SUMMARY
CD133+ populations of human glioblastoma multiforme (GBM) cells are reportedly enriched for tumor stem cells (TSCs) or tumor-initiating cells (TICs). Approximately 40% of freshly isolated GBM specimens, however, do not contain CD133+ tumor cells, raising the possibility that CD133 may not be a universal enrichment marker for GBM TSCs/TICs. Here we demonstrate that stage-specific embryonic antigen 1(SSEA-1/LeX)+ GBM cells fulfill the functional criteria for TSC/TIC, since (1) SSEA-1+ cells are highly tumorigenic in vivo, unlike SSEA-1- cells; (2) SSEA-1+ cells can give rise to both SSEA-1+ and SSEA-1− cells, thereby establishing a cellular hierarchy; and (3) SSEA-1+ cells have self-renewal and multilineage differentiation potentials. A distinct subpopulation of SSEA-1+ cells was present in all but one of the primary GBMs examined (n = 24), and most CD133+ tumor cells were also SSEA-1+, suggesting that SSEA-1 may be a general TSC/TIC enrichment marker in human GBMs.
INTRODUCTION
The cancer stem cell hypothesis posits that tumorigenic potential is largely restricted to a subset of self-renewing tumor cells with stem cell-like properties designated as tumor stem cells (TSCs) or tumor-initiating cells (TICs) (Clarke et al., 2006; Jordan et al., 2006; Reya et al., 2001). Since these TSCs/TICs represent only a subpopulation within the whole of the tumor, there is great interest in finding cell-surface markers that will allow the prospective identification and isolation of these cells (Uchida et al., 2000; Vescovi et al., 2006). The AC133 (later designated as CD133/PROM1) antigen was originally identified as a surface antigen expressed in hematopoietic stem cell populations (Corbeil et al., 1998; Miraglia et al., 1997). Weissman and colleagues have shown that human fetal brain cells expressing AC133 antigen have neural stem cell (NSC)-like properties (Uchida et al., 2000). Several groups have demonstrated that cell sorting for CD133 expression can enrich for TSC/TIC populations in brain tumors (Bao et al., 2006; Galli et al., 2004; Piccirillo et al., 2006; Singh et al., 2004) as well as in colon cancer (O’Brien et al., 2007; Ricci-Vitiani et al., 2007) and prostate cancer (Richardson et al., 2004). These CD133+ cells appear to be responsible for tumor initiation in vivo and are relatively resistant to radiation compared to the remaining bulk of tumor cells, suggesting their potential role in tumor recurrence (Bao et al., 2006).
Whether CD133 can serve as a universal TSC enrichment marker for all tumors, however, has been questioned following a series of recent papers. Several groups have reported the absence of CD133+ cell populations in primary GBM-derived cells (Beier et al., 2007; Joo et al., 2008; Ogden et al., 2008; Wang et al., 2008). Similarly, it has been proposed that CD44 can be an enrichment marker for colon and prostate cancer TICs (Dalerba et al., 2007; Patrawala et al., 2007). In these studies, tumor cells expressing both CD133 and CD44 cells were not a significant population (Dalerba et al., 2007; Shmelkov et al., 2008), suggesting that there may exist more than one marker that can prospectively enrich for a TSC/TIC population. Alternatively, it is also possible that TSC/TIC population may not exist in some tumors. Indeed, two groups have provided evidence that almost all tumor cells can function as TSCs/TICs in lymphoma and human melanoma (Kelly et al., 2007; Quintana et al., 2008). Determining whether these two possibilities exist in human GBMs has significant biologic and clinical implications.
Over the last few years, we have been isolating TSC/TIC-like cells from various primary GBMs by culturing freshly dissociated GBM cells from patients in media favoring the growth of normal NSCs (Lee et al., 2006; Lee et al., 2008). In nearly half of these in vitro-established TSC/TIC lines, as well as acutely isolated tumor cells from fresh GBM patient tumors (total of 24 samples), we failed to detect a distinct CD133+ populations. Nevertheless, these “CD133-negative” tumors contain cells with stem cell-like properties that are highly tumorigenic in orthotopic transplantation SCID models, similar to CD133+ TSC/TIC lines from CD133+ tumors. In search of an alternative and/or more general enrichment marker for GBM TSCs/TICs, we have identified distinct subpopulations of cells expressing a neural stem/progenitor cell marker, stage-specific embryonic antigen 1 (SSEA-1/CD15/Lewis X [LeX]) (Capela and Temple, 2002, 2006). We now demonstrate that the selection for SSEA-1+ cells enriches for glioma TSC/TIC subpopulations in all of the GBMs that are devoid of CD133+ cells. These SSEA-1+ cells give at least a 100-fold tumorigenic enrichment in mouse xenograft models compared to SSEA-1− cells, as well as capabilities for self-renewal and multilineage differentiation. We propose that SSEA-1, along with CD133, is a marker that enriches for TSCs/TICs in primary human GBMs.
RESULTS
Expression of SSEA-1 in Acutely Isolated GBM Cells and Established TSC/TIC Lines
We have established TSC/TIC-like cells from various primary GBMs by culturing freshly dissociated cells in neurobasal serum-free media with N2/B27 supplement in the presence of basic FGF and EGF (NBE media) (Lee et al., 2006, 2008). We have used rather strict criteria for defining TSC/TIC lines, requiring that they are clonogenic in vitro, express stem cell markers, be capable of neuronal and/or glial differentiation, be tumorigenic in vivo in serial transplantation, and be able to generate xenograft tumors that recapitulate the biological and genomic features of the parental GBM.
In an attempt to phenotype various established TSC/TIC lines, we first determined the expression of CD133 by fluorescence-activated cell sorting (FACS) analysis (Table 1 and Figure 1). We found that seven cell lines out of 12 different GBM TSC/TIC lines had very distinct subpopulations ofCD133+cells. The percentage of CD133+ cells ranged from 1.7% to 63.5% (Table 1), similar to what has been previously reported (Piccirillo et al., 2006; Singh et al., 2004). By contrast, the remaining five cell lines did not contain any cells with detectable levels of CD133 expression, consistent with other reports (Beier et al., 2007; Joo et al., 2008; Ogden et al., 2008; Wang et al., 2008). Similar to the GBM lines containing CD133+ cells, these cell lines are highly tumorigenic, grew as neurospheres (see Figure S1A available online), and expressed high levels of stem cell-associated proteins such as Nestin (Lendahl et al., 1990), Sox2 (Suh et al., 2007; Yuan et al., 1995), Bmi1(Lessard and Sauvageau, 2003), and Ezh2 (Shenet al., 2008; Sher et al., 2008; Valk-Lingbeek et al., 2004) (Figure S1C). These data, which are consistent with those of others (Beier et al., 2007; Joo et al., 2008; Ogden et al., 2008; Wang et al., 2008), suggest that CD133 may not be a universal enrichment marker for TSC/TIC in GBMs.
Table 1.
Expression of CD133 and/or SSEA-1 in Various Primary Human GBMs and Their Derivative Tumor Cells
| TSC Name |
Percent Expression at the Earliest Passages | Percent Expression during In Vitro Expansion | Percent Expression of Xenograft Tumors | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total CD133+ |
Total SSEA-1+ |
CD133+/ SSEA-1+ |
SSEA-1+ in CD133+ |
Passages | Total CD133+ |
Total SSEA-1+ |
CD133+/ SSEA-1+ |
SSEA-1+ in CD133+ |
Passages | Total CD133+ |
Total SSEA-1+ |
CD133+/ SSEA-1+ |
SSEA-1+ in CD133+ |
|||||||||
| (1) Established TSC/TIC Lines |
0308 | 38.2 | 57.9 | 17.3 | 45.3 | p5 | 22.2 ± 6.5 | 60.1 ± 17.5 | 12.0 ± 1.5 | 56.5 ± 12.3 | p10–13 | 10.2 ± 6.5 | 17.6 ± 14 | 2.5 ± 2.9 | 19.8 ± 16.3 | |||||||
| 0206 | 3.7 | 87.5 | 3.2 | 86.5 | P2 | 7.4 ± 1.3 | 64.7 ± 22.0 | 6.6 ± 1.3 | 89.2 ± 1.4 | p5–7 | 3.5 ± 0.2 | 63.5 ± 13.6 | 3.4 ± 0.4 | 97.7 ± 14.6 | ||||||||
| 0827 | 2.1 | 74.4 | 2.0 | 95.2 | P1 | 2.4 ± 2.5 | 46.0 ± 8.4 | 1.2 ± 1.2 | 50.2 ± 3.1 | p2–6 | 2.3 ± 0.4 | 38.3 ± 8.5 | 1.5 ± 0.1 | 65.8 ± 6.0 | ||||||||
| 1031 | 1.7 | 1.0 | ND | ND | P0 | 9.4 ± 8.4 | 52.4 ± 20.7 | 7.9 ± 8.4 | 74.2 ± 23.2 | p8–10 | 2.7 ± 1.0 | 31.2 ± 9.3 | 1.2 ± 1.1 | 40.2 ± 24.8 | ||||||||
| 0822 | 63.5 | 85.8 | 60.4 | 95.1 | P4 | 57.3 ± 15.5 | 87.6 ± 7.3 | 54.8 ± 14.0 | 95.8 ± 1.5 | p11–12 | ND | ND | ND | ND | ||||||||
| 0117 | 22.3 | 7.7 | 2.5 | 11.2 | P4 | 5.7 ± 0.7 | 45.0 ± 16.7 | 2.3 ± 0.5 | 41.0 ± 3.5 | p14–15 | ND | ND | ND | ND | ||||||||
| 0211 | 28.0 | 0 | 0 | 0 | p7 | 34.6 ± 10.8 | 0 | 0 | 0 | p7–15 | 33.8 ± 13.3 | 0 | 0 | 0 | ||||||||
| 1228 | 0 | 6.8 | 0 | 0 | p3 | 0 | 41.9 ± 9.0 | 0 | 0 | p7–19 | 0 | 8.8 ± 4.3 | 0 | 0 | ||||||||
| 0131 | 0 | 3.4 | 0 | 0 | p3 | 0 | 2.5 ± 1.9 | 0 | 0 | p8–14 | 0 | 3.1 ± 1.7 | 0 | 0 | ||||||||
| 0707 | 0 | 0.7 | 0 | 0 | p5 | 0 | 3.5 ± 1.3 | 0 | 0 | p6–10 | 0 | 2.8 | 0 | 0 | ||||||||
| 0905 | 0 | 56.6 | 0 | 0 | p1 | 0 | 86.6 ± 16.6 | 0 | 0 | p2–8 | 0 | 56.9 ± 27.3 | 0 | 0 | ||||||||
| 0909 | 0 | 3.0 | 0 | 0 | p1 | 0 | 94.2 | 0 | 0 | p2 | ND | ND | ND | ND | ||||||||
| (2) Freshly Isolated GBM Cells |
1106 | 10.8 | 18.1 | 7.9 | 73.1 | p0 | ||||||||||||||||
| K4 | 5.2 | 5.6 | 3.9 | 75.0 | p0 | |||||||||||||||||
| 447 | 10.4 | 70.5 | 6.7 | 64.2 | p0 | |||||||||||||||||
| 492 | 12.4 | 10.8 | 2.4 | 18.9 | p0 | |||||||||||||||||
| 453 | 8.5 | 18.7 | 5.3 | 62.3 | p0 | |||||||||||||||||
| 460 | 6.6 | 70.3 | 4.9 | 74.5 | p0 | |||||||||||||||||
| 1218 | 0 | 13.4 | 0 | 0 | p0 | |||||||||||||||||
| 449 | 0 | 5.9 | 0 | 0 | p0 | |||||||||||||||||
| 461 | 0 | 32.2 | 0 | 0 | p0 | |||||||||||||||||
| 0605 | 0 | 2.4 | 0 | 0 | p0 | |||||||||||||||||
| 0420RP | 0 | 63.3 | 0 | 0 | p0 | |||||||||||||||||
| 475 | 0 | 7.7 | 0 | 0 | p0 | |||||||||||||||||
GBM tumor lines were presented as two groups: (1) the established TSC/TIC lines that have been expanded in vitro and (2) acutely dissociated tumor cells isolated from primary patient GBMs that have not been expanded in culture (labeled as passage 0 [p0]). Due to the limited number of cells, the expression of CD133 and/or SSEA-1 was determined only in later passages in some of the established GBM lines. We reported the expression values at the earliest passage technically possible in these cases. Expression of CD133 and/or SSEA-1 was determined at various passages during the in vitro expansion phase as well as the xenograft tumors. ‘‘Total CD133+’’ or ‘‘Total SSEA-1+’’ indicates the percentage of the positive cells in the whole population. ‘‘CD133+/SSEA-1+’’ indicates the percentage of double-positive cells in the whole population. ‘‘SSEA-1+ in CD133+’’ indicates the percentage of double-positive cells out of the CD133+ cells. ND, not determined.
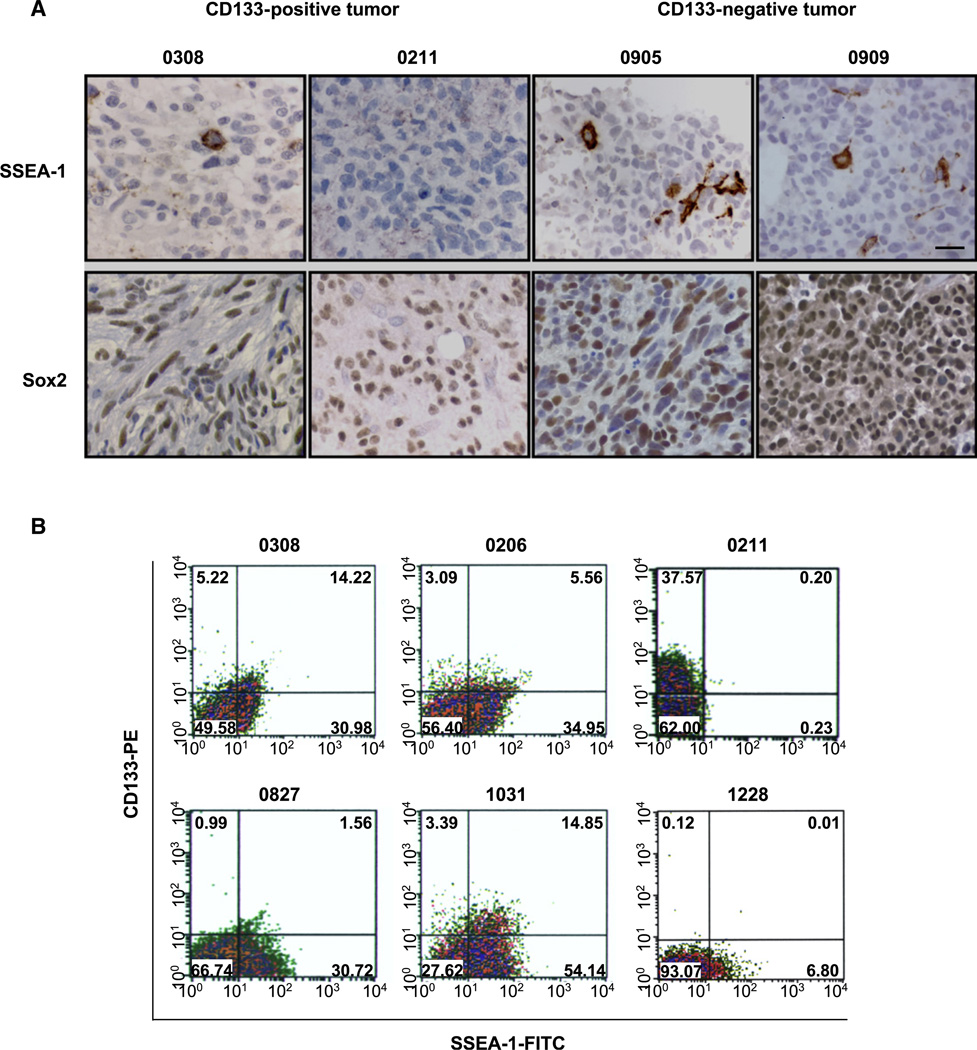
Figure 1. Expression of SSEA-1 in Primary Human GBM Tissues and Their Derivative TSCs/TICs.
(A) Immunohistochemical detection of SSEA-1 and Sox2 in paraffin section from four different patient GBM tissues. Immunopositive cells were visualized by brown DAB staining. The number above represents the designated name for the parental patient GBM tumor. Scale bar represents 20 micron.
(B) Flow cytometry analysis of CD133 (PE labeled, y axis) and SSEA-1 (FITC labeled, x axis) in various TSCs/TICs. Numbers in each quadrate indicate percentage of cells.
In order to identify a potential general TSC/TIC enrichment marker(s) applicable to most GBMs, we first screened several surface antigens expressed on normal NSCs. One such candidate molecule was SSEA-1/LeX. We identified distinct SSEA-1+ cell populations in all but one established TSC/TIC line (11 of 12 cell lines in total, the 0211 line being the only exception). In addition, we determined the expression level of SSEA-1 in acutely isolated GBM tumor cells that were not passaged in vitro. Acutely isolated tumor cells from all tested GBMs contain distinct SSEA-1+ populations (26.6% ± 26.3%), suggesting universal expression of SSEA-1 in GBMs (Table 1). In order to determine whether these SSEA-1+ cells exist in human GBMs in situ, we performed immunohistochemical analysis of patient-derived GBM paraffin sections (Figure 1). Consistent with the in vitro data, a subpopulation of cells with distinct cell membrane-associated staining was detected in GBM patient paraffin sections (Figure 1A). The one GBM in which we did not find SSEA-1+ staining was the tumor from which the only SSEA-1− TSC/TIC line was derived (0211 cells), adding credibility to both the fidelity of antibody staining and the consistency of SSEA-1 status in early passage cells and the primary tumor (Figure 1A). By contrast, we could not obtain reliable immunohistochemical staining of CD133 despite multiple trials using different antibodies and modification of staining conditions (data not shown).
Next, we performed CD133/SSEA-1 dual FACS staining of the TSC/TIC lines in order to determine whether there is significant overlap between these subpopulations (Figure 1B and Table 1). In six out of seven established TSC/TIC lines containing CD133+ cells, a significant proportion of CD133 cells (67.8% ± 22.1%) was also positive for SSEA-1. The percentages of SSEA-1+ and CD133+ cells were widely variable between different GBMs; however, more than 95% of GBMs contained SSEA-1+ cells, regardless of the presence of CD133+ cells (Table 1).
In Vitro Characterization of SSEA-1+ Cells from Various GBM-Derived TSCs/TICs
It was reported that CD133 expression in the brains, determined by immunohistochemical staining of AC133 antibodies, is restricted to the early fetal stages but absent in adult brains including in the subventricular zone (SVZ), where NSCs reside (Pfenninger et al., 2007). Two recent papers, however, have reported broad expression of CD133 mRNA in the brains of embryonic and adult CD133 promoter knockin mice (Shmelkov et al., 2008; Zhu et al., 2009). Whether these data are applicable to the human and whether mRNA expression correlates with immunopositivity of CD133 antibodies that recognize glycosylation-dependent epitopes remain unanswered questions (Coskun et al., 2008; Mirzadeh et al., 2008; Shmelkov et al., 2008; Uchida et al., 2000; Zhu et al., 2009). By contrast, SSEA-1 expression is persistent throughout most stages of neural development and in the adult SVZ (Capela and Temple, 2002, 2006). Thus, given the frequency of SSEA-1 expression in GBMs, we chose to further characterize the SSEA-1+ subpopulation from various GBM-derived TSCs/TICs.
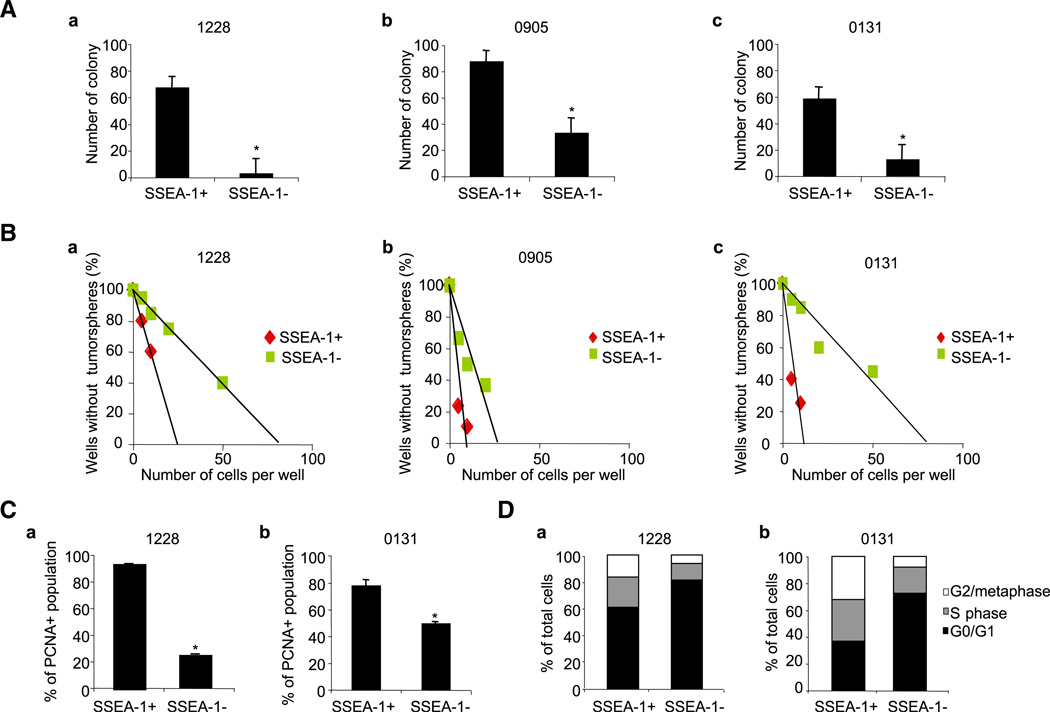
First, we determined the clonogenicity of the SSEA-1+ versus the SSEA-1− subpopulation from various GBM-derived TSCs/TICs in vitro. SSEA-1-dependent cell sorting was performed using FACS, followed by confirmation of the purity of the separated cell populations (SSEA-1+, 79.3%–96.7%; and SSEA-1−, 98.0%–99.7%) and their viability. SSEA-1+ cells derived from three different GBM lines were consistently more clonogenic than their respective SSEA-1− cells in a standard soft-agar colony-forming assay (Figure 2A) and limiting dilution assay (Figure 2B). Additionally, PCNA staining and cell-cycle analysis revealed that SSEA-1+ cells have significantly more PCNA staining (Figure 2C) and a much larger S/G2/M phase population (Figure 2D) than do SSEA-1− cells. Taken together, these data indicate that SSEA-1+ cells are enriched for cells actively traversing the cell cycle with clonogenic potential.
Figure 2. Characterization of SSEA-1+ and SSEA-1− Cells In Vitro from Various GBM-Derived TSCs/TICs.
(A) Soft agar colony-forming assay to determine clonogenicity of SSEA-1+ versus SSEA-1− cells from three different GBM lines.
(B) Limiting dilution sphere-forming assay. Three cell lines used in (A) were plated into 96-well plates with various seeding densities (5–50 cells per well, 30 wells per each condition). Data from a representative experiment were shown. R2 > 0.83.
(C and D) PCNA staining (C) and cell-cycle analysis (D) of 1228 and 0131 cells by flow cytometry. Error bars represent SD (performed in triplicates); *p < 0.05.
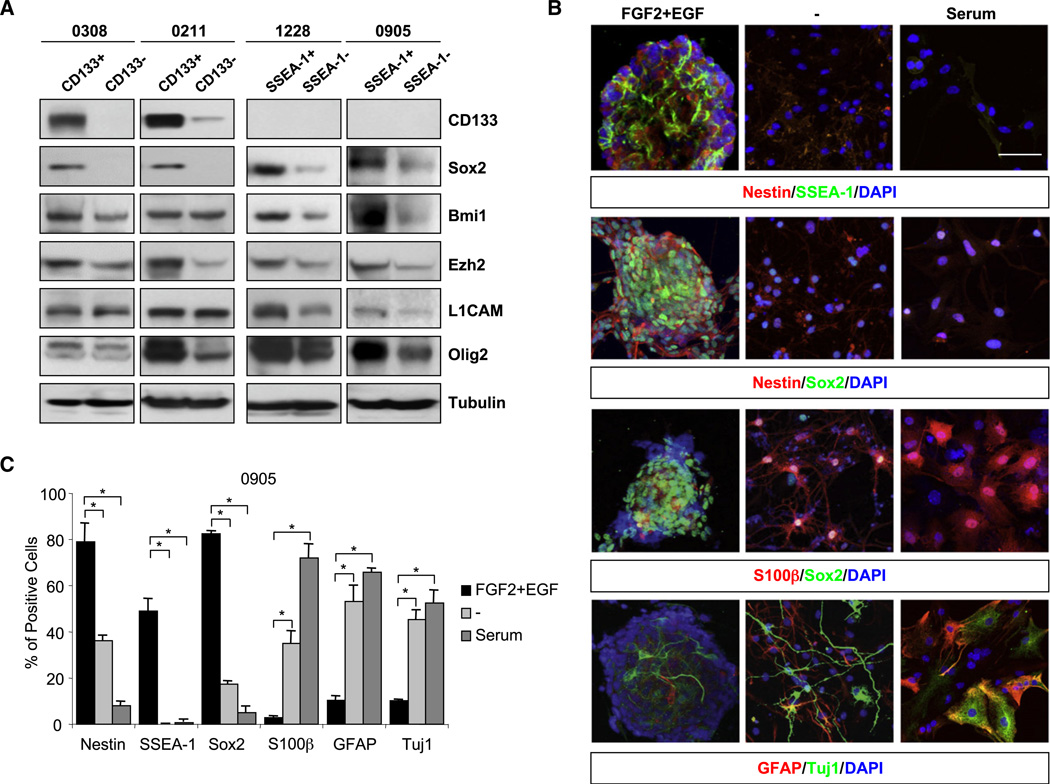
Second, we determined whether the SSEA-1+ subpopulation is enriched for cells expressing stem cell-associated markers by Western blot analysis (Figure 3). We fractionated GBM cells maintained in NBE condition by the expression status of CD133 or SSEA-1 (Figure 3A). CD133- or SSEA-1-dependent cell sorting was performed by using immunomagnetic selection, followed by FACS confirmation of the purity (higher than 90%) and the viability of sorted populations. Consistent with the previous reports (Bao et al., 2008; Ligon et al., 2007; Liu et al., 2006; Singh et al., 2004), CD133+ populations revealed higher expression of various stem cell-associated markers. Similar to CD133+ populations, SSEA-1+ subpopulations showed higher levels of stem cell marker expression including SOX2 (Yuan et al., 1995), Bmi1 (Lessard and Sauvageau, 2003), Ezh2 (Lee et al., 2008; Valk-Lingbeek et al., 2004), L1CAM (Bao et al., 2008), and Olig2 (Ligon et al., 2007) compared to respective negative populations (Figure 3A).
Figure 3. SSEA-1+ Cells Express a High Level of Stem Cell-Associated Proteins and Have Differentiation Potentials In Vitro.
(A) Expression of stem cell-associated proteins in CD133+/− or SSEA-1+/− subpopulation from four different GBM lines. Cells cultured in NBE media were separated into two groups either by CD133 or by SSEA-1, and then proteins were extracted for Western blot analysis. α-tubulin was used as a loading control. Note the absence of CD133 protein expression in 1228 and 0905 lines.
(B) Immunocytochemical analysis of 0905 GBM line cultured in differentiation-inducing condition. Each column represents the same culture condition. “FGF2+EGF” indicates the NBE condition used in the proliferation of these cells. “–” and “serum” indicate the growth factor withdrawal and the addition of serum, respectively. Antibodies used in each condition are shown below. DAPI staining (blue) was used to identify nuclei. White bars represent 50 micron.
(C) Quantitation of immunopositive cells in (B). Error bars represent SD; *p < 0.01.
Others and we have previously demonstrated that GBM TSCs/TICs have the potential to differentiate along glial and/or neuronal lineages with concurrent loss of stem cell marker expression (Galli et al., 2004; Lee et al., 2006, 2008; Singh et al., 2004). In order to determine whether SSEA-1+ cells have such differentiation potential, we performed immunocytochemical analysis by using various GBM TSC/TIC lines cultured in the absence of FGF2/EGF or in the presence of serum. Under these differentiation-inducing conditions, SSEA-1-expressing neurosphere cells underwent significant morphological changes, including extension of processes and spread-out cell shapes (Figure 3B). In order to determine the relative expression of stem cell and differentiation markers during differentiation, we processed these cells for immunocytochemical analysis and quantitated immunopositive cells (Figure 3C and Figure S2). The differentiation conditions induced significantly increased expression of the astroglial differentiation markers GFAP/S100β and/or the neuronal marker TuJ1, with concurrent loss of the stem/progenitor cell markers such as Sox2 and Nestin. Similar to other stem/progenitor cell markers, SSEA-1 expression was significantly decreased under differentiation conditions (Figure 3C).
We also examined whether SSEA-1+ cells expressed other proposed neural stem/progenitor cell markers. Integrin alpha6, Integrin beta1 (Hall et al., 2006), and Olig2 have been reported to be highly expressed in normal NSCs and progenitor cells. In GBMs, it has been reported that most CD133+ cells are also Olig2+ (Ligon et al., 2007). Most SSEA-1+ TSC/TIC cells were Integrin beta1+ under NBE conditions with significant downregulation of both Integrin beta1 and SSEA-1 under differentiation condition (absence of FGF2/EGF) (Figure S3). Similar to Integrin beta1, Integrin alpha6 and Olig2-expressing cells were also more enriched in SSEA-1+ cells under NBE conditions. Moreover, most of SSEA-1+ cells were Integrin alpha6+ (84%), beta1+ (94%), and Olig2+ (75%), and their expressions were significantly decreased under differentiation conditions (Figure S3). Taken together, these data suggest that SSEA-1+ cells express stem cell-associated markers and can differentiate toward a more mature phenotype.
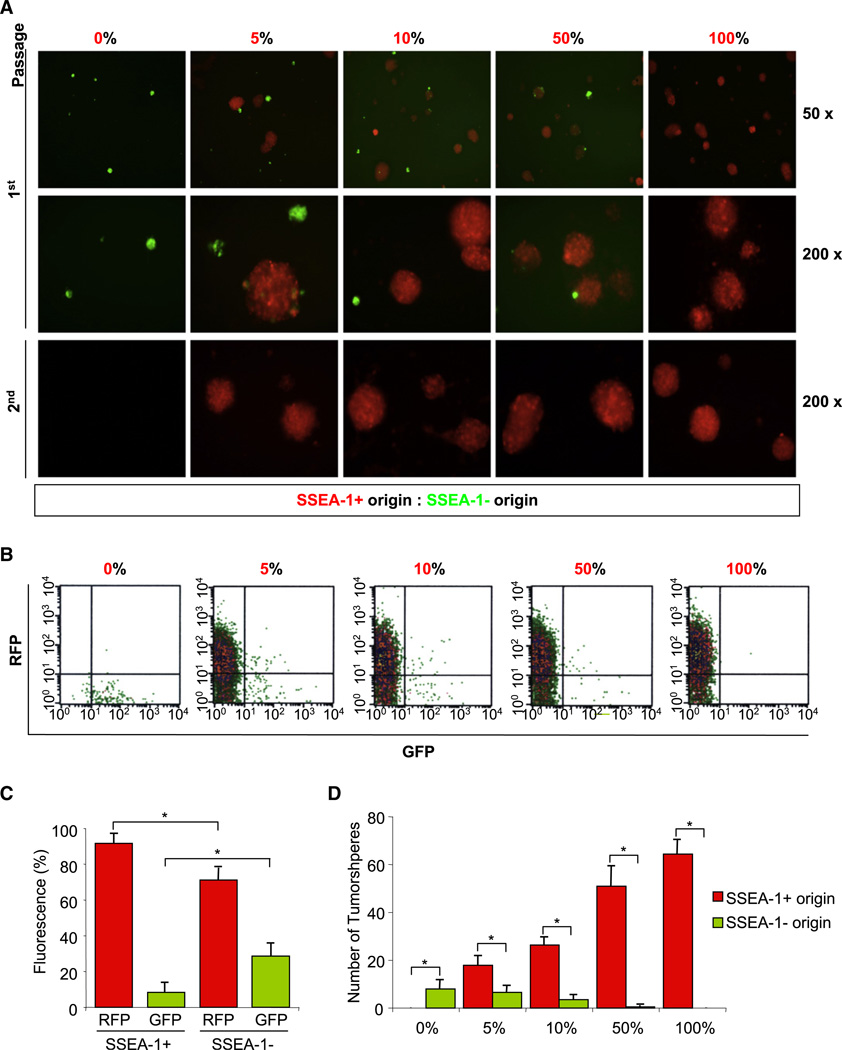
In order to address the cellular hierarchy of SSEA-1+ glioma cells, we performed lineage-tracking experiments (Figure 4). GBM TSCs/TICs were transduced with lentiviral vectors expressing red fluorescence protein (RFP) or green fluorescence protein (GFP). Genetically labeled SSEA-1 + cells (expressing RFP) and SSEA-1− cells (expressing GFP) were mixed in various ratios and cultured at low-cell density allowing for clonal expansion. After 2 weeks of culture, the remaining cells were examined for GFP/RFP expression as well as SSEA-1 expression (Figure 4). Even in the wells in which only 5% of the initial cells were expressing RFP, most of the sphere-forming and SSEA-1+ cells after 2 weeks of cultures were RFP+, indicating that these cells were derived from SSEA-1+ cells (Figure 4C). Additionally, secondary sphere formation assay was performed to further compare relative clonogenicity of SSEA-1+ and SSEA-1− cells. Almost all secondary spheres were originated from SSEA-1+ cells (Figure 4A). Similar results were obtained from nongenetic labeling methods (Figure S4). Taken together, these data strongly indicate that SSEA-1+ GBM cells are highly clonogenic and sustain in vitro growth of the total tumor cell population by generating both SSEA-1+ and SSEA-1− cells.
Figure 4. Lineage Tracking of SSEA-1+ versus SSEA-1− GBM Cells.
(A) Representative microphotograph of the resultant cells that were cultured for 2 weeks after the mixture of 0905-derived SSEA-1+ (red) and SSEA-1− (green) at the indicated ratios. Cells were harvested at a 2 week time point and prepared for secondary sphere-forming assay (the bottom row) or quantitation by flow cytometry (B). These cells were further stained with SSEA-1-PE antibody, and the representative data from the mixture of 5% SSEA-1+ and 95% SSEA-1− cells are shown (C). Spheres generated after the initial 2 week cultures were counted in each condition (D). Error bars represent SD; *p < 0.05.
Tumorigenic Potential of SSEA-1+ Cells in SCID Orthotopic Transplantation Model
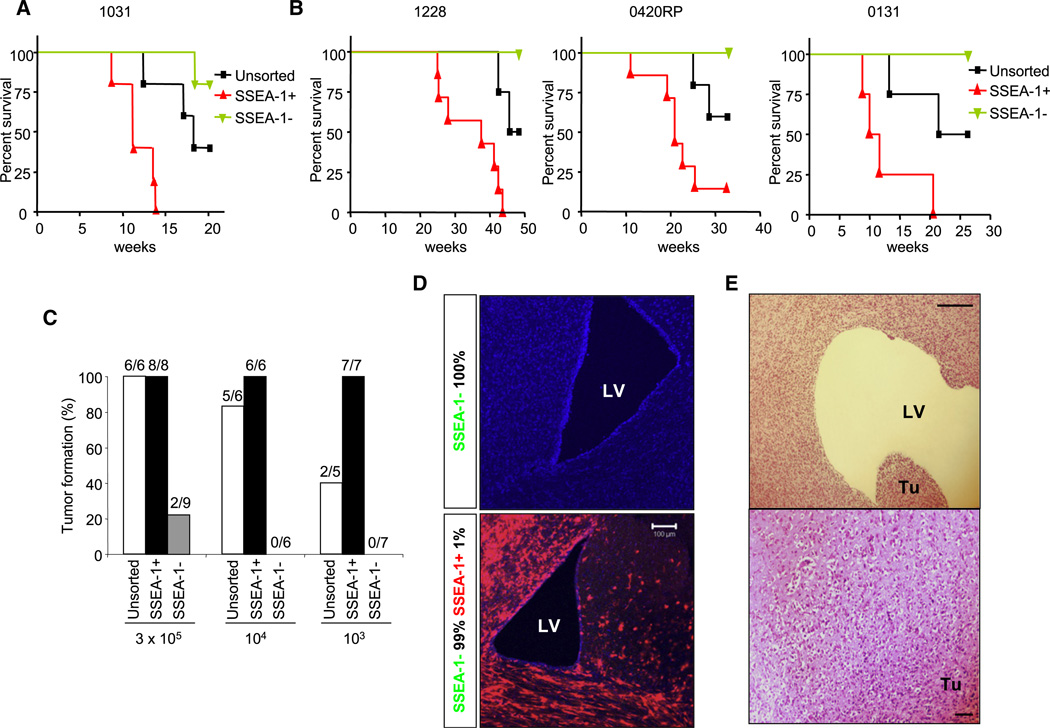
In order to determine whether the subpopulation of SSEA-1+ cells is enriched for tumorigenic potential in vivo, we injected magnetic bead-sorted SSEA-1+, SSEA-1−, and unsorted cells into the brains of neonatal NOD-SCID mice (Figure 5). Cells from four different GBMs were evaluated for tumorigenic potential of SSEA-1+ subpopulations. Acutely isolated (i.e., without in vitro expansion) SSEA-1+ cells from fresh GBM tissues generated tumors significantly more efficiently than SSEA-1− cells (Figure 5A). We also evaluated the tumorigenic potential of SSEA-1+ cells derived from in vitro-established TSC/TIC lines. Only unsorted and SSEA-1+ cells consistently formed tumors in mice, whereas SSEA-1− cells failed to generate tumors. Importantly, the onset of tumors was significantly faster, and the overall survival of tumor-bearing animals was significantly shorter in SSEA-1+ cell-injected mice than in mice injected with unsorted cells (Figure 5B). Next, in order to estimate the relative enrichment of tumorigenic potential in SSEA-1+ cells compared to SSEA-1− cells, we performed additional in vivo tumorigenicity titration assays by varying the number of injected 1228 cells (Figure 5C). As few as 1000 SSEA-1+ cells were enough to initiate tumors, whereas 300,000 cells of SSEA-1− cells were required to make occasional tumors (two out of nine mice after 12 months of injection). The cell numbers required for tumor generations in SSEA-1− cells were more than two orders of magnitude greater, suggesting that SSEA-1+ cells are more than 100-fold enriched for tumorigenic potential. In order to provide definite evidence of enriched tumorigenic potential of SSEA-1+ cells, we performed in vivo lineage-tracing experiments by using genetically labeled SSEA-1+ cells (RFP transduced) and SSEA-1− cells (GFP transduced) (Figure 5D). Even in the situation in which only 1% of RFP-expressing cells was mixed with 99% GFP-labeled SSEA-1− cells and then injected into the brains of adult SCID mice, the resultant tumors were composed almost entirely of RFP-expressing cells.
Figure 5. In Vivo Tumorigenic Potential of SSEA-1+ Cells in SCID Mouse Orthotopic Xenograft Models.
(A) Kaplan-Maier survival graphs of animals injected with total (unsorted), SSEA-1+, or SSEA-1− population of acutely isolated GBM cells (1031) at passage zero.
(B) Kaplan-Maier survival graphs of 1228, 0420 RP, and 0131 GBM cells (from left to right). P values were determined by log rank test: p < 0.01.
(C) In vivo tumorigenicity titration of SSEA-1+ versus SSEA-1− 1228 GBM cells. Tumorigenic potential of subpopulation was evaluated by the tumor formation rate.
(D) Lineage tracking of SSEA-1+ versus SSEA-1− GBM cells in vivo. GFP-transduced SSEA-1− cells and RFP-transduced SSEA-1+ GBM cells were mixed at 100: 0 (upper) and 99: 1 ratio (lower) and injected into the brains of adult SCID mice. Immunofluorescence microphotographs were taken to detect GFP and RFP. DAPI (blue) was used to visualize nuclei.
(E) Representative microphotograph of secondary tumor xenograft induced by serial transplantation of SSEA-1+ 1228 GBM cells. Scale bars represents 100 micron.
Furthermore, the SSEA-1+ cells from these tumors sustained their tumorigenic potential in serial transplantation experiments in which SSEA-1+ cells from xenograft tumors were isolated by sorting and then transplanted back into the brains of secondary mice (Figure 5E). Taken together, these data demonstrate that SSEA-1+ cells are highly enriched in tumorigenic potential and are capable of forming secondary tumors following in vivo passage.
In order to determine the fate of these injected SSEA-1+ and SSEA-1− tumor cells well before the formation of apparent tumor masses, we performed immunohistochemical analysis using a human-specific antibody. We detected distinct human tumor cells infiltrating widely into brain parenchyma and adjacent to the ventricular walls of mice injected with SSEA-1+ cells. By contrast, there were significantly fewer human cells in brains injected with SSEA-1− cells (Figure S5).
Characterization of SSEA-1+ Cells in SCID Xenograft Tumors In Situ
If the SSEA-1+ population of cells has TSC/TIC properties in vivo, one would expect that SSEA-1+ cells would persist and retain stem cell-like properties in tumors generated by both unsorted and SSEA-1+ GBM-derived cells. We therefore determined the SSEA-1 expression of cells acutely dissociated from these xenograft tumors. Similar to the parental tumor, tumor xenografts derived from SSEA-1+ cells displayed a mixture of both SSEA-1+ cells and SSEA-1− cells, demonstrating the ability of SSEA-1+ cells to generate both SSEA-1+ and SSEA-1- cells in vivo, as we had demonstrated in vitro (Figure 6A and Table 1). Since it is technically possible that we could have missed a rare subpopulation of CD133+ cells in our initial tumor-inducing cell population, we also examined these xenograft tumors for the expression of CD133+ cells. Importantly, we could not find any CD133+ cells in any of our xenograft tumor cells (Figure 6A and Figure S6). Thus, these data further support the notion that SSEA-1+ cells function as TICs in these CD133− GBM tumors.
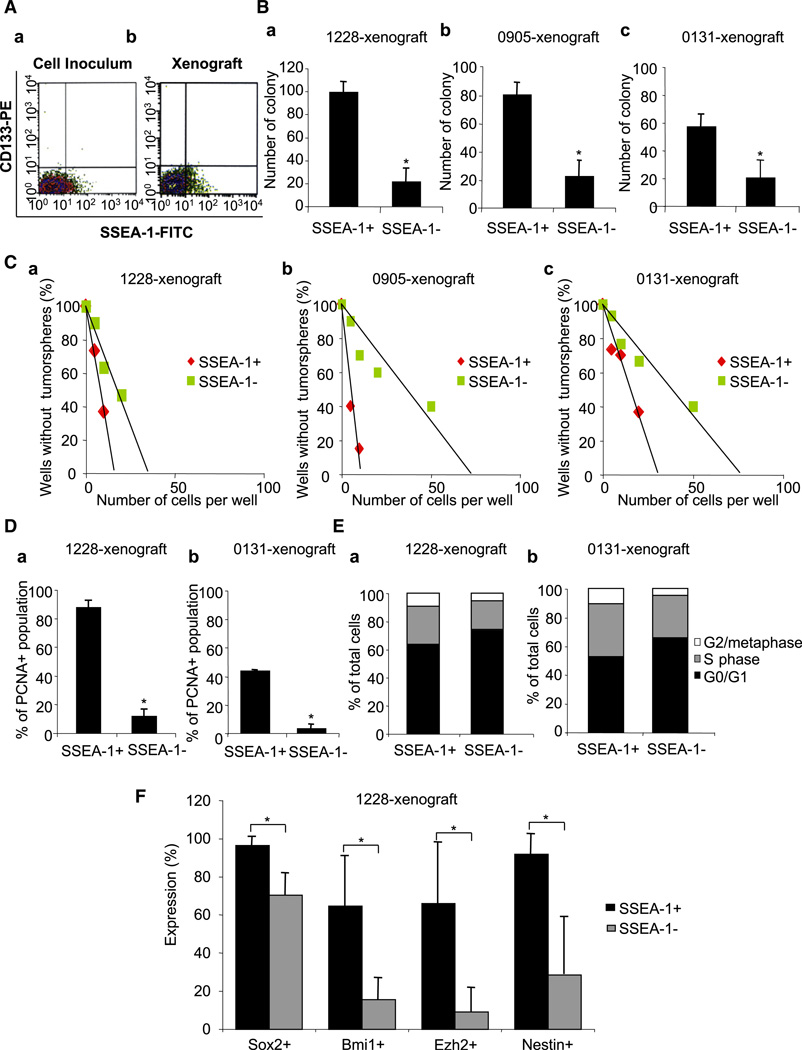
Figure 6. Characterization of SSEA-1+ Cells in SCID Xenograft Tumors In Situ.
(A) FACS analysis to determine the percentage of cells expressing CD133 and/or SSEA-1. Cells prepared for the injection into mice (Aa) and isolated from the resultant xenograft tumors (Ab) were used for the analysis. As a representative example, data from 1228 line were shown. Soft agar colony-forming assay (B) and limiting dilution sphere forming assay (C) were performed in order to determine clonogenicity of SSEA-1+ versus SSEA-1− cells that were acutely isolated from xenograft tumors. These three different tumors were derived from injection of three different GBM lines (1228, 0905, and 0131), respectively. R2 > 0.94. PCNA staining (D) and cell-cycle analysis (E) of xenograft tumors derived from 1228 and 0131 cells were performed by flow cytometry. (F) Expression of stem cell-related genes in SSEA-1+ versus SSEA-1− population of xenograft tumor cells, determined by FACS staining. The tumors were generated by the injection of 1228 SSEA-1+ cells. Error bars represent SD (performed in triplicates); *p < 0.05.
In order to further characterize the tumor cells within the xenograft tumors, we performed clonogenicity assay, PCNA staining, and cell-cycle analysis by using freshly dissociated cells from xenograft tumors (Figures 6B–6E). All SSEA-1+ cells isolated from three different TSC/TIC line-derived xenograft tumors were highly clonogenic compared to their respective SSEA-1− counterparts (Figures 6B and 6C), similar to our in vitro data (Figures 2A and 2B). SSEA-1+ cells acutely isolated from xenograft tumors had significantly greater PCNA staining (Figure 6D) and a larger S/G2/M phase population (Figure 6E) than did SSEA-1− cells, indicating the higher proliferation potential of SSEA-1+ cells in vivo. Furthermore, SSEA-1+ cells maintained high-level expression of the stem cell markers compared to the SSEA-1− cells, as determined by comparative flow cytometry (Figure 6F). In sum, these data implicate that in vivo SSEA-1+ cells are highly clonogenic and proliferative and express markers consistent with a TSC/TIC phenotype.
DISCUSSION
Early reports suggested that CD133 expression was synonymous with, or at least a prerequisite for, glioma-derived TSCs/TICs (Bao et al., 2006; Dirks, 2006; Piccirillo et al., 2006; Singh et al., 2004; Trumpp and Wiestler, 2008). More recent reports, however, have suggested that CD133 may not be an absolute indicator of such cells (Beier et al., 2007; Bidlingmaier et al., 2008; Griguer et al., 2008; Joo et al., 2008; Wang et al., 2008). These apparent discrepancies in the literature could be explained in part by poor reliability of antibodies detecting CD133+ populations (Bidlingmaier et al., 2008; Potgens et al., 2002) and/or the recent observation that expression of CD133 itself may be reversibly modulated by environmental factors such as hypoxia and cellular stress (Chen et al., 2007; Griguer et al., 2008). Another possibility, however, is that CD133 is not a universal marker for TSCs/TICs in every GBM cell.
Although CD133+ GBM cells meet the functional criteria of TSCs/TICs, we have found that approximately 40% of extensively analyzed glioblastomas did not contain CD133+ cells either upon immediate tumor dissociation or during in vitro cell expansion under stem cell culture conditions. Nevertheless, these CD133− GBMs still harbor a subpopulation of TSCs/TICs cells as defined by their ability to generate neurospheres in culture; capability of bilineage differentiation; clonogenicity in soft agar; and ability to form highly invasive, malignant, and serially transplantable tumors in immunodeficient animals.
In order to enrich for the presumptive subpopulation of TSCs/TICs in the CD133− tumors, we reasoned that these cells might express markers found on normal neural stem/progenitor cells. Thus, along with other antigens, we evaluated cells for the expression of SSEA-1. The antigen SSEA-1, also known as CD15 or LeX, is a fucose-containing trisaccharide highly expressed on embryonic stem cells in the developing brain and adult SVZ (Capela and Temple, 2002, 2006). SSEA1+ cells exist throughout the major developmental stages of the central nervous system, and their relative abundance correlates well with the prevalence of neural stem/progenitor cells (Capela and Temple, 2002, 2006). Consistent with the persistent expression of SSEA-1 throughout developmental phases of the CNS, we found SSEA-1 expression in TSC/TIC lines from 23 of 24 evaluated tumors (96%), whereas only 13 of 24 GBM tumors (54%) harbored CD133+ cells.
Not only was SSEA-1 expression seen in the majority of TSC/TIC lines, but the selection of SSEA-1-expressing cells enriched for a population of cells that specifically harbored the properties that meet all criteria for TSCs/TICs. Thus, SSEA-1+ cells formed neurospheres, were clonogenic in soft agar, could generate GFAP+ and Tuj1+ cells following induction of differentiation, and were highly tumorigenic in vivo. Furthermore, SSEA-1+ cells could generate both SSEA-1+ and SSEA-1− cells in vitro and in vivo, suggesting a hierarchical lineage and the ability to generate tumors heterogeneous for SSEA-1 expression much like the primary GBM tumors in situ. Finally, SSEA-1+ cells sorted from these SSEA-1+ cell-generated orthotopic tumors could successively generate secondary tumors with the exact same characteristics as the primary tumor, thereby further confirming their identity as TSCs/TICs.
As is the case for CD133, SSEA-1 does not enrich for a population of glioblastoma TSCs/TICs in every tumor, since one of our TSC/TIC lines contains CD133+ cells but not SSEA-1+ cells. Nevertheless, SSEA-1 should prove to be a useful enrichment marker for TSCs/TICs from most glioblastomas, especially those that are CD133−. Since the significant overlap exists between CD133+ cells and SSEA-1+ cells, SSEA-1 can be useful as a TSC/TIC enrichment marker in CD133+ cell-containing GBMs as well. Our preliminary data showed that in some of these tumors, both SSEA-1-/CD133+ cells and SSEA-1+/CD133− cells are much more tumorigenic as compared to SSEA-1−/CD133− cells. Precise characterization of these subpopulations merits further investigation.
Through a series of eloquent experiments, Read and coworkers recently demonstrated the existence of CD15+ tumor-propagating cells in an animal model of medulloblastoma with the identification of CD15+ cells within a subset of human medulloblastoma specimens (Read et al., 2009). Our results, along with Read et al., suggest that CD15 (SSEA-1) can be a general TSC/TIC enrichment marker in human brain tumors. Additionally, our findings are solely based on primary human glioblastoma specimens, rather than being based largely on a brain tumor animal model, adding to the potential clinical relevance.
Similar to hematopoietic stem cells, probably the most thoroughly characterized stem cell population, a combination of markers will ultimately best define glioma TSCs/TICs (Dick, 2008; Kim et al., 2006; Weissman et al., 2001). Given the complex genetic and epigenetic heterogeneity of GBMs, it is unlikely that the expression of a single marker such as CD133 or SSEA-1 will define TICs in all tumors. Furthermore, if tumors originate from stem/progenitor cells at different points along the normal developmental pathway, TSCs/TICs from different subsets of gliomas may have different immunophenotypes and characteristic stem cell markers. Whether SSEA-1+ tumors represent a specific subtype of glioblastomas compared to CD133+ tumors is impossible to determine conclusively at this point due to the relatively small numbers of the established cell lines evaluated to date. Additionally, whether CD133 and/or SSEA-1 have functional properties in TSCs/TICs is an important question worthy of further study.
In conclusion, SSEA-1 appears to be an enrichment marker for glioblastoma-derived TSCs/TICs from CD133− tumors. Whether SSEA-1+ glioblastomas represent a distinct subset of glioblastomas compared to CD133+ tumors and whether there are additional markers that will further refine the identification of the specific TSC/TIC remain to be seen. Finally, it will be interesting to see whether SSEA-1 might represent a TSC/TIC marker for malignancies other than brain tumors.
EXPERIMENTAL PROCEDURES
TIC Cultures
Following informed consent, tumor samples classified as glioblastoma, based on the World Health Organization (WHO) criteria, were obtained from patients undergoing surgical treatment at the National Institutes of Health (NIH) in accordance with the appropriate Institutional Review Boards (Kleihues et al., 2002). Within 1–3 hr after surgical removal, tumors were washed and enzymatically dissociated into single cells. Red blood cells were removed by differential centrifugation. Tumor cells were cultured in NBE media consisting of neurobasal media (Invitrogen), N2 and B27 supplements (0.5 × each; Invitrogen), and human recombinant bFGF and EGF (25 ng/ml each; R&D Systems). For induction of differentiation, cells were cultured in NBE media without growth factors or DMEM media (Invitrogen) with 10% fetal bovine serum (Invitrogen). Uncoated plastic dishes were used for neurosphere formation assays of NBE cells. For adherent culture of NBE cells, the plates were precoated with polyornithine/laminin mixture (Invitrogen).
FACS Analysis
Patient GBM-and xenograft-derived tumor cells as well as in vitro-cultured cells were dissociated into single-cell suspensions and labeled with the following antibodies: anti-CD133-PE (Miltenyi Biotec), SSEA-1-FITC (MMA clone, BD), Sox2 (R&D Systems, MAB2018), Bmi1 (Upstate), Ezh2 (PharMingen), GFAP (DAKO), Tuj1 (Covance), Integrin alpha6 (Chemicon), Integrin beta1-PE (R&D Systems), and Olig2 (Santa Cruz Biotechnology, sc-48817) antibody. For the SSEA-1 staining, one million cells were labeled with 2 µg of antibodies at 4°C for 10 min. Concentrations of other antibodies and the staining conditions were followed per the manufacturers’ recommendations. For nonconjugated primary antibodies, we subsequently incubated with PE- or FITC-conjugated secondary antibodies (BD). Antibodies against mouse immunoglobulin conjugated to PE or FITC were used as antibody isotype controls (BD). The stained cells were analyzed on the FACS Vantage SE flow cytometer (BD).
Cell Sorting
For flow cytometric cell sorting, cells were dissociated into single-cell suspensions and labeled with anti-SSEA-1-FITC antibody (BD) and/or anti-CD133-PE antibody (Miltenyi Biotec). These labeled cells were then physically sorted using the FACS Vantage SE flow cytometer (BD). Cell Quest Acquisition and Analysis software (BD) was used to acquire and quantify the fluorescence signal distributions and intensities from individual cells. For magnetic cell sorting, single-cell suspensions were labeled with anti-SSEA-1-FITC antibody (BD), incubated with anti-FITC microbeads (Miltenyi Biotec), and then separated by using MACS separation system (Miltenyi Biotec). We have followed the manufacturer’s protocol in these steps. In order to ensure high purity of sorted populations, we have used two separation columns consecutively. We confirmed the purity of cells by FACS analysis of tumor cells after cytofluorimetric or magnetic sorting.
Soft Agar Colony-Forming Assay
The standard protocol was used with a minor modification. Briefly, low-melting-point agar (Difco) was melted and mixed with NBE media at a 1:1 ratio to make a supporting bottom layer (1%) in a 2 ml/well (6-well plate). The bottom agar layer was allowed to solidify at room temperature for 20 min. The top layer containing 0.4% agar (2 ml/well) was prepared by mixing stock agar solutions with 100,000 cells in NBE media and then laid on top of the supporting agar layer. Growth factors were added every 2 days. Cell colonies were allowed to form at 37°C for 2–3 weeks. More than three wells were prepared for the each condition, and colonies in each well were counted.
Limiting Dilution Assay
In vitro NBE-cultured cells and xenograft-derived tumor cells were dissociated into single-cell suspensions, sorted for SSEA-1 expression, and then plated into 96-well plates with various seeding densities (5–50 cells per well). Cells were incubated at 37°C for 2–3 weeks. At the time of quantification, each well was examined for the formation of tumor spheres.
PCNA Staining and Cell-Cycle Analysis
In vitro NBE-cultured cells and xenograft-derived tumor cells were prepared as single-cell suspensions and labeled with anti-SSEA-1-FITC antibody (BD) for surface antigen labeling. The cells were fixed with methanol for 30 min at −20°C, permeabilized, and then stained with anti-PCNA antibody (Chemicon) followed by anti-mouse IgG-PE secondary antibody (BD). For cell-cycle analysis, cells were incubated in DAPI (4′C,6-diamidino-2-phenylindole) (Invitrogen) and analyzed by flow cytometry.
Antibodies for Western Blot Analysis and Immunohistochemistry
The following antibodies were used as primary antibodies: CD133 (Abcam); Nestin and Sox2 (R &D Systems); Bmi1 (Upstates); Ezh2 (PharMingen); L1CAM (Neomarker); Olig2 (Santa Cruz Biotechnology; sc-48817); α-tubulin (Sigma); SSEA-1-FITC (BD); GFAP (Dako); Tuj1 (Covance); and S100β, Integrin beta1, and Human ribonucleoprotein (Chemicon, 1281).
Lentiviral Vector Preparation
Lentiviruses were produced in 293FT cells with packaging mix (ViraPower Lentiviral Expression Systems, Invitrogen) according to the manufacturer’s instructions. Lentiviruses were concentrated by ultracentrifugation, and viral titer was determined by serial dilution. pLenti6.2-GW/EmGFP Expression control vector was purchased from Invitrogen. For the construction of RFP-expressing lentiviral vector, MaxFP-Red DNA fragment from pMaxFP-Red-C vector (Amaxa) was inserted into pLenti6.2/V5-DEST Gateway Vector (Invitrogen).
Lineage Tracking
We have used both genetic labeling and nongenetic labeling methods for lineage-tracking experiments. TSCs/TICs derived from various GBMs were genetically labeled using lentiviral vectors expressing either RFP or GFP (multiplicity of infection, 5) and further sorted to ensure uniform labeling. SSEA-1+ (RFP) and SSEA-1− (GFP) cells were sorted by using SSEA-1 FACS, reconstituted at the desired ratios, and then cultured in a low-cell density allowing for clonal expansion. After 2 weeks of culture, the remaining cells were examined for GFP/RFP expression by fluorescent microscope, and the resulting tumor spheres were counted. These tumor spheres were dissociated into single cells for FACS analysis and cultured again for secondary tumor sphere formation assay.
For nongenetic labeling of tumor cells, SSEA-1+ and SSEA-1− cells were sorted using magnetic beads and labeled with green color dye (CellTracker Green CMFDA, Invitrogen). Labeled SSEA-1+ or SSEA-1− cells were mixed with unlabeled SSEA-1− or SSEA-1+ populations, respectively, at a 4:6 (SSEA-1+:SSEA-1−) ratio. At each time point during the 5 day culture period, cells were stained with SSEA-1-PE antibody, and the labeled cells were detected by using flow cytometry.
Intracranial Tumor Cell Injection into SCID Mice
An intracranial orthotopic model was utilized for evaluation of TIC tumorigenicity (Uchida et al., 2000). Unsorted or sorted SSEA-1+ and SSEA-1− cells were resuspended in 2 µl of HBSS and injected stereotactically into the lateral ventricles of cryoanesthetized neonatal SCID mice at postnatal day 1 or into the striatum of adult mice by using stereotactic device (coordinates, 2 mm anterior, 2 mm lateral, 2.5 mm depth from the dura). Following injection, neonatal mice were returned to their mothers and allowed to grow to adulthood. There was no injection procedure-related animal lethality. The animals were killed at given time points for the analysis of tumor histology and immunohistochemistry. Brains were perfused with 4% paraformaldehyde by cardiac perfusion and further fixed at 4°C overnight.
Statistical Analysis
All values are shown as mean ± standard deviation (SD). Kaplan-Maier survival analysis was performed in Prism 4.0 software.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Dr. Dragan Maric (NINDS FACS Facility) for his valuable contributions to this work. We also thank Dr. Carolyn Smith (NINDS Light Imaging Facility) and Dr. Hong Sug Kim for their valuable contributions to this work.
Footnotes
SUPPLEMENTAL DATA
The Supplemental Data include six figures and can be found with this article online at http://www.cell.com/cell-stem-cell/supplemental/S1934-5909(09)00104-0.
REFERENCES
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J. Mol. Med. 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev. Biol. 2006;291:300–313. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, Panchision DM. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Weigmann A, Huttner WB. AC133 hematopoietic stem cell antigen: human homologue of mouse kidney prominin or distinct member of a novel protein family? Blood. 1998;91:2625–2626. [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc. Natl. Acad. Sci. USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- Dirks PB. Cancer: stem cells and brain tumours. Nature. 2006;444:687–688. doi: 10.1038/444687a. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR, Jr, Gillespie GY. CD133 is a marker of bioenergetic stress in human glioma. PLoS ONE. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N. Engl. J. Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, et al. Identification of A2B5+CD133− tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–514. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- Pfenninger CV, Roschupkina T, Hertwig F, Kottwitz D, Englund E, Bengzon J, Jacobsen SE, Nuber UA. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Potgens AJ, Schmitz U, Kaufmann P, Frank HG. Monoclonal antibody CD133-2 (AC141) against hematopoietic stem cell antigen CD133 shows crossreactivity with cytokeratin 18. J. Histochem. Cytochem. 2002;50:1131–1134. doi: 10.1177/002215540205000814. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, Febbo PG, Wechsler-Reya RJ. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–147. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher F, Rossler R, Brouwer N, Balasubramaniyan V, Boddeke E, Copray S. Differentiation of neural stem cells into oligodendrocytes: involvement of the polycomb group protein Ezh2. Stem Cells. 2008;26:2875–2883. doi: 10.1634/stemcells.2008-0121. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J. Clin. Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A, Wiestler OD. Mechanisms of disease: cancer stem cells—targeting the evil twin. Nat. Clin. Pract. Oncol. 2008;5:337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int. J. Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.