ABSTRACT
In Australia, pneumococcal vaccine is provided free to all adults aged ≥65 years and Indigenous people aged 15–65 years, and is subsidized for non-Indigenous adults <65 years of age with risk factors. This study aimed to explore pneumococcal vaccination uptake in older patients attending 550 Australian general practices from 2010–2017 by patient sociodemographics, presence of comorbidities and practice characteristics. Study 1: a cross-sectional analysis of ‘active’ patients aged ≥65 years in each year was performed to calculate annual pneumococcal vaccination uptake. Study 2: a cohort of 58,589 ‘every year’ patients aged 60–65 years in 2010 was analyzed to identify the number of patients immunized during the study period. Logistic regression models assessed associations between vaccination, patient and practice characteristics. Annual pneumococcal vaccine uptake varied by patient’s age (65–74 or ≥75 years), presence of comorbidities and regularity of practice visits (range 36% to 76%), and it declined slowly from 2011–2016 amongst all groups. Cohort analyses showed that 69% of those aged 60–65 years in 2010 had a recorded pneumococcal vaccination by 2017 (peak age of vaccination = 66 years), and vaccination was more likely among those with comorbidities, ex-smokers and frequent attenders to practices. Findings demonstrate that the NPS MedicineInsight database provides estimates of vaccination uptake consistent with past surveys, reproducible every year and at low cost. It has the advantage of additional clinical information compared to the Australian Immunization Register. Whilst vaccination uptake was adequate among ‘every year’ patients, interventions are needed to improve pneumococcal vaccination for all older Australians.
KEYWORDS: Pneumococcal vaccines, general practice, vaccination coverage, aged, primary health care, comorbidity
Introduction
Invasive pneumococcal disease, caused by different serogroups of the bacteria Streptococcus pneumoniae, is an important contributor to morbidity and mortality worldwide. Severe forms of the disease (i.e. pneumonia, meningitis and septicemia) are more prevalent in infants, the elderly, and among those with impaired immune systems.1–3
Vaccination is an essential element of the global effort to prevent pneumococcal disease and understanding gaps in vaccination uptake is essential when trying to improve rates.1,4 In Australia, pneumococcal polysaccharide vaccine (PPV) is provided free to all adults aged 65 years and over, and Indigenous people aged 15–65 years as part of the Australian Immunisation Programs (AIP)5 and is subsidized under the Pharmaceutical Benefits Schedule (PBS) for non-Indigenous adults < 65 years of age with comorbidities.6 For non-Indigenous adults without comorbidities, a single dose of the PPV is recommended at 65 years of age, and for those aged > 65 years who did not receive a dose when turning 65 years, a single catch-up dose of PPV should be offered as soon as possible. For Indigenous adults, a first dose is recommended when reaching the age of 50 years. Routine revaccination with PPV is recommended only for Indigenous adults or those with comorbidities. In these cases up to 3 doses (i.e. 2 revaccinations) of PPV in adulthood are recommended depending on the risk factors.6
Although in late 2016 the Australian Immunization Register (AIR) was extended to include data on all vaccinations performed in adults,7 under-reporting of vaccines given in general practice is believed to be occurring.8 To date, no report on pneumococcal vaccination has been undertaken using AIR data.7 In the past, vaccination uptake in Australia has been estimated using intermittent community surveys, which have limitations such as high cost and self-reported data.9,10 A systematic review of Australian studies from 1992–2013, estimated that pneumococcal vaccination coverage for people ≥65 years ranged from 50.3% to 72.8% after the introduction of free vaccine,9 similar to the 54% found in the national immunization survey.10 However, all available data is derived from cross-sectional studies, most of them in specific groups, and with limited data on sociodemographic features or other patient characteristics which may be associated with vaccination uptake.
Given that the vast majority of all child NIP vaccines5,11 and vaccinations for those aged ≥65 years10,12 are delivered through general practice in Australia,12 using electronic health records (EHR) from a longitudinal general practice database may have some advantages.13 In 2015 the National Prescribing Service (NPS) MedicineWise made available the MedicineInsight platform for research purposes.14 With data from EHR collected since 2010 from approximately 650 general practices and four million patients, it is a rich source of information about the use of medicines,15 clinical investigations,16 chronic diseases17 and vaccinations.18 Using MedicineInsight dataset we sought a contemporary, verifiable, estimate for annual pneumococcal vaccination uptake (Study 1, time series analysis), and explore sociodemographic characteristics, underlying medical conditions, consultation attendance and practice characteristics associated with uptake when, according to their age, patients become eligible for the free vaccination scheme,5 in order to identify the potential for improvement in vaccine delivery (Study 2, retrospective cohort analysis). Additionally, we sought evidence about a strategy that has been promoted of using patients’ attendance for their annual influenza vaccination as an opportunity to administer the pnenumococcal vaccine.
Patients and methods
Data source
For both studies we used data from the NPS MedicineInsight, a general practice database developed and managed by NPS MedicineWise.19 De-identified EHR data is extracted from participating general practices around Australia, varying by size, billing methods, and range and type of services offered. All patients receive a unique identifying number within a practice, so each patient is connected to the same practice throughout the period of data extraction. This generates a large, longitudinal database, with patients of all ages, from all States and geographic regions.
Sample
Study 1 (time series analysis) aimed to investigate annual pneumococcal vaccination uptake from 2010 to 2016 (i.e. the percentage of patients ever vaccinated) among all ‘active’ patients attending the practice that year and aged ≥ 65 years. ‘Active’ patients were defined according to the Royal Australian College of General Practitioners as any person who had at least three consultations at the practice in two consecutive years.20 We also analyzed the proportion of ‘active’ patients aged ≥65 years who received the first pneumococcal vaccination each year.
Study 2 (retrospective cohort analysis) aimed to investigate factors associated with the uptake of pneumococcal vaccination (i.e. first recorded dose) when, according to their age, patients became eligible for the free vaccination scheme.5 The cohort sample included all patients aged between 60 and 65 years in 2010, who had at least one consultation in every year from 2010 to 2017 (identified as ‘every year’ patients in this study). The same cohort sample was used to analyze the administration of a pneumococcal and an influenza vaccine on the same day in 2017 (for those who had not received pneumococcal vaccination until 2016).
Data extraction
A data extraction algorithm was used to identify pneumococcal vaccines in the dataset for both studies (i.e. time series and retrospective cohort analysis), which contained all immunizations recorded between 2010 and 2017. Administration of a vaccine can be recorded by GPs and practice nurses from either a drop down menu or as free text.19 For that reason, pneumococcal vaccination was identified by the vaccine brand names (Pneumovax® or Prevenar 13®) and also by combining the terms ‘pneumo’ and ‘vax’, ‘vacc’ or ‘injection’, as well as synonyms or possible misspelling of these terms. We also searched the dataset for records of pneumococcal vaccine administered before 2010, because previous studies have shown that many practices in the MedicineInsight database have EHR going back to 2000.15,17
Moreover, to assess the proportion of the cohort who had not received the pneumococcal vaccine until 2016 but were vaccinated against pneumococcal and influenza on the same day in 2017 (second part of Study 2), information on influenza vaccination was also obtained using a similar data extraction algorithm. The immunization field was searched for the term ‘flu’ or brand names of this vaccine (i.e. Afluria®, Agrippal®, Fluad®, Fluarix, FluQuadri™, Influvac®, Vaxigrip®).
Outcomes
In Study 1, annual pneumococcal vaccination uptake for the period 2010–2016 was estimated as the proportion of ‘active’ patients attending the practice that year and aged ≥ 65 years who received the vaccine. Two different frequency measures were estimated: 1) ‘ever vaccinated’, defined as the proportion of ‘active’ patients who, according to the EHR, received at least one dose of pneumococcal vaccine between 2000 and the correspondent year, and 2) ‘vaccinated that year’, defined as the proportion of ‘active’ patients who were vaccinated against pneumococcal in that year and had no previous vaccination identified in the EHR.
In Study 2, pneumococcal vaccination rate was defined as the proportion of ‘every year’ patients (at least one annual consultation between 2010 and 2017) who were aged 60–65 years in 2010 who received their first dose of the vaccine (i.e. no previous adult vaccination).We also estimated the proportion of patients who received pneumococcal and influenza vaccines at the same visit in 2017, among those who had not received pneumococcal vaccine until 2016.
Covariates
For patients, covariates included gender, Indigenous status (Aboriginal and/or Torres Strait Islander: yes/no), patient's Index of Relative Socio-economic Advantage and Disadvantage (IRSAD) quintile and comorbidity. IRSAD is a macroeconomic indicator of relative economic and social advantage/disadvantage position within an area/postcode compared to the rest of the country.21 Chronic conditions that can lead to an increased risk of invasive pneumococcal disease, as listed in the Australian Immunisation Handbook,6 were extracted from MedicineInsight (diagnosis or reason for encounter) using algorithms from a previous study.17 These included: cardiac disease, chronic respiratory or neurological conditions, immunocompromising conditions (e.g. those with malignancy or HIV infection), chronic liver disease, diabetes mellitus, chronic renal failure and hemoglobinopathies.6 Individuals with at least one of these conditions were considered as having comorbidity. Covariates for general practices included: state, rurality (classified as major cities; inner regional; outer regional/remote/very remote), and the practice IRSAD quintiles.
The median number of visits per year to the practice was also included as a covariate. Duplicate entries for the same patient on the same date were excluded.
Statistical analysis
For Study 1, annual pneumococcal vaccination uptake estimates from 2010 to 2016 among ‘active’ patients aged ≥65 years were presented graphically, including: 1) the proportion of patients who were ‘ever vaccinated’ between 2000 and the correspondent year and 2) the proportion of patients ‘vaccinated that year’ (i.e. no previous pneumococcal vaccination in the EHR).
In Study 2, the association between pneumococcal vaccination and practice or patient’s characteristics among a cohort of individuals aged 60–65 years in 2010 was assessed using logistic regression with robust standard errors for taking into account clustering within the practice. Practice characteristics were mutually adjusted, while patient variables were adjusted for practice characteristics, gender, and median number of consultations per year. Results are presented as adjusted proportions. All analyses were performed in the statistical software Stata 15.0 (StataCorp, Texas, USA). The Human Research Ethics Committee of the University of Adelaide exempted this study from ethical review because it used existing and non-identifiable data.
Results
Study 1 shows that population sample size of ‘active’ patients aged ≥ 65 years (mean: 68.3, SD: 8.1) for the time series analysis varied by year (2010: 220,801, 2011: 257,737, 2012: 300,581, 2013: 348,845, 2014: 400,056, 2015: 456,184, 2016: 470,034, 2017: 453,215). The pneumococcal vaccination uptake (i.e. ‘ever vaccinated’) in each calendar year from 2010 to 2016 was 63%, 60%, 56%, 54%, 53%, 52% and 52%, respectively (Figure 1). It varied between 36% to 76%, depending on the patient’s age and presence of comorbidities, and in general decreased over time in all groups (p < .001). The lowest rate was observed among those aged 65–74 years without comorbidity, and the highest among those aged ≥75 years with comorbidities. Moreover, the decrease in pneumococcal vaccination uptake in patients without comorbidities was more pronounced (p < .001).
Figure 1.
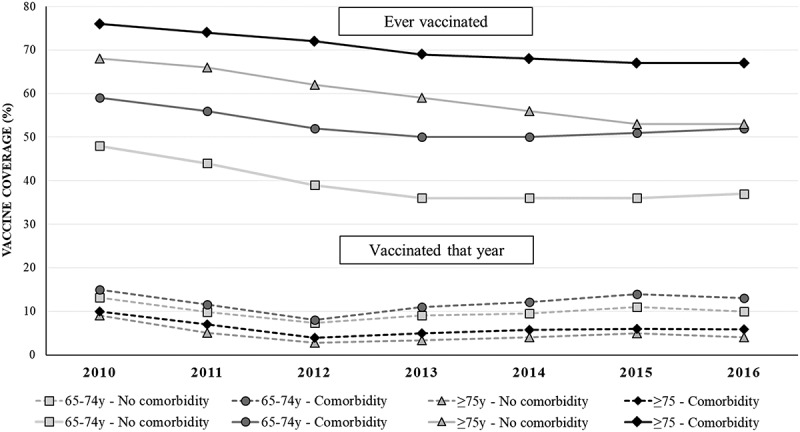
Pneumococcal vaccine uptake in each year among ‘active’ patients aged ≥ 65 years that year. Results from the time series analysis (Study 1). Australia, 2010–2016.
Figure 1 also shows the proportion of elderly individuals ‘vaccinated that year’. In 2016 it varied from 3% to 15%. The highest rate was for those aged 65–74 years with a comorbidity, while those aged ≥ 75 years were less likely to be immunized, independent of having or not comorbidity.
Figure 2 shows the pneumococcal vaccination rate for patients aged 60–65 years in 2010 (Study 2, cohort analysis). Of the 259,236 eligible patients, 58,549 (22.6%) had at least one annual consultation between 2010 and 2017 (‘every year’ patients). This cohort of ‘every year’ patients had similar sex and IRSAD distribution compared to all individuals aged 60–65 years in the database (see Supplementary Table 1). The cumulative incidence of vaccination uptake by the end of 2017 among ‘every year’ patients was 69% (n = 40,160).
Figure 2.
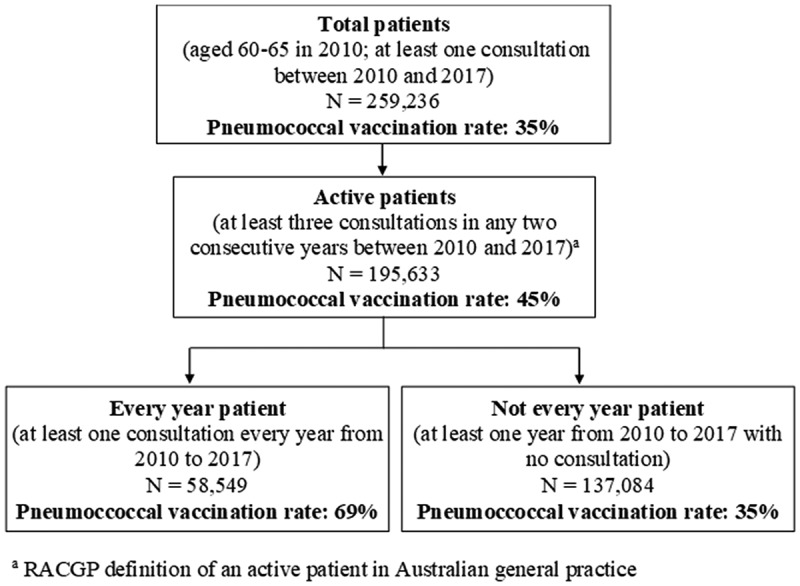
Flowchart of the selection of patients included in the cohort sample (Study 2) with respective pneumococcal vaccination rates. Australia, 2010–2017.
Approximately 80% of those ‘every year’, vaccinated, patients received their first dose after they turned 65 years, with a peak at 66 years (Figure 3). The same figure also shows that before the age of 65 years, those receiving the pneumococcal vaccine were up to five times more likely to have a comorbidity.
Figure 3.
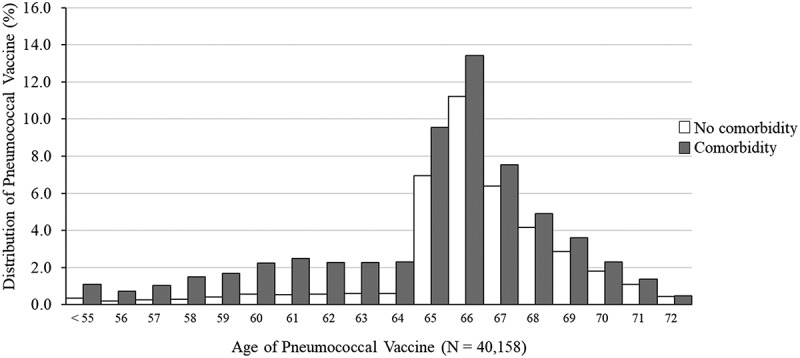
Age of first pneumococcal vaccination among ’every year’ patients aged 60–65 years in 2010 (at least one consultation per year from 2010 to 2017) with and without comorbidities. Results from cohort data analysis (Study 2). Australia, 2010–2017.
Table 1 shows pneumococcal vaccine uptake among ‘every year’ patients was 5% lower among practices located in outer/remote/very remote areas compared to inner regional or major cities and 10% lower in the second highest IRSAD quintile areas (65. and 4%) compared to the lowest quintile (71.7%). Small differences were observed according to gender or patient’s IRSAD, while uptake was highest among those with comorbidities (71.8%) or ex-smokers (71.2%).
Table 1.
Pneumococcal vaccination ratesa among ’every year‘ patients aged 60–65 years in 2010 (at least one consultation per year from 2010 to 2017), according to patient and practice’s characteristics. Results from cohort data analysis (n = 58,549). Australia, 2010–2017.
| Variable | Sample distribution (%) | Proportion of vaccinated (%, 95%CI) | p-value |
|---|---|---|---|
| Practice’s Characteristics | |||
| Rurality | < 0.0001 | ||
| Major cities | 56.2 | 68.5 (68.0;69.1) | |
| Inner regional | 31.1 | 69.7 (69.0;70.4) | |
| Outer regional/Remote/Very remote | 12.8 | 66.2 (64.9;67.4) | |
| IRSAD Quintile | < 0.0001 | ||
| Very high | 11.7 | 69.8 (68.7;71.0) | |
| High | 27.2 | 65.4 (64.6;66.2) | |
| Middle | 19.9 | 68.7 (67.8;69.6) | |
| Low | 21.0 | 68.9 (68.1;69.7) | |
| Very Low | 20.2 | 71.7 (70.8;72.5) | |
| Patient’s Characteristics | |||
| Gender | 0.0051 | ||
| Male | 43.5 | 68.0 (67.4;68.6) | |
| Female | 56.5 | 69.1 (68.6;69.6) | |
| IRSAD quintile | 0.1444 | ||
| Very high | 22.0 | 67.7 (66.5;68.9) | |
| High | 16.2 | 67.7 (66.7;68.8) | |
| Middle | 24.1 | 69.4 (68.5;70.3) | |
| Low | 17.9 | 68.9 (68.0;69.9) | |
| Very Low | 19.8 | 69.1 (68.0;70.2) | |
| Comorbiditiesb | < 0.0001 | ||
| No | 42.6 | 64.4 (63.8;65.0) | |
| Yes | 57.4 | 71.8 (71.3;72.3) | |
| Smoking status | < 0.0001 | ||
| Non smoker | 53.5 | 68.2 (67.7;68.7) | |
| Smoker | 6.9 | 64.9 (63.4;66.3) | |
| Ex smoker | 35.6 | 71.2 (70.6;71.8) | |
| Not recorded | 4.1 | 57.4 (55.4;59.4) | |
aAnalyses of practice’s characteristics are adjusted for each other and median yearly consultations; analyses of patient’s characteristics are adjusted for gender, practice’s characteristics and median yearly consultations.
bComorbidities include: cardiac disease, chronic neurological disease, chronic respiratory disease, chronic liver disease, immunocompromising conditions or other chronic diseases (including chronic renal failure, diabetes mellitus or hemoglobinopathies).
Among ‘every year’ patients who had never received a pneumococcal vaccine up to 2016 (n = 22,120), we examined the pattern of immunization in 2017 (Figure 4). Of the 3,731 pneumococcal vaccinations in that year, 87% (n = 3,244) were given between March and August, coinciding with the peak of influenza vaccinations. Overall, 53% (n = 1,980) of those receiving the pneumococcal vaccine in 2017 were also immunized against influenza on the same day, while 10,205 eligible patients only received the influenza vaccine, and 8,184 did not receive any immunization during that year.
Figure 4.
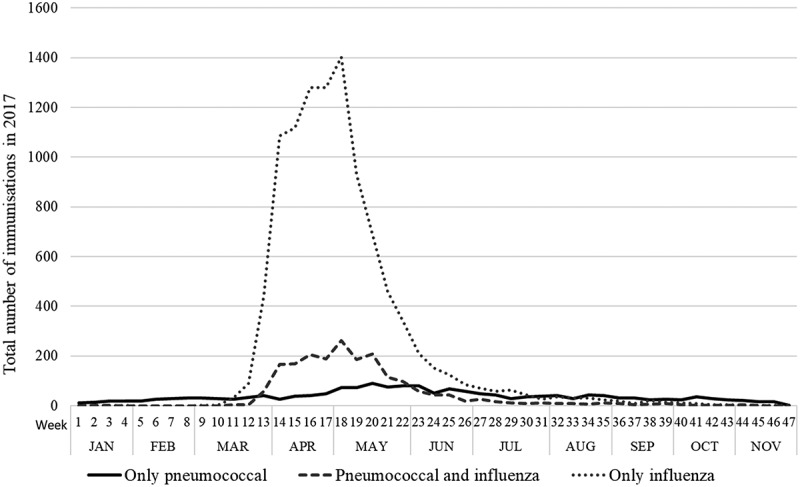
Total number of pneumococcal and influenza immunizations among ‘every year’ patients aged 60–65 years in 2010 (at least one consultation per year from 2010 to 2016), among those consulting in 2017 and without a previous record of a pneumococcal vaccine (N = 22,120). Results from cohort data analysis (Study 2).
Discussion
The proportion of older Australians (aged 65–74 years or ≥ 75 years) with or without comorbidities, immunized against pneumococcus, declined slowly from 2010 (range 76%-48%) to 2017 (range 69%-39%). However, the number of vaccinations given each year remained relatively stable. Vaccination uptake was higher in patients with comorbidities and those aged ≥75 years, and most patients were immunized in the years immediately after they turn 65 years, which coincides with free access to the vaccine under the NIP.5 Whilst pneumococcal vaccination uptake varied slightly according to practice and patient characteristics, only the presence of comorbidities or being an ex-smoker affected rates appreciably. Finally, as expected, there is evidence of co-administration of seasonal influenza and pneumococcal vaccination and this knowledge could inform strategies and guide the allocation of resources to increase vaccination rates. In fact, the concept of ‘make mine a double’, combining influenza and pneumococcal vaccination, may be useful in encouraging higher uptake of the latter.
The finding that pneumococcal vaccination uptake among those aged ≥75 years with comorbidities declined, albeit slowly, is of concern. This decline is consistent with data from the New South Wales Health Survey22 and may be related to publicity about severe injection site reactions in 2011 and the subsequent removal of the recommendation of revaccination after 5 years in people without a medical risk factor. For patients with preexisting conditions, however, up to two additional doses are still indicated, depending on the medical history and age.5
Results of the cohort sample demonstrated that vaccination uptake might be linked to how often people visit different practices to obtain care, since Australians are not registered with one practice. Higher uptake in those we labeled ‘every year’ compared to ‘not every year’ patients suggest that being associated with one general practice and therefore having more opportunities for vaccination might play a role. Supporting this idea, the vaccination uptake among ‘every year’ patients (69%) was similar to that found among patients aged 65 years or over in England (70%), where patients are registered with only one practice.23 A previous Australian study found a pneumococcal vaccination uptake of 73%, slightly higher than ours, but data was obtained from health provider forms (BEACH study).24 In the cohort, patients who were intermittent or infrequent attenders at a practice (‘not every year’ patients) may be receiving care from another practice, including pneumococcal vaccination, which is not recorded at their MedicineInsight practice, leading to lower pneumococcal uptake estimates.
We found that most pneumococcal vaccinations are given at or just after the age of 65 years. This may indicate a high level of awareness about the NIP, probably influenced by repeated prompts that appear on the GPs screen at every consultation once the patient turns 65 years (generated automatically by the installed medical software programs and based on the EHR), informing about the availability of (free) pneumococcal vaccination and its importance. The higher uptake in those with comorbidities also suggests that the message about who is at higher risk is reasonably well known, with many of these patients getting vaccinated before the age of 65 years. Understanding why many are either not offered, delay acceptance or refuse pneumococcal vaccination is more difficult to explain, especially in the context of high influenza vaccination uptake in those aged 65 years or over. Authors of a review on vaccine hesitancy suggest that social, religious, political, cultural and emotional factors play a role and these are influenced by changes in scientific, cultural and medico-legal environments, in addition to vaccine policies, health professional, media and communication.25 A National Immunization Survey undertaken in 2016 found that a third of Australians aged 70 years or over do not think they need to be immunized against influenza, pneumonia, shingles or whooping cough.26,13 Moreover, the BEACH study showed that among 331 unvaccinated patients at risk of pneumococcus infection (42% of those investigated in that study), 46% were considered not at risk by the GP and 39% had a recorded vaccine objection.24
Our results demonstrate the potential of MedicineInsight data to monitor vaccination uptake, especially because such data is potentially available on a weekly basis, results are consistent with previous surveys, and the dataset provides medical history and socioeconomic information from patients and practices. We found that pneumococcal vaccination does not vary appreciably by most practice and patient characteristics, although having comorbidity or being an ex-smoker increased rates. This contrasts with findings that the use of medications15,27 or tests16 in Australia can vary with sociodemographic factors, but it is similar to findings for influenza vaccination uptake in general practice.18 Being available free (after turning 65 years), easily accessible through general practice and able to be offered opportunistically – for instance at the time of influenza vaccination – makes it available to everyone. In European countries, being an immigrant, living alone or in rural areas, and being unmarried have been associated with lower uptake of pneumococcal vaccination.28
This study has some limitations. First, the records extracted and used in the analysis are entered and maintained for clinical purposes. Therefore, the quality and accuracy may vary by clinician or practice. For instance, the information can be recorded as free text instead of using standardized medical codes. However, to increase data quality algorithms used for data extraction included not only medical codes, but also a combination of terms related to pneumococcus vaccination as well as possible misspelling of these terms.29 Second, Australians do not have to register with a single practice. They can seek care from different practices and may have received pneumococcal vaccination or other medical care at another practice, hospital or clinic. It is possible that the information does get recorded in the MedicineInsight practice but only if the GP has asked about and entered that information. This is probably more likely to occur for ‘every year’ patients and this may account for some of the differences found in our results. Third, we cannot rule out double-counting of patients who visit more than one MedicineInsight practice, but estimates suggest the likelihood of this is low given the broad distribution of MedicineInsight practices across geographic areas.19 Fourth, our study cannot account for patients who refuse pneumococcal vaccination, because the EHR used by Australian GPs do not provide any structured way to record this, but a previous study reported a rate of 7%.24 This will influence the feasibility of attaining higher vaccination rates, but should be balanced against reports that provider recommendation is the most influential factor in a patient’s decision to vaccinate.10 Lastly, our population is likely to be biased toward patients who frequently attend a single practice and therefore may not be fully representative of all older Australians.
Despite these limitations, this study has several strengths: the large number of patients, practices and GPs included (with numbers increasing over time, but maintaining the same distribution14), linkage of vaccination data with clinical, sociodemographic and practice information, the addition of influenza vaccination data and the ability to create a longitudinal cohort. Also, our results demonstrated that estimates for vaccine uptake vary by how a ‘patient’ is defined, and this has important ramifications for the interpretation of any primary care data. In the future, the Australian Immunization Register may also provide reliable estimates, but currently MedicineInsight appears to be an important data source, which can be used to provide estimates of vaccination uptake that is reproducible every year, has a low cost, with results consistent with past surveys.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2019.1682844.
References
- 1.Pneumococcal disease . 2014 [accessed 2019 Jun 2013]. https://www.who.int/immunization/diseases/pneumococcal/en/.
- 2.Toms C, de Kluyver R , the Enhanced Invasive Pneumococcal Disease Surveillance Working Group for the Communicable Diseases Network Australia. Invasive pneumococcal in Australia, 2011. and 2012. Commun Dis Intell 2016;40(2):E267–E284. [DOI] [PubMed] [Google Scholar]
- 3.Drijkoningen JJC, Rohde GGU.. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20:45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 4.Djennad A, Ramsay ME, Pebody R, Fry NK, Sheppard C, Ladhani SN, Andrews NJ. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2019;6:42–50. doi: 10.1016/j.eclinm.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australian Immunization Programs . https://beta.health.gov.au/initiatives-and-programs/national-immunisation-program.
- 6.Australian Immunisation Handbook . 2019. https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/pneumococcal-disease.
- 7.Australian Government. Department of Health . Using the Australian Immunisation Register. [Available from https://beta.health.gov.au/health-topics/immunisation/health-professionals/using-the-australian-immunisation-register] Accessed on 12/05/2019. [Google Scholar]
- 8.National Centre for Immunisation Research and Surveillance (NCIRS) . Evaluation of the National Shingles Vaccination Program Process and early impact evaluation. 2009. [Available from: http://ncirs.org.au/sites/default/files/2019-04/Shingles%20Program%20Evaluation%20Report_1%20March%202019_Final%20for%20web.pdf]. Accessed on 12/05/2019. [Google Scholar]
- 9.Dyda A, Karki S, Hayen A, MacIntyre CR, Menzies R, Banks E, Kaldor JM, Liu B. Influenza and pneumococcal vaccination in Australian adults: a systematic review of coverage and factors associated with uptake. BMC Infect Dis. 2016;16(1):515. doi: 10.1186/s12879-016-1820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Australian Institute of Health and Welfare 2011 . 2009 adult vaccination survey: summary results. Canberra, Australia: AIHW; 2011. Cat. no. PHE 135. [Google Scholar]
- 11.Hull BL, Hendry A, Dey A, Brotherton J, Macartney K, Beard F. Annual Immunisation coverage report 2017. National Centre of Immunisation Research and Surveillance; 2017. [Google Scholar]
- 12.Newspoll Omnibus Survey . Summary report. Canberra, Australia: Flu Vaccinations, Department of Health;2014. June. [Google Scholar]
- 13.Regan AK, Gibbs RA, Effler PV. An audit of the reliability of influenza vaccination and medical information extracted from eHealth records in general practice. Vaccine. 2018;36(23):3195–98. doi: 10.1016/j.vaccine.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 14.Busingye D, Gianacas C, Pollack A, Chidwick K, Merrifield A, Norman S, Mullin B, Hayhurst R, Blogg S, Havard A, et al. Data resource profile: MedicineInsight, an Australian national primary health care database. Int J Epidemiol. 2019.doi: 10.1093/ije/dyz147 [DOI] [PubMed] [Google Scholar]
- 15.Bernardo CDO, Gonzalez-Chica D, Stocks N. Influenza-like illness and antimicrobial prescribing in Australian general practice from 2015 to 2017: a national longitudinal study using the MedicineInsight dataset. BMJ Open. 2019;9(4):e026396. doi: 10.1136/bmjopen-2018-026396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Chica D, Stocks N. Changes to the frequency and appropriateness of vitamin D testing after the introduction of new Medicare criteria for rebates in Australian general practice: evidence from 1.5 million patients in the NPS MedicineInsight database. BMJ Open. 2019; 8;9(3):e024797. doi: 10.1136/bmjopen-2018-024797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Chica DA, Vanlint S, Hoon E, Stocks N. Epidemiology of arthritis, chronic back pain, gout, osteoporosis, spondyloarthropathies and rheumatoid arthritis among 1.5 million patients in Australian general practice: NPS MedicineWise MedicineInsight dataset. BMC Musculoskelet Disord. 2018;19(1):20. doi: 10.1186/s12891-018-1941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Oliveira Bernardo C, González-Chica DA, Chilver M, Stocks N. Influenza immunisation coverage from 2015 to 2017: A national study of adult patients from Australian general practice. Vaccine. 2019;37:4268–74. doi: 10.1016/j.vaccine.2019.06.057. [DOI] [PubMed] [Google Scholar]
- 19.Medicinewise NPS. General practice insights report July 2016-June 2017: a working paper. Sydney: NPS MedicineWise, 2018. [Google Scholar]
- 20.The Royal Australian College of General Practitioners . Standards for general practices. 4th edition. 2015. [Available from https://www.racgp.org.au/FSDEDEV/media/documents/Running%20a%20practice/Practice%20standards/4th%20edition/Standards-4th-edtion.pdf]. Accessed on 09/01/2019. [Google Scholar]
- 21.Australian Bureau of Statistics (ABS) . Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia. [Available from. http://www.abs.gov.au/ausstats/abs@.nsf/mf/2033.0.55.001]. Accessed on 09/01/2019. [Google Scholar]
- 22.NSW Government. HealthStats NSW. Influenza and pneumococcal disease immunisation . [Available from http://www.healthstats.nsw.gov.au/Indicator/com_flupneumoimmu_age]. Accessed on 17/06/2019. [Google Scholar]
- 23.Public Health England . Pneumococcal Polysaccharide Vaccine (PPV) coverage report, England, April 2017 to March 2018. Health Protection Report. 2018;12(27). [Google Scholar]
- 24.Harrison C, Britt H. Pneumococcal vaccination among patients at general practice encounters 2013. Sydney: FMRC, University of Sydney; 2014. (Byte from BEACH No: 2014). [Google Scholar]
- 25.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA. Vaccine hesitancy. Hum Vaccines Immunother. 2013;9(8):1763–73. doi: 10.4161/hv.24657.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Australian Government. Department of Health. National Immunisation Research - Qualitative (2016) and quantitative (2017) research reports. https://beta.health.gov.au/resources/publications/national-immunisation-research-qualitative-2016-and-quantitative-2017. Accessed on 2018 Dec 18.
- 27.Stocks N, Ryan P, McElroy H, Allan J. Statin prescribing in Australia: socioeconomic and sex differences. A cross-sectional study. MJA. 2004;180:229–31. [PubMed] [Google Scholar]
- 28.Jain A, van Hoek AJ, Boccia D, Thomas SL. Lower vaccine uptake amongst older individuals living alone: A systematic review and meta-analysis of social determinants of vaccine uptake. Vaccine. 2017;35(18):2315–28. doi: 10.1016/j.vaccine.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM, RECORD Working Committee . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pneumococcal disease . 2014 [accessed 2019 Jun 2013]. https://www.who.int/immunization/diseases/pneumococcal/en/.
- Australian Immunization Programs . https://beta.health.gov.au/initiatives-and-programs/national-immunisation-program.


