Abstract
COVID-19 pandemic caused by SARS-CoV-2, continues to manifest with severe acute respiratory syndrome among the adults, however, it offers a convincing indication of less severity and fatality in pediatric age group (0–18 years). The current trend suggests that children may get infected but are less symptomatic with less fatality, which is concordant to earlier epidemic outbreaks of SARS-CoV and MERS-CoV, in 2002 and 2012, respectively. According to the available data, children appear to be at lower risk for COVID-19, as adults constitute for maximum number of the confirmed cases (308,592) and deaths (13,069) as on 22nd March (https://www.worldometers.info/coronavirus). However, rapid publications and information of the adult patients with COVID-19 is in progress and published, on the contrary, almost no comprehensive data or discussion about the COVID-19 in children is available. Therefore, in this review, we outline the epidemiology, clinical symptoms, diagnosis, treatment, prevention, possible immune response and role of thymus in children to combat the COVID-19 outbreak.
Keywords: Children, Coronaviruses, COVID-19, Immunity, Pandemic, Thymus
Abbreviations: ARDS, Acute Respiratory Distress Syndrome; COVID-19, Coronavirus Disease-2019; MERS-CoV, Middle East Respiratory Syndrome Coronavirus; pp1ab, Polyprotein 1ab; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; WHO, World Health Organization
1. Introduction
From last two decades, coronaviruses have led to three major outbreaks that started from 2002 with SARS-CoV, then MERS-CoV in 2012 and currently SARS-CoV-2 (COVID-19). The rapid spread of the disease around the globe which originated from Wuhan city of China, has been declared as pandemic by WHO on 11 March 2020. SARS-CoV-2 is a new human infecting Beta-coronavirus belonging to a large family of zoonotic coronaviruses that are enveloped and single stranded RNA viruses, leading to zoonotic diseases. Zoonotic diseases, also called zoonosis are the diseases that are caused by the organisms/infective agents carried by the animals, transferred from animals to humans resulting in illnesses. They might belong to any class of viruses, bacteria, protozoa, and fungi. During these interactions, there are numerous ways these sources of infections like Coronaviruses, can be contracted to humans from animals via direct contact, food borne, water borne or vectors, resulting in zoonotic diseases (CDC July 2017). The commonly circulating corona viruses in humans are NL63, -HKU1, -OC43 and HCoV2-229E. Although CoVs can quickly bring mutations, leading to novel CoVs, that could transmit from animals to humans (Velavan and Meyer, 2020).
This virus comprises of densely glycosylated spike (S) proteins which enables it to penetrate and bind to the angiotensin-converting enzyme 2 receptor (ACE2) of the human host cell, similar as found previously in SARS-CoV (Del Rio et al., 2020). The polyprotein 1ab (pp1ab), consists of nonstructural proteins 1–16, which is similar to other members of subgenus, Sarbecovirus. Analysis of the pp1ab protein in COVID-19 showed that the protein sequence remains unchanged in samples studied from different locations during the outbreak. A unique deletion of eight amino acids in the virulence factor of the COVID-19 was detected in an isolate from an asymptomatic Japanese patient (Cardenas-Conejo et al., 2020). Additionally, 42 amino acid signatures in the pp1ab was identified that was only present in COVID-19. Such studies indicates that COVID-19 might have originated by genetic drift from a bat (bat-SL-CoV-RaTG13) (Cárdenas‐Conejo et al., 2020, Wu et al., 2020). Genetic drift is a kind of mutation that results from a variation in the gene pool over a period. It generally happens when alleles that are the variants of a gene either increase or decrease randomly with time (Honnay, 2013). It’s been found that the effect of genetic drift is stronger in small population, that’s why even a smaller event may result in larger impacts. The same is true for the pathogenicity of the microorganisms especially viruses, that frequently mutates, and impact of genetic drift is high (Kennedy and Dwyer, 2018).
The other CoVs like HCoV2-229E, NL63, and OC43.15 which mostly circulate in humans, were also originally found in bats, camels and cattle, however no information is known about HCoV-HKU1(Huynh et al., 2012, Davis et al., 2018). Bats act as the prime reservoir to most of the CoVs, circulating in animals, which are not linked with the human infections. Such viruses have ability to mutate rapidly and transform into a novel CoV that can transmit to humans (de Wit et al., 2016). Such condition had occurred in China (2002), when novel CoVs transmitted from bat to humans, resulted in a severe respiratory syndrome (SARS-CoV) (Luk et al., 2019, Drosten et al., 2003). In Saudi Arabia (2012) another CoVs were transmitted from camels to humans, known as Middle East Respiratory Syndrome coronavirus (MERS-CoV) (Luk et al., 2019). The current 2019 novel SARS-CoV-2, which emerged in China is a Betacoronavirus associated to the lineage B or sarbecovirus, a subgenus. Sequencing of SARS-CoV-2 revealed 87–89% nucleotide similarity with bat SARS-related CoV (bat-SL-CoVZC45). The epidemic of SARS-CoV-2 was first announced from Wuhan city of China on 31st Dec 2019, having a local seafood market as a primary source of the virus. From here the infected humans either symptomatic or asymptomatic carried forward and transmitted the virus by direct contact or respiratory droplets to cities and other countries, which finally led to a pandemic (Zhu et al., 2020, Chan et al., 2020).
1.1. Epidemiology of COVID-19 in children
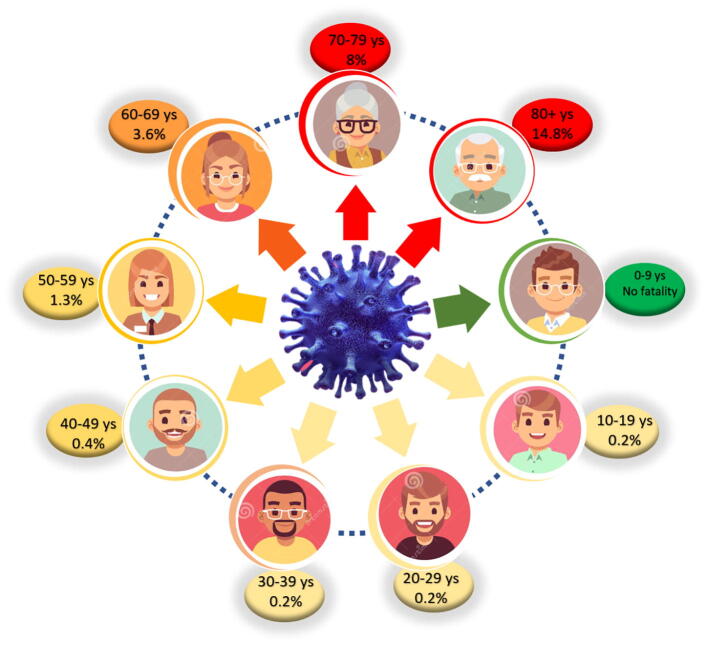
Preparatory epidemiological information pointed that the symptomatic manifestation of COVID-19 in children is rare. As per the reports from Chinese Centers for Disease Control and Prevention as of March 2020, only 2% cases of less than 19 years have been found affected by SARS-CoV-2 (Table 1 & Fig. 1) (Wu and McGoogan, 2020a, Wu and McGoogan, 2020b). A study was conducted having 3 case series of SARS-CoV-2 infected children. The 1st series had 20 children from Zhejiang (until 31st Jan 2020), the 2nd series had 34 children from Shenzhen (19th Jan to 7th Feb 2020) and the 3rd series with nine infants from various provinces of China (Wang et al., 2020b, Chen et al., 2020b, Wei et al., 2020). Most of the clinical details were obtained from the 2nd series, like, no children had any underlying disease, respiratory symptoms were seen in 65% children, mild symptoms in 26% and 9% were found asymptomatic. The 1st case series was presented with mild to moderate fever, cough, rhinitis, diarrhea, headache and poor feeding, cyanosis, dyspnea in severe cases. The 3rd series presented with fever in four infants. Almost all the children with manifested symptoms recovered within couple of weeks. No death is reported in children infected with SARS-CoV-2 till date (Chen et al., 2020b).
Table 1.
Rate of fatality among pediatric age group by COVID-19.
| S. No | Name of Country | Total number of cases | Age distribution % Ages 0–10/10–18 |
Case fatality | WEBLINK |
|---|---|---|---|---|---|
| 1 | Global cases till 16 March | 153,523 5735(Deaths) |
Ages 1–10 0% Ages 10–20 1.2% |
https://www.worldometers.info/coronavirus/ | |
| 2 | China | 81,048 | Ages 0–10 0.2% Ages 10–19 1.2% |
NA | https://www.statista.com/statistics/1095024/china-age-distribution-of-wuhan-coronavirus-covid-19-patients/ |
| 3 | Italy | 21,157 | 1.2% | NA | https://www.statista.com/statistics/1101680/coronavirus-cases-development-italy/ |
| 4 | South Korea | 7979 | Below age 10 0.9% Ages 10–19 5.2% |
NA | https://www.statista.com/statistics/1102730/south-korea-coronavirus-cases-by-age/ |
Fig. 1.
Illustration of fatality rate (percentage) among different age group in COVID-19. The sources of data presented is https://www.worldometers.info/coronavirus/https://www.statista.com/statistics/1095024/china-age-distribution-of-wuhan-coronavirus-covid-19-patients/.
Considering the epidemiology of the previous two outbreaks, it was identified that, although children are susceptible to infection by SARS-CoV, younger children comparatively had shown milder disease as compared to the 12 years of age and adolescents. It was reported that adolescent somehow resembled the clinical and radiological findings as adults and showed decline in their conditions as well (Leung et al., 2004a). As reported in a review by Banikab et al., 2015, Al-Sehaibany, 2017, MERS-CoV is predominantly prevalent among adults, but couple of cases among pediatric group were also reported, although as in case of SARs-CoV and COVID 19, most of them were asymptomatic. The diagnosis was mostly done during screening process of the adult family members, who were MERS-CoV positive.
Although there are no reasonable cases of children affected with COVID-19, but they could act as the carriers of virus. It is clearly evidenced that children are susceptible to SARS-CoV-2 contagion, but considerably without prominent disease, giving the chance that children could act as mediators for viral transmission and amplification. Thus, public and social health policies such as avoiding contacting with elderly group would be decided to avoid transfer and keep vulnerable people safe (Kelvin and Halperin, 2020). There are “silent patients” which may stay undiagnosed and are able to propagate the virus to considerable groups of people. It seems that children may not be as susceptible to COVID-19, as adults, and even after contracting the virus, they may remain asymptomatic (Guoqing et al., 2020).
1.2. Clinical presentation of COVID-19 in children
Study conducted by Wang et al., Wei et al., and Chen et al., has proved that the children develop milder symptoms compared to adults which is also seen early in outbreak of SARS-CoV and MERS-CoV infections (Hon et al., 2003, Alfaraj et al., 2019, Kwan et al., 2004, Wang et al., 2020b, Wei et al., 2020, Chen et al., 2020a). Additionally this study is the indication that mild symptoms or absence of severe symptoms in children may lead to misdiagnosis and will lead to skip the required test for SARS-CoV-2 and therefore, asymptomatic children might spread the disease(Guan et al., 2020). A study has revealed that SARS-CoV-2 can be found in feces long after throat and nose swabs test negative. However, the maximum number of SARS-CoV-2 infected children, have been found as a part of family cluster outbreak. This is also in accordance with the previous outbreaks of SARS-CoV and MERS-CoV, which reported to have 50–80% and 32% of children, infected by household contact, respectively (Al-Tawfiq et al., 2016, Wang et al., 2020b).
Children have equal chances of becoming infected with SARS-CoV-2 as adults, although would have milder symptoms or completely asymptomatic as suggested by a recently published study in March 2020 (Wang et al., 2020b). Although, the role of children in spreading the virus is still to be unraveled. Also, till date, there is no evidence of vertical transmission of SARS-CoV-2, from mother to the infant (Chen et al., 2020a).
1.3. Diagnosis
Real time polymerase chain reaction (RT-PCR) of respiratory tract secretion is the basic diagnostic method for COVID-19 disease, which can detect higher loads of virus from lower respiratory tract secretion as compared to higher tract (Lee et al., 2017, Vabret et al., 2003). Therefore, the initial negative suspected cases should be repeated for lower respiratory secretion. RT-PCR is used for genes that encodes the surface spike glycoprotein and internal RNA-dependent polymerase (Zhou et al., 2017). Whole genome sequencing is also being used as a molecular detection test for the SARS-CoV-2 (Chan et al., 2020).
1.4. Treatment
There is no specific treatment recommended for children by neither WHO, nor the US CDC. However, the aim of the treatment in children with COVID-19 is the prevention of organ failure, ARDS and hospital acquired infections. This is achieved by supportive treatment, which includes adequate intake of fluid, calories and ventilator support (Chen et al., 2020b). The recommended treatment for HCoVs infected children is the oral lopinavir/ritonavir along with corticosteroids and aerosolized interferon alpha-2b and also intravenous immunoglobulin, in case of severe cases. Lopinavir and ritonavir belong to the class of medications named as protease inhibitors. They are recommended to use in combination with other antivirals. Lopinavir/ritonavir may be considered for use as part of an investigational protocol for patients with COVID-19. Although, there is no evidence or recommendation from WHO about the benefit of above-mentioned therapies (Cao et al., 2020). Most of the children with SARS-CoV-2 infection had been treated with only lopinavir/ritonavir without using immunoglobulins (Wang et al., 2020b). Other potential therapeutic options include monoclonal antibodies, protease inhibitors like chloroquine, RNA synthesis inhibitors and the vaccines
The inhibition of the spike glycoprotein, responsible for interaction between host cell and virus, has been achieved by using monoclonal antibodies. This treatment minimizes the attachment of CoVs with host cell and therefore mortality rate in infected people is decreased. Many other human cell receptors for HCoVs like angiotensin-converting enzyme 2, aminopeptidase N, dipeptidyl peptidase 4 and O-acetylated sialic acid, are potential proteins for monoclonal based therapy (Jiang et al., 2014, Barton et al., 2014, Huang et al., 2015)
Enveloped viruses, also known as endosomal viruses, which are specialized to fuse through endosomal pathways can be minimized by inhibiting specific protease enzymes. In case of CoVs, papain proteases (PLpro) plays an important role in replication and could be a potential target for therapy. Several PLpro proteases have been reported so far, which differ among the CoVs species, making a narrow spectrum PLpro inhibitors, hence act as a potent antiviral drug against specific CoVs (Báez-Santos et al., 2015, Que et al., 2003, Lee et al., 2015).
Another way of reducing fusion of SARS-CoV and human cell, is using chloroquine, which increases the endosomal pH and hence inhibits the infection. Additionally, chloroquine has been reported to interfere via glycosylation of SARS-CoV receptors and also suppresses the entry of virus (Savarino et al., 2006, Wang et al., 2020a).
Inhibitors of enzymes like helicase which brings the unwinding of double stranded RNA during the replication is of great importance in treating the COVID-19. Additionally, Immucillin-A, is an adenosine analog that inhibits polymerase of viral RNA in a variety of viruses like SARS-CoV and MERSCoV, DRACO (double-stranded RNA activated caspase oligomerizer) initiates the apoptosis of infected cells by targeting double-stranded RNA of virus, without involving healthy cells can also be considered in treating CoVs (Rider et al., 2011) (Warren et al., 2014, Lundin et al., 2014, Rappe et al., 2018)
Various vaccines are in progress against HCoVs for preventing the infection, minimizing severity of disease and viral shedding. Spike glycoprotein S and its receptor-binding domain are the main targets for vaccine development. Nonetheless, CoVs has got the privilege of rapid mutation, which is a challenge in the progress of vaccine development (He et al., 2006, Hashem et al., 2019).
1.5. Prevention
Rapid and impactful measures for infection control are needed to minimize the spread of SARS-CoV-2. One of the most challenging tasks with COVID-19, is the containment of nosocomial transmission which is a potential threat among the children. Health care setups appear to enhance the chance of viral transmission because of infective droplets producing procedures like suctioning of the airways and intubation. Adequate and appropriate hospital sanitization is of paramount importance to control and restraint the hospital acquired COVID-19 infection. A well-known fact is the need of strict hand sanitization which can be achieved by using ethanol, isopropanol, etc. and minimal social gatherings (Bin et al., 2016, Leung et al., 2004b).
2. Possible reasons for immunity against viral infection in children
2.1. Effect of environmental factors on the immune system in children
The idea of immune memory provides previous exposures form existing immune function. The exposures include not only specific antigens exciting adaptive (specific) immune memory, but also preserved antigens related to molecular patterns, that mold innate (non-specific) immune responses for long period of life. It is not surprising that there is a major scale of external agents and environmental factors that influence and modulate the immune system, which is most easily identified in early life, a period of fast variable environments (MacGillivray and Kollmann, 2014).
The immune system is supposed to respond to harmful antigens such as viruses, bacteria and other pathogens. However, normal microbial flora, chemical agents, stress, and other factors can also elicit, frame or interact with the immune system. It is commonly reasonable that some of these interactions are physiological and requisites for a healthy immune system. While there are group of factors negatively influence, such as, immunosuppressive influence of UV irradiation, the artificially immunotoxin agents, such as polycyclic aromatic hydrocarbons, autoimmune or allergies affect negatively. Positive effects are derived from naturally contacting with natural bacterial flora, good lifestyle, and diet. There is a great communication between the environment and the immune system. Many environmental factors can be of effects on immune system such as such as bacteria, sun exposure, age, exercise, stress and air pollution (Esser, 2016). It is thought that prior exposure to moderate coronaviruses may play a part in kids’ comparative COVID-19 edge over adults. Children presence in environments of schoolyard, may be continuously producing antibodies to pathogens and those antibodies are variably enough to fight off COVID-19.
There is a significant modification of vaccine antibody levels among children and early life exposition to drinking water arsenic and lead in blood. Special differences were reported in children related to difference based on gender, only female group was susceptible to metal-related alteration in antibody concentrations. Weight against age, nutritional condition, did not show the link between vaccine antibody and the metal mixture (Welch et al., 2020).
2.2. The role of angiotensin-converting enzyme 2 receptor (ACE2) of the human host cell
SARS-CoV-2 enter the host cell through binding of viral spike proteins to ACE2 receptors which exist on the host cell membrane. This increases the potential of infection susceptibility via expression of the target ACE2 receptor in exposed epithelium to virus. Children have more ACE level in serum than adults. Considering the opposite relation between ACE and ACE2, this variability reflects a lower level of ACE2 expression in children in comparison to adults. While adolescents had higher concentrations of blood ACE than adults and is interesting to observe that COVID-19 in the age group 10–19 years is only found in 1% in the Chinese group. Epidemiological proofs show that SARS-CoV-2 in children is less intense than adults (Rodriguez et al., 1981, Skarstein, 2020, Wan et al., 2020)
One of the most common parameters for the severity of the COVID-19 is the chronic illness, like high blood pressure, diabetes, and cardiovascular disease (Mizumoto et al., 2015). Based on this fact, children appear to be at lower risk, due to absence of such aliments at young age. This trend has been also seen during SARS-CoV outbreak in 2002, with a relatively lower mortality rate and mild symptoms, compared to adults (Hon et al., 2003). Innate immunity of the human body is critical and is the first line of defense for battling the infectious diseases. This defense mechanism shows an exponential decline in the later life span, which leads to increase in the incidence of infectious disease. For example, diseases like chickenpox and measles are fatal to adults, almost 25 times more than children (Rawson et al., 2001).
The adaptive immune mechanism is a slowly evolved defense system that recognizes specific agents and is prolonged in its action against the pathogens (Tregoning and Schwarze, 2010). Research suggests that if a child has a strong innate defense to current virus, the chances of fight against the infection is more effective, with manifestation of only mild symptoms. The fact is that children also get caught with the respiratory infection, however they fight robustly and recover faster than the adults. The probable justification could be healthy respiratory tissues due to nonsmoking, less exposure to air pollution, etc. As also seen in previous outbreaks like SARS and MERS in 2003 and 2012, respectively, that the underlying diseases was one of the major causes for the increased rate of death among the adults. The co-occurring diseases could be diabetes, cardiovascular, obesity or any autoimmune disease. Among the adults, an acute respiratory distress syndrome (ARDS) is a condition, which is caused by detrimental immune response (de Wit et al., 2016). The intricate imbalance of the immune system over activates the lungs and hence accumulating fluid in the alveoli. Under normal conditions, RBCs rush into these air sacs to pick up new oxygen, however on inflammation of these alveoli, the RBCs stop functioning, making it difficult to breathe. Therefore, the people with ARDS have higher death risk of coronavirus as seen in SARS coronavirus. This is also one of the reasons that frequency of fatality of COVID-19 is less in children, owing to non-presence of complicated immunological response, compared to adults. However, it is a subject of priority for the researchers to unravel the mechanism of action and outcome in children against the CoVs infection.
2.3. Interferon as a first line of innate immunity against viral infection
Interferon (IFN) is a glycoproteins cluster, synthesized by host infected cells as an antiviral response once the body is infected by virus. IFN is the first defense line in innate immunity against viral infection. Genetics and variable protein structures are bases to classify IFN into IFN-α, IFN-β, IFN-γ, etc. Interferons play essential role as antiviral factors via two mechanisms: (1) As innate immunity, IFN stimulates production of antiviral effector proteins, thus inhibit replication of invaded virus in the cells which are released to protect neighboring normal cells from viral invasion; (2) activate T cellular immunity through supporting the proliferation and activation of an important T lymphocyte clonal named cytotoxic T lymphocyte, later activates natural killer (NK) and macrophage cells to remove the virus. Deficiency of body endogenous IFN may lead to a decrease in an antiviral response. Children are subjected to viral infection since their immature immunity revealing lower levels of specific cellular and humoral immunity and IFN production (Shen and Yang, 2020). Earlier stimulation of interferons in children and their minimal developed immune system may be the cause of their zero or near to zero accident average. Treatment with interferon-inducers can decrease the death rate of SARS-CoV at the very earlier stages of the disease. Additionally, interferon-γ to an interferon-I, as a synergistic combination treatment, may amplify the advantages. At the later stages of the disease, considering the immunopathogenic over-reactions leads to possible cytokine storm (Shahabi nezhad et al., 2020, Kelvin and Halperin, 2020).
2.4. Physiological role of thymus in children against viral infection
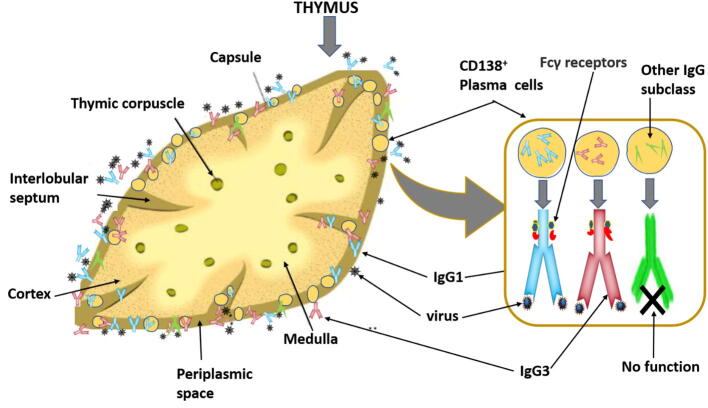
A study published by Miyamoto et al. (2004), reported that pandemic disease is dependent on acquired/vaccinated immunity and weather conditions that are catastrophic to several viruses such as severe acute respiratory syndrome corona virus (Miyamoto et al., 2004). The less effect of COVID-19 on children is not because of vaccines, as there is no significant cross reactivity against SARS-CoV, which indicates that decreased symptoms of SARS-CoV in children may be attributed to other reasons (Yu et al., 2007). Thymus gland may play a key role in this regard, as it is active in the childhood than adulthood. It is known that thymus housed mainly T lymphocyte, Sarah et al., (2016) reported that there are plasma cells generated by B lymphocyte which are residing in thymus, they studied the phonemic of these resident cells and their potential protective function against viral infections. It was found that starting from the first year of life, CD138(+) plasma cells begin to accumulate in perivascular space of thymus (PST), where they secrete immunoglobulin G (IgG) without additional activation and secrete IgG1 and IgG3 exclusively, the two main antibodies that able to fix the complement on IgG subclasses (Fig. 2). Plasma cells in thymus comprise a high recurrence of cells reactive to popular viral proteins. It was revealed that an unknown function of the PST has an effective niche for viral-specific plasma cells. The PST is placed between the thymic epithelial zones and the blood circulation (Nuñez et al., 2016).
Fig. 2.
Role of thymus gland in viral infection. Illustration shows CD138+ plasma cells (PC) accumulated in thymic perivascular space, where they constitutively produce IgG1 and IgG3 which are reactive to common viral proteins and IgG subclass with no function.
IgG3 and IgG1 are the main secreted IgG subclass by thymic plasma cells, with negligible IgG2 and IgG4 producing plasma cells. IgG3 and IgG1subclass have been found associated with the immunity against the common viral infections. Specific antibodies against viral antigens are produced by maximum plasma cells in infants less than 1 year and pediatric age group between 1 and 15 years. Moreover, the occurrence of plasma cells was increased in case of influenza and measles in children compared with the newborn thymus. Furthermore, it was concluded that the expression of chemokine receptor and CXCR3 elevates in the thymic B cells with increasing age and is localized in the thymic perivascular spaces (Kao et al., 2015, Bordon, 2017).
3. Conclusion
The COVID-19 disease has emerged as a pandemic and the current data reveals that children do not develop serious disease, that requires hospitalization compared to adults. The possible reasons for disparity in clinical severity between children and adults could be the difference in cytokine profile (having less interleukin 6 and myeloperoxidase) in the lungs of children. Additionally, thymus has a significant role with its resident plasma cells secreting functional antibodies fixing complements, which highly promote the immunity in the starting of early age. Also, the other possible factors involved in decreased clinical severity in pediatric age group could be less exposure to pollutants and increased incidence of viral upper respiratory tract infections, which may result in better immune response due to cross reactive antibodies against SARS-CoV-2. However, further research is needed to understand the underlying mechanisms leading to decreased mortality in the pediatric age group.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Authors sincerely acknowledge all the front-line health and other emergency workers for their devotion and inexhaustible service to combat the COVID-19 pandemic.
Funding
Deanship of scientific Research, Imam Abdulrahman Bin Faisal University, 31441 Dammam, Saudi Arabia under the Project No Covid19-2020-010-IRMC.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Suriya Rehman, Email: surrehman@iau.edu.sa, https://scholar.google.com/citations?view_op=search_authors&mauthors=suriya+rehman&hl=en&oi=ao.
Ebtesam A. Al-Suhaimi, Email: ealsuhaimi@iau.edu.sa.
References
- Alfaraj Sarah H., Al-Tawfiq Jaffar A., Altuwaijri Talal A., Memish Ziad A. Middle East respiratory syndrome coronavirus in pediatrics: a report of seven cases from Saudi Arabia. Front. Med. 2019;13:126–130. doi: 10.1007/s11684-017-0603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sehaibany Fares. Middle East respiratory syndrome in children Dental considerations. Saudi Med. J. 2017;38(4):339–343. doi: 10.15537/smj.2017.4.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq Jaffar A., Kattan Rana F., Memish Ziad A. Middle East respiratory syndrome coronavirus disease is rare in children: an update from Saudi Arabia. World J. Clin. pediatrics. 2016;5:391. doi: 10.5409/wjcp.v5.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Santos Yahira M., St John Sarah E., Mesecar Andrew D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banikab, Khandaker, Rashid Middle east respiratory syndrome coronavirus “MERS-CoV”: current knowledge gaps. Paediatric Respiratory Rev. 2015;16:197–220. doi: 10.1016/j.prrv.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton Christopher, Calvin Kouokam J., Lasnik Amanda B, Foreman Oded, Cambon Alexander, Brock Guy, Montefiori David C., Vojdani Fakhrieh, McCormick Alison A., O'Keefe Barry R. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Seo Yu, Heo Jung Yeon, Song Min-Suk, Lee Jacob, Kim Eun-Ha, Park Su-Jin, Kwon Hyeok-il, Kim Semi, Kim Young-il, Si Young-Jae. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin. Infect. Dis. 2016;62:755–760. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordon Yvonne. Antibody responses: a thymic niche for plasma cells. Nat. Rev. Immunol. 2017;17:78. doi: 10.1038/nri.2017.2. [DOI] [PubMed] [Google Scholar]
- Cao Bin, Wang Yeming, Wen Danning, Liu Wen, Wang Jingli, Fan Guohui, Ruan Lianguo, Song Bin, Cai Yanping, Wei Ming, Li Xingwang, Xia Jiaan. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Conejo Y., Linan-Rico A., Garcia-Rodriguez D.A., Centeno-Leija S., Serrano-Posada H. An exclusive 42 amino acid signature in pp1ab protein provides insights into the evolutive history of the 2019 novel human-pathogenic coronavirus (SARS-CoV2) J. Med. Virol. 2020 doi: 10.1002/jmv.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) July 14, 2017.
- Chan Jasper Fuk-Woo, Yuan Shuofeng, Kok Kin-Hang, To Kelvin Kai-Wang, Chu Hin, Yang Jin, Xing Fanfan, Liu Jieling, Yip Cyril Chik-Yan, Poon Rosana Wing-Shan. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Huijun, Guo Juanjuan, Wang Chen, Luo Fan, Xuechen Yu., Zhang Wei, Li Jiafu, Zhao Dongchi, Dan Xu., Gong Qing. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Zhi-Min, Jun-Fen Fu., Shu Qiang, Chen Ying-Hu, Hua Chun-Zhen, Fu-Bang Li Ru., Lin Lan-Fang Tang, Wang Tian-Lin, Wang Wei. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatrics. 2020:1–7. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Brian M., Foxman Betsy, Monto Arnold S., Baric Ralph S., Martin Emily T., Uzicanin Amra, Rainey Jeanette J., Aiello Allison E. Human coronaviruses and other respiratory infections in young adults on a university campus: prevalence, symptoms, and shedding. Influenza Other Respir. Viruses. 2018;12:582–590. doi: 10.1111/irv.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit Emmie, van Doremalen Neeltje, Falzarano Darryl, Munster Vincent J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio Carlos, Malani Preeti N. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020 doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- Drosten Christian, Günther Stephan, Preiser Wolfgang, Van Der Werf Sylvie, Brodt Hans-Reinhard, Becker Stephan, Rabenau Holger, Panning Marcus, Kolesnikova Larissa, Fouchier Ron AM. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Esser Charlotte., editor. Environmental Influences on the Immune System. Springer; Vienna, Austria: 2016. [Google Scholar]
- Guan Wei-jie, Zheng-yi Ni YuHu, Liang Wen-hua, Chun-quan Ou, He Jian-xing, Liu Lei, Shan Hong, Lei Chun-liang, Hui David S.C. Clinical characteristics of coronavirus disease 2019 in China. New England J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guoqing Qian, Yang Naibin, Ma Ada Hoi Yan, Wang Liping, Li Guoxiang, Chen Xueqin, Chen Xiaomin. A COVID-19 Transmission within a family cluster by presymptomatic infectors in China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem Anwar M., Algaissi Abdullah, Agrawal Anurodh Shankar, Al-Amri Sawsan S., Alhabbab Rowa Y., Sohrab Sayed S., Almasoud Abdulrahman S., Alharbi Naif Khalaf, Peng Bi-Hung, Russell Marsha. A highly immunogenic, protective, and safe adenovirus-based vaccine expressing middle east respiratory syndrome coronavirus S1-CD40L fusion protein in a transgenic human dipeptidyl peptidase 4 mouse model. J. Infect. Dis. 2019;220:1558–1567. doi: 10.1093/infdis/jiz137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Yuxian, Li Jingjing, Lanying Du., Yan Xuxia, Guangan Hu., Zhou Yusen, Jiang Shibo. Identification and characterization of novel neutralizing epitopes in the receptor-binding domain of SARS-CoV spike protein: revealing the critical antigenic determinants in inactivated SARS-CoV vaccine. Vaccine. 2006;24:5498–5508. doi: 10.1016/j.vaccine.2006.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon K.L.E., Leung C.W., Cheng W.T.F., Chan P.K.S., Chu W.C.W., Kwan Y.W., Li A.M., Fong N.C., Ng P.C., Chiu M.C. Clinical presentations and outcome of severe acute respiratory syndrome in children. The Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnay, Olivier, 2013. Genetic Drift Chapter In book: Brenner's Encyclopedia of Genetics, pp. 251–253. https://doi.org/10.1016/B978-0-12-374984-0.00616-1.
- Huang Xingchuan, Dong Wenjuan, Milewska Aleksandra, Golda Anna, Qi Yonghe, Zhu Quan K., Marasco Wayne A., Baric Ralph S., Sims Amy C., Pyrc Krzysztof. HCoV-HKU1 Spike protein uses O-acetylated sialic acid as an attachment receptor determinant and employs HE protein as a receptor-destroying enzyme. J. Virol. 2015 doi: 10.1128/JVI.00854-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh Jeremy, Li Shimena, Yount Boyd, Smith Alexander, Sturges Leslie, Olsen John C., Nagel Juliet, Johnson Joshua B., Agnihothram Sudhakar, Edward Gates J. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J. Virol. 2012;86:12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Liwei, Wang Nianshuang, Zuo Teng, Shi Xuanling, Poon Kwok-Man Vincent, Yongkang Wu., Gao Fei, Li Danyang, Wang Ruoke, Guo Jianying. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008140. 234ra59-34ra59. [DOI] [PubMed] [Google Scholar]
- Kao Daniela, Danzer Heike, Collin Mattias, Groß Andrea, Eichler Jutta, Stambuk Jerko, Lauc Gordan, Lux Anja, Nimmerjahn Falk. A monosaccharide residue is sufficient to maintain mouse and human IgG subclass activity and directs IgG effector functions to cellular Fc receptors. Cell Rep. 2015;13:2376–2385. doi: 10.1016/j.celrep.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Kelvin Alyson A., Halperin Scott. COVID-19 in children: the link in the transmission chain. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy David A., Dwyer Greg. Effects of multiple sources of genetic drift on pathogen variation within hosts. PLOS Biology. 2018 doi: 10.1371/journal.pbio.2004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan Mike Yat Wah, Chan Wai Ming, Ko Po Wan, Leung Chi Wai, Chiu Man Chun. Severe acute respiratory syndrome can be mild in children. Pediatric Infectious Dis.J. 2004;23:1172–1174. [PubMed] [Google Scholar]
- Lee Jee-Soo, Ahn Ji Soo, Yu Byeong Su, Cho Sung Im, Kim Man Jin, Choi Jong Moon, Seo Soo Hyun, Park Sung Sup, Seong Moon-Woo. Evaluation of a real-time reverse transcription-PCR (RT-PCR) assay for detection of Middle East respiratory syndrome coronavirus (MERS-CoV) in clinical samples from an outbreak in South Korea in 2015. J. Clin. Microbiol. 2017;55:2554–2555. doi: 10.1128/JCM.00667-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Hyun, Lei Hao, Santarsiero Bernard D., Gatuz Joseph L., Cao Shuyi, Rice Amy J., Patel Kavankumar, Szypulinski Michael Z., Ojeda Isabel, Ghosh Arun K. Inhibitor recognition specificity of MERS-CoV papain-like protease may differ from that of SARS-CoV. ACS Chem. Biol. 2015;10:1456–1465. doi: 10.1021/cb500917m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung Chi-wai, Kwan Yat-wah, Ko Po-wan, Chiu Susan S., Loung Po-yee, Fong Nai-chung, Lee Lai-ping, Hui Yim-wo, Law Helen K.W., Wong Wilfred H.S., Kwok-hung Chan J.S., Peiris Malik, Lim Wilina W.L., Lau Yu-lung, Chiu Man-chun. Severe acute respiratory syndrome among children. Off. J. Am. Acad. Pediatrics. 2004;113(6):535–543. doi: 10.1542/peds.113.6.e535. [DOI] [PubMed] [Google Scholar]
- Leung T.F., Ng P.C., Cheng F.W.T., Lyon D.J., So K.W., Hon E.K.L., Li A.M., Li C.K., Wong G.W.K., Nelson E.A.S., Hui J., Sung R.Y.T., Yam M.C., Fok T.F. Infection control for SARS in a tertiary paediatric centre in Hong Kong. J. Hosp. Infect. 2004;56:215–222. doi: 10.1016/j.jhin.2003.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk Hayes K.H., Li Xin, Fung Joshua, Lau Susanna K.P., Woo Patrick C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infection Genetics Evol. 2019 doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin Anna, Dijkman Ronald, Bergström Tomas, Kann Nina, Adamiak Beata, Hannoun Charles, Kindler Eveline, Jonsdottir Hulda R., Muth Doreen, Kint Joeri. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle East respiratory syndrome virus. PLoS pathogens. 2014;10 doi: 10.1371/journal.ppat.1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray Duncan M., Kollmann Tobias R. The role of environmental factors in modulating immune responses in early life. Front. Immunol. 2014;5:434. doi: 10.3389/fimmu.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Hideki, Sahara Keiji, Sugieda Masaaki. Seroepidemiological analysis of influenza pandemics in Shizuoka Prefecture and all Japan. Int. Congr. Ser. 2004;1263:413–416. doi: 10.1016/j.ics.2004.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto Kenji, Endo Akira, Chowell Gerardo, Miyamatsu Yuichiro, Saitoh Masaya, Nishiura Hiroshi. Real-time characterization of risks of death associated with the Middle East respiratory syndrome (MERS) in the Republic of Korea, 2015. BMC Med. 2015;13:228. doi: 10.1186/s12916-015-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez Sarah, Moore Carolina, Gao Baoshan, Rogers Kortney, Hidalgo Yessia, Del Nido Pedro J., Restaino Susan, Naka Yoshifumi, Bhagat Govind, Madsen Joren C., Bono María Rosa, Zorn Emmanuel. The human thymus perivascular space is a functional niche for viral-specific plasma cells. Sci. Immunol. 2016;1:eaah4447. doi: 10.1126/sciimmunol.aah4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que T.L., Wong V.C.W., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. [PubMed] [Google Scholar]
- Rappe Julie Christiane Françoise, de Wilde Adriaan, Di Han, Müller Christin, Stalder Hanspeter, V’kovski Philip, Snijder Eric, Brinton Margo A., Ziebuhr John, Ruggli Nicolas. Antiviral activity of K22 against members of the order Nidovirales. Virus Res. 2018;246:28–34. doi: 10.1016/j.virusres.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson Helen, Crampin Amelia, Noah Norman. Deaths from chickenpox in England and Wales 1995–7: analysis of routine mortality data. BMJ. 2001;323:1091–1093. doi: 10.1136/bmj.323.7321.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider Todd H., Zook Christina E., Boettcher Tara L., Wick Scott T., Pancoast Jennifer S., Zusman Benjamin D. Broad-spectrum antiviral therapeutics. PLoS ONE. 2011;6:e22572. doi: 10.1371/journal.pone.0022572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Shin, Abernathy Kendig. Serum angiotensin-converting enzyme activity in normal children and in those with sarcoidosis. J. Pediatr. 1981;99:68–72. doi: 10.1016/s0022-3476(81)80959-6. [DOI] [PubMed] [Google Scholar]
- Savarino Andrea, Di Trani Livia, Donatelli Isabella, Cauda Roberto, Cassone Antonio. New insights into the antiviral effects of chloroquine. Lancet. Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabi nezhad F., Mosaddeghi P., Negahdaripour M., Dehghani Z., Farahmandnejad M., Moghadami M., Nezafat N., Masoompour S.M. Therapeutic approaches for COVID-19 based on the dynamics of interferon-mediated immune responses. Preprints. 2020 [Google Scholar]
- Shen K., Yang Y. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J. Pediatr. 2020 doi: 10.1007/s12519-020-00344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstein Kolberg E. ACE2, COVID19 and serum ACE as a possible biomarker to predict severity of disease. J. Clin. Virol. 2020 doi: 10.1016/j.jcv.2020.104350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning John S., Schwarze Jürgen. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret Astrid, Mourez Thomas, Gouarin Stéphanie, Petitjean Joëlle, Freymuth François. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin. Infectious Dis. 2003;36:985–989. doi: 10.1086/374222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan Thirumalaisamy P., Meyer Christian G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020 doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Manli, Cao Ruiyuan, Zhang Leike, Yang Xinglou, Liu Jia, Mingyue Xu., Shi Zhengli, Zhihong Hu., Zhong Wu., Xiao Gengfu. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.F., Yuan J., Zheng Y.J., Chen J., Bao Y.M., Wang Y.R., Wang L.F., Li H., Zeng J.X., Zhang Y.H. 'Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen', Zhonghua er ke za zhi. Chin. J. Pediatrics. 2020;58:E008. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. [DOI] [PubMed] [Google Scholar]
- Warren Travis K., Wells Jay, Panchal Rekha G., Stuthman Kelly S., Garza Nicole L., Van Tongeren Sean A., Dong Lian, Retterer Cary J., Eaton Brett P., Pegoraro Gianluca. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Min, Jingping Yuan Yu, Liu Tao Fu, Xue Yu, Zhang Zhi-Jiang. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B.M., Branscum A., Geldhof G.J. Evaluating the effects between metal mixtures and serum vaccine antibody concentrations in children: a prospective birth cohort study. Environ. Health. 2020 doi: 10.1186/s12940-020-00592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Canrong, Liu Yang, Yang Yueying, Peng Zhang Wu., Zhong Yali Wang, Wang Qiqi, Yang Xu., Li Mingxue, Li Xingzhou, Zheng Mengzhu, Chen Lixia, Li Hu.a. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. 'Characteristics of and important lessons from the Coronavirus Disease 2020. (COVID-19) Outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yu Y., Jin H., Chen Z., Yu Q.L., Ma Y.J., Sun X.L., Wang B. Children's vaccines do not induce cross reactivity against SARS-CoV. J. Clin. Pathol. 2007;60:208–211. doi: 10.1136/jcp.2006.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Jie, Li Cun, Zhao Guangyu, Chu Hin, Wang Dong, Yan Helen Hoi-Ning, Poon Vincent Kwok-Man, Wen Lei, Wong Bosco Ho-Yin, Zhao Xiaoyu. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3 doi: 10.1126/sciadv.aao4966. eaao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Na., Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Roujian Lu. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunyou Wu, McGoogan Jennifer M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]