafoli D., Marri N., Giangolini G. Prevalence and characterization of methicillin-resistant Staphylococcus aureus carrying mecA or mecC and methicillin-susceptible Staphylococcus aureus in dairy sheep farms in central Italy. J. Dairy Sci. 2017;100(10):7857–7863. doi: 10.3168/jds.2017-12940. 2017 Oct Epub 2017 Aug 2. [DOI] [PubMed] [Google Scholar]
- Giangaspero A., Paoletti B., Iorio R., Traversa D. Prevalence and molecular characterization of Giardia duodenalis from sheep in central Italy. Parasitol. Res. 2005;96(1):32–37. doi: 10.1007/s00436-005-1317-7. [DOI] [PubMed] [Google Scholar]
- Gonzales-Barron U., Gonçalves-Tenório A., Rodrigues V., Cadavez V. Foodborne pathogens in raw milk and cheese of sheep and goat origin: a meta-analysis approach. Curr. Opin. Food Sci. 2017;18:7–13. [Google Scholar]
- Grešíková M., Sekeyová M., Stúpalová S., Nečas S. Sheep milk-borne epidemic of tick-borne encephalitis in Slovakia. Intervirology. 1975;5(1–2):57–61. doi: 10.1159/000149880. [DOI] [PubMed] [Google Scholar]
- Horrocks S.M., Anderson R.C., Nisbet D.J., Ricke S.C. Incidence and ecology of Campylobacter jejuni and coli in animals. Anaerobe. 2009;15(1–2):18–25. doi: 10.1016/j.anaerobe.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Huang H., Brooks B.W., Lowman R., Carrillo C.D. Campylobacter species in animal, food, and environmental sources, and relevant testing programs in Canada. Can. J. Microbiol. 2015;61(10):701–721. doi: 10.1139/cjm-2014-0770. [DOI] [PubMed] [Google Scholar]
- Hussain M., Stitt V., Szabo E., Nelan B. Toxoplasma gondii in the food supply. Pathogens. 2017;6(2):21. doi: 10.3390/pathogens6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison M.L., Walters L.D., Avery S.M., Munro F., Moore A. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 2005;71(3):1231–1236. doi: 10.1128/AEM.71.3.1231-1236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E., Ladero V., Chico I., Maldonado-Barragán A., López M., Martín V. Antibiotic resistance, virulence determinants and production of biogenic amines among enterococci from ovine, feline, canine, porcine and human milk. BMC Microbiol. 2013;(13):288. doi: 10.1186/1471-2180-13-288. 2013 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K., Hunt K., Dalmasso M. Listeria monocytogenes in milk products. In: Garg N., editor. Microbes in Food and Health. Springer International Publishing; Cham: 2016. pp. 289–315. [Google Scholar]
- Juffs H., Deeth H. Food Standards Austalia New Zealand; Canberra: 2007. Scientific Evaluation of Pasteurisation for Pathogen Reduction in Milk and Milk Products; p. 152. [Google Scholar]
- Karagiannis I., Mellou K., Gkolfinopoulou K., Dougas G., Theocharopoulos G., Vourvidis D. Outbreak investigation of brucellosis in Thassos, Greece, 2008. Eurosurveillance. 2012;17(11):20116. [PubMed] [Google Scholar]
- Keyhani M. Presence of the Mycobacterium tuberculosis in the milk of experimentally reproduced sheep tuberculosis. Bulletin de l’offince international des épizooties. 1970;73(11):993–998. [PubMed] [Google Scholar]
- Klinger I., Rosenthal I. Public health and the safety of milk and milk products from sheep and goats. Revue Scientifique et Technique-office International des Epizooties. 1997;16(2):482–488. doi: 10.20506/rst.16.2.1034. [DOI] [PubMed] [Google Scholar]
- Koppenaal H. Een uitbraak met Campylobacter fetus na het eten van rauwmelkse schapenkaas. Ned. Tijdschr. 2017;161(D1704):1–7. [PubMed] [Google Scholar]
- Kouijzer I.J.E., Kampschreur L.M., Wever P.C., Hoekstra C., van Kasteren M.E.E., de Jager-Leclercq The value of 18F-FDG PET/CT in diagnosis and during follow-up in 273 patients with chronic q fever. J. Nucl. Med. 2018;59(1):127–133. doi: 10.2967/jnumed.117.192492. [DOI] [PubMed] [Google Scholar]
- Kriz B., Benes C., Daniel M. Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997-2008) Epidemiol. Mikrobiol. Imunol. 2009;58(2):98–103. [PubMed] [Google Scholar]
- Labuda M., Elečková E., Ličková M., Sabó A. Tick-borne encephalitis virus foci in Slovakia. Int. J. Med. Microbiol. 2002;291:43–47. [PubMed] [Google Scholar]
- Lahou E., Uyttendaele M. Growth potential of Listeria monocytogenes in soft, semi-soft and semi-hard artisanal cheeses after post-processing contamination in deli retail establishments. Food Control. 2017;76:13–23. [Google Scholar]
- Leedom J.M. Milk of nonhuman origin and infectious diseases in humans. Clin Infect Diseases. 2006;43(5):610–615. doi: 10.1086/507035. [DOI] [PubMed] [Google Scholar]
- Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LNV . 2008. Convenant to Reduce Antibiotic Resistance in Livestock Farming in the Netherlands (in Dutch)http://www.rijksoverheid.nl/documentenen-publicaties/kamerstukken/2008/12/08/convenant-antibioticaresistentiedierhouderij Available: [Google Scholar]
- Lollai S.A., Ziccheddu M., Duprè I., Piras D. Characterization of resistance to tetracyclines and aminoglycosides of sheep mastitis pathogens: study of the effect of gene content on resistance. J. Appl. Microbiol. 2016;121(October (4)):941–951. doi: 10.1111/jam.13229. 2016 Epub 2016 Aug 25. [DOI] [PubMed] [Google Scholar]
- Luptakova L., Benova K., Rencko A., Petrovova E. DNA detection of Toxoplasma gondii in sheep milk and blood samples in relation to phase of infection. Vet. Parasitol. 2015;208(3):250–253. doi: 10.1016/j.vetpar.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Macori G., Giacinti G., Bellio A., Gallina S., Bianchi D.M., Sagrafoli D. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the ovine dairy chain and in farm-related humans. Toxins (Basel) 2017;9(5) doi: 10.3390/toxins9050161. 2017 May 16 pii: E161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen M.J.J., Friesema I.H.M., Haagsma J.A., van Pelt W. RIVM Rapport 2017-0097. Rijkstinstituut voor Volksgezondheid en Milieu; Bilthoven: 2017. Disease burden of food-related pathogens in the Netherlands, 2016; p. 58. [Google Scholar]
- MARAN . 2018. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2017.https://www.wur.nl/upload_mm/7/b/0/5e568649-c674-420e-a2ca-acc8ca56f016_Maran%202018.pdf 2018 Available: [Google Scholar]
- Miguel E., Chevalier V., Ayelet G., Ben Bencheikh M.N., Boussini H., Chu D.K. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Euro Surveill. 2015;22(13) doi: 10.2807/1560-7917.ES.2017.22.13.30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas M., Minas A., Gourgulianis K., Storunara A. Epidemiological and clinical aspects of human brucellosis in central Greece. Japanese Journal of Infetcious Diseases. 2007;60:362–366. [PubMed] [Google Scholar]
- Monis P.T., Thompson R.C. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect. Genet. Evol. 2003;3(4):233–244. doi: 10.1016/j.meegid.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Mousavi S., Dehkordi F.S., Rahimi E. Virulence factors and antibiotic resistance of Helicobacter pylori isolated from raw milk and unpasteurized dairy products in Iran. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014;20(51) doi: 10.1186/1678-9199-20-51. 2014 Dec 4 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MPI . Ministry for Primary Industries; Wellington, New Zealand: 2013. Assessment of the Microbiological Risks Associated With the Consumption of Raw Milk; p. 97. MPI Technical Paper No: 2014/12. [Google Scholar]
- Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2015;4(2) doi: 10.1128/microbiolspec.VMBF-0016-2015. Review. 2016 Apr PMID: 27227291 Free PMC Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NethMap . 2019. Consumption of Antimicrobial Agents and Antimicrobial Resistance Among Medically Important Bacteria in the Netherlands in 2018.https://www.wur.nl/upload_mm/a/7/9/89640bbc-53a2-40f0-ba4a-a9a34a7bf416_Nethmap%20Maran%202019.pdf 2019 Available: [Google Scholar]
- Ng’ang’a C.M., Bukachi S.A., Bett B.K. Lay perceptions of risk factors for Rift Valley fever in a pastoral community in northeastern Kenya. BMC Public Health. 2016;16:32. doi: 10.1186/s12889-016-2707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas D.E., Jacobsen K.H., Waters N.M. Risk factors associated with human Rift Valley fever infection: systematic review and meta-analysis. Trop. Med. Int. Health. 2014;19(12):1420–1429. doi: 10.1111/tmi.12385. [DOI] [PubMed] [Google Scholar]
- NSW Food Authority . 2016. Approval of High Pressure Processing (HPP) of Milk.http://www.foodauthority.nsw.gov.au/news/newsandmedia/departmental/2016-06-03-HPP-milk (03-06-2016) Available 28.05.2018. [Google Scholar]
- NVWA BuRO . Netherlands Food and Consumer Product Safefety Authority, Office for Risk Assessment and Research (NVWA BuRO); Utrecht (the Netherlands): 2017. Advice on the Suitability of Alternatives for Pasteurisation to Safeguard Microbial Food Safety of Milk; p. 11. [Google Scholar]
- Obaidat M.M., Bani Salman A.E., Roess A.A. High prevalence and antimicrobial resistance of mecA Staphylococcus aureus in dairy cattle, sheep, and goat bulk tank milk in Jordan. Trop. Anim. Health Prod. 2018;50(2):405–412. doi: 10.1007/s11250-017-1449-7. 2018 Feb Epub 2017 Oct 23. [DOI] [PubMed] [Google Scholar]
- Offerdahl D.K., Clancy N.G., Bloom M.E. Stability of a tick-borne flavivirus in milk. Front. Bioeng. Biotechnol. 2016;4(MAY) doi: 10.3389/fbioe.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . 2009. Bucellosis.http://www.oie.int/en/animal-health-in-the-world/animal-diseases/Brucellosis/ available: [Google Scholar]
- Onni T., Sanna G., Larsen J., Tola S. Antimicrobial susceptibilities and population structure of Staphylococcus epidermidis associated with ovine mastitis. Vet. Microbiol. 2011;148(1):45–50. doi: 10.1016/j.vetmic.2010.07.024. 2011 Feb 24 Epub 2010 Aug 5. [DOI] [PubMed] [Google Scholar]
- Opsteegh M., van Roon A., Wit B., Hagen-Lenselink B., van Duijkeren E., Dierikx C. RIVM Rapport 2018-0059. 2018. Surveillance zoönosen in de melkgeiten- en melkschapenhouderij in 2016 (surveillance zoonoses in dairy goat and dairy sheep industry in 2016) [report in Dutch] [Google Scholar]
- Ortigosa M., Irigoyen A., Urdin M., García S., Ibañez F.C. Torre P. Sources of enterococci in idiazábal-type cheese. Int. J. Food Microbiol. 2008;125(2):146–152. doi: 10.1016/j.ijfoodmicro.2008.03.035. 2008 Jul 15 Epub 2008 Apr 8. [DOI] [PubMed] [Google Scholar]
- Panelli S., Brambati E., Bonacina C., Feligini M. Updating on the fungal composition in Sardinian sheep’s milk by culture-independent methods. J. Dairy Res. 2014;81(2):233–237. doi: 10.1017/S0022029914000090. [DOI] [PubMed] [Google Scholar]
- Pappas G., Papadimitriou P., Christou L., Akritidis N. Future trends in human brucellosis treatment. Expert Opin. Investig. Drugs. 2006;15(10):1141–1149. doi: 10.1517/13543784.15.10.1141. [DOI] [PubMed] [Google Scholar]
- Peel M.M., Palmer G.G., Stacpoole A.M., Kerr T.G. Human lymphadenitis due to Corynebacterium pseudotuberculosis: report of ten cases from Australia and review. Clin. Infect. Dis. 1997;24(2):185–191. doi: 10.1093/clinids/24.2.185. [DOI] [PubMed] [Google Scholar]
- Pexara A., Solomakos N., Govaris A. Q fever and prevalence of Coxiella burnetii in milk. Trends Food Sci. Technol. 2018;71:65–72. [Google Scholar]
- Pires A.F.A., Patterson L., Maier G. The Principles and Practice of Q Fever: The One Health Paradigm. 2017. Coxiella burnetii implications for food safety; pp. 279–288. [Google Scholar]
- Porcellato D., Aspholm M., Skeie S.B., Monshaugen M., Brendehaug J., Mellegård H. Microbial diversity of consumption milk during processing and storage. Int J Food Microbiology. 2018;266:21–30. doi: 10.1016/j.ijfoodmicro.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Punpanich W., Srijuntongsiri S. Pasteurella (Mannheimia) haemolytica septicemia in an infant: a case report. J. Infect. Dev. 2012;6(7):584–587. doi: 10.3855/jidc.1998. [DOI] [PubMed] [Google Scholar]
- Ranadheera C.S., Naumovski N., Ajlouni S. Non-bovine milk products as emerging probiotic carriers: recent developments and innovations. Curr. Opin. Food Sci. 2018;22:109–114. [Google Scholar]
- Raynal-Ljutovac K., Lagriffoul G., Paccard P., Guillet I., Chilliard Y. Composition of goat and sheep milk products: an update. Small Rumin. Res. 2008;79(1):57. [Google Scholar]
- RIVM . 2013. Brucellose.http://lci.rivm.nl/richtlijnen/brucellose available: [website in Dutch] [Google Scholar]
- Rocha C.E., Mol J.P.S., Garcia L.N.N., Costa L.F., Santos R.L., Paixão T.A. Comparative experimental infection of Listeria monocytogenes and Listeria ivanovii in bovine trophoblasts. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0176911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelis J., Lianou A., Kakouri A., Delbès C., Rogelj I., Bogovic-Matijasić B., Montel M.C. Changes in the microbial composition of raw milk induced by thermization treatments applied prior to traditional Greek hard cheese processing. J. Food Prot. 2009;72(4):783–790. doi: 10.4315/0362-028x-72.4.783. [DOI] [PubMed] [Google Scholar]
- Sanciu G., Marogna G., Paglietti B., Cappuccinelli P., Leori G., Rappelli P. Outbreak of mastitis in sheep caused by multi-drug resistant Enterococcus faecalis in Sardinia. Italy. Epidemiol Infect. 2012;13:1–3. doi: 10.1017/S0950268812000647. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santman-Berends I., Luttikholt S., Van den Brom R., Van Schaik G., Gonggrijp M., Hage H., Vellema P. Estimation of the use of antibiotics in the small ruminant industry in the Netherlands in 2011 and 2012. PLoS One. 2014;9(8):e105052. doi: 10.1371/journal.pone.0105052. 2014 Aug 12 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santman-Berends I.M.G.A., Brouwer-Middelesch H., Van Wuijckhuise L., de Bont-Smolenaars A.J.G., Van Schaik G. Surveillance of cattle health in the Netherlands: monitoring trends and developments using routinely collected cattle census data. Prev. Vet. Med. 2016;134:103–112. doi: 10.1016/j.prevetmed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Schoder D., Melzner D., Schmalwieser A., Zangana A., Winter P., Wagner M. Important vectors for Listeria monocytogenes transmission at farm dairies manufacturing fresh sheep and goat cheese from raw milk. J. Food Prot. 2011;74(6):919–924. doi: 10.4315/0362-028X.JFP-10-534. [DOI] [PubMed] [Google Scholar]
- Scott L.C., Menzies P.I. Antimicrobial resistance and small ruminant veterinary practice. Vet. Clin. North Am. Food Anim. Pract. 2011;27:23–32. doi: 10.1016/j.cvfa.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Sherman D.M. The spread of pathogens through trade in small ruminants and their products. Revue Scientific et Technique-Office International des Epizooties. 2011;30(1):207–217. doi: 10.20506/rst.30.1.2036. [DOI] [PubMed] [Google Scholar]
- Solomakos N., Govaris A., Angelidis A.S., Pournaras S., Burriel A.R., Kritas S.K., Papageorgiou D.K. Occurrence, virulence genes and antibiotic resistance of Escherichia coli O157 isolated from raw bovine, caprine and ovine milk in Greece. Food Microbiol. 2009;26(8):865–871. doi: 10.1016/j.fm.2009.06.002. 2009 Dec Epub 2009 Jun 10. [DOI] [PubMed] [Google Scholar]
- Spanu V., Spanu C., Virdis S., Cossu F., Scarano C., De Santis E.P. Virulence factors and genetic variability of Staphylococcus aureus strains isolated from raw sheep’s milk cheese. Int. J. Food Microbiol. 2012;153(1–2):53–57. doi: 10.1016/j.ijfoodmicro.2011.10.015. 2012 Feb 1 Epub 2011 Oct 29. [DOI] [PubMed] [Google Scholar]
- Spanu V., Scarano C., Cossu F., Pala C., Spanu C., De Santis E.P. Antibiotic resistance traits and molecular subtyping of Staphylococcus aureus isolated from raw sheep milk cheese. J. Food Sci. 2014;79(10):M2066–71. doi: 10.1111/1750-3841.12590. 2014 Oct Epub 2014 Sep 3. [DOI] [PubMed] [Google Scholar]
- Steiner T. Treating foodborne illness. Infect. Dis. Clin. North Am. 2013;27(3):555–576. doi: 10.1016/j.idc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Steward K.F., Robinson C., Holden M.T.G., Harris S.R., Ros A.F., Pérez G.C. Diversity of Streptococcus equi subsp. Zooepidemicus strains isolated from the Spanish sheep and goat population and the identification, function and prevalence of a novel arbutin utilisation system. Vet. Microbiol. 2017;207:231–238. doi: 10.1016/j.vetmic.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Streinu-Cercel A. Invasive fungal infections. Germs. 2012;2(2):35. doi: 10.11599/germs.2012.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Arashima Y., Kato K., Ogawa M., Kono K., Watanabe K., Saito T. A case of Pasteurella haemolytica sepsis in a patient with mitral valve disease who developed a splenic abscess. Scand. J. Infect. Dis. 2003;35(10):764–765. doi: 10.1080/00365540310016385. [DOI] [PubMed] [Google Scholar]
- Turchi B., Pero S., Torracca B., Fratini F., Mancini S., Galiero A., Cerri D. Occurrence of Clostridium spp. In ewe’s milk: enumeration and identification of isolates. Dairy Sci. Technol. 2016;96(5):693–701. [Google Scholar]
- Ungemach F.R., Müller-Bahrdt D., Abraham G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int. J. Med. Mic. 2006;296:33–38. doi: 10.1016/j.ijmm.2006.01.059. [DOI] [PubMed] [Google Scholar]
- van Asselt E.D., van der Fels‐Klerx H.J., Marvin H.J.P., van Bokhorst‐van de Veen H., Nierop Groot M. Overview of food safety hazards in the European dairy supply chain. Compr. Rev. Food Sci. Food Saf. 2017;16:59–75. doi: 10.1111/1541-4337.12245. [DOI] [PubMed] [Google Scholar]
- van Bokhorst-van de Veen H., Minor M., Zwietering M., Nierop Groot M. Wageningen UR Food and Biobased Research; Wageningen: 2015. Microbial Hazards in the Dairy Chain: a Literature Study. Report 1553; p. 93. [Google Scholar]
- Van den Brom R., Lievaart-Peterson K., Luttikholt S., Peperkamp K., Wouda W., Vellema P. Abortion in small ruminants in the Netherlands between 2006 and 2011. Tijdschr. 2012;137(7):450–457. [PubMed] [Google Scholar]
- Van den Brom R., van Engelen E., Roest H.I., van der Hoek W., Vellema P. Coxiella burnetii infections in sheep or goats: an opinionated review. Vet. Microbiol. 2015;181(1–2):119–129. doi: 10.1016/j.vetmic.2015.07.011. [DOI] [PubMed] [Google Scholar]
- van Engelen E., Luttikholt S., Peperkamp K., Vellema P., Van den Brom R. Small ruminant abortions in the Netherlands during lambing season 2012-2013. Vet. Rec. 2014;174(20):506. doi: 10.1136/vr.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verraes C., Claeys W., Cardoen S., Daube G., De Zutter L., Imberechts H. A review of the microbiological hazards of raw milk from animal species other than cows. Int. Dairy J. 2014;39(1):121–130. [Google Scholar]
- Verraes C., Vlaemynck G., Van Weyenberg S., De Zutter L., Daube G., Sindic M. A review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J. 2015;50:32–44. [Google Scholar]
- Wagner M., Melzner D., Bagò Z., Winter P., Egerbacher M., Schilcher Outbreak of clinical listeriosis in sheep: evaluation from possible contamination routes from feed to raw produce and humans. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52(6):278–283. doi: 10.1111/j.1439-0450.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- Willis C., Jørgensen F., Aird H., Elviss N., Fox A., Jenkins C. An assessment of the microbiological quality and safety of raw drinking milk on retail sale in England. J. Appl. Microbiol. 2018;124(2):535–546. doi: 10.1111/jam.13660. [DOI] [PubMed] [Google Scholar]
- Zeman P., Januska J., Orolinova M., Stuen S., Struhar V., Jebavy L. High seroprevalence of granulocytic ehrlichiosis distinguishes sheep that were the source of an alimentary epidemic of tick-borne encephalitis. Wien. Klin. Wochenschr. 2004;116(17):614–616. doi: 10.1007/s00508-004-0191-0. [DOI] [PubMed] [Google Scholar]