ABSTRACT
Vaccination has been one of the major revolutions in the history of human health. Vaccination programs have targeted entire populations such as infants or elderly subjects as a matter of being efficient with time and resources. These general populations are heterogeneous in terms of factors such as ethnicity, health status, and socio-economics. Thus, there have been variations in the safety and effectiveness profiles of certain vaccinations according to current population-wide strategies. As the concept of precision medicine has been raised in recent years, many researchers have suggested that vaccines could be administered more precisely in terms of particular target populations, vaccine formulations, regimens, and dosage levels. This review addresses the concept and framework of precision immunization, summarizes recent and representative clinical trials of among specific populations, mentions important factors to be addressed in customizing vaccinations, and provides suggestions on the establishment of precision immunization with the goal of maximizing the effectiveness of vaccines in general.
KEYWORDS: Precision immunization, immunization strategy, clinical trials, effectiveness, safety
Introduction
Vaccination is often considered as the most economical and effective strategy for the prevention and control of most infectious diseases. As a result of vaccination, smallpox has been eradicated worldwide, the incidence of polio has decreased by 99%, and the process of elimination of measles is progressing.1 Vaccination has been ranked as one of the ten greatest public health achievements during the 20th century by the Centers for Disease Control and Prevention.2 However, most vaccines are evaluated only in healthy populations both during development and post-marketing. Further, only a standard immunization schedule is approved for the whole population. Therefore, some vaccines may be incapable of eliciting a robust specific immune response against the targeted pathogen or disease or might be associated with significant increase in adverse reactions in specific populations or individuals. Therefore, it is critical to develop personalized immunization strategies based on the type of vaccines and the individual characteristics of the immune system. Further, the difference in responses with age, genetics, underlying diseases, or other factors must also be taken into consideration.
Precision immunization, based on customized vaccination for a specific individual, is essential to ensure that more individuals are protected through vaccination. Although precision immunization has not been widely implemented yet, substantial efforts are being made by countries, vaccine manufacturers, and health-care workers to evaluate more specific immunization strategies for different populations. For instance, the national immunization schedules in the United States,3,4 the United Kingdom,5 and Australia6 all include vaccination recommendations for specific risk groups, providing clinical advice for health professionals on the safest and most effective use of vaccines in their practice. Considering that a vaccine may be given to individuals who might not benefit from it, or it might represent an increased risk due to specific health issues, vaccine manufacturers routinely assess many of these concerns during the development of the vaccines or after its registration. In 2017, China’s first vaccination evaluation clinic was established in Shanghai. The newly established vaccination evaluation clinic has ten senior professional experts from the infectious diseases department, immunization program department, and special needs diagnosis and treatment center. It mainly provides professional vaccination guidance services for children with consultation needs, premature infants, and children with special diseases. This is a model for training health-care personnel to assess the benefit/risk profile of the intervention and the specific health condition of the individuals they attend to.
According to our understanding, we define precision immunization as a personalized immunization strategy based on individual characteristics of vaccine recipients; it may involve various dosage and doses of vaccines, different vaccine types, vaccination timings, intervals between vaccine doses, or formulations, in order to maximize the benefit and minimize the risk of the vaccination. Unlike the conventional immunization strategy setting for a whole population, the focus of precision immunization is individual. The implementation of precision immunization strategy will contribute to expanding the use of vaccines while preventing diseases and improving human health more efficiently. Although researchers are working toward precision immunization, the gains are fragmentary and no systematic framework for implementing it is currently available. Moreover, the majority of vaccine clinical trials that have been conducted in specific populations have been focused merely on sub-populations. Besides, the factors considered were relatively single, and the researchers failed to take precision immunization into account from an overall perspective. Herein, we propose the concept and framework of precision immunization, which is novel to vaccinology. Based on the practice of human vaccination and our prediction of the future direction of vaccination, we put forward the concept of precision immunization for the first time. In establishing the framework of precision immunization, vaccines have been classified according to their specific status, and the progress of vaccination in special individuals has been systematically summarized, which will benefit both health-care workers and related individuals. In addition, we have also summarized the critical factors and correlative evidence in the implementation process of precision immunization, which is a new aspect of our review that may, to some extent, clear the direction of precision immunization for researchers in their future practices.
Implementation process of precision immunization
Currently, precision immunization is still in its infancy. The specific implementation process of precision immunization is shown in Figure 1. The first and most important step would be to establish a large database comprising efficacy, immunogenicity, and safety data from various vaccine clinical trials. In addition, massive real-world evidence from vaccination practice must be included, using big data to guide or recommend precision immunization. When a person wants or needs to be vaccinated, the associated information about him/her may be collected, after which, valuable information from this database may be integrated and extracted so as to develop a most suitable precision immunization regime. Healthy individuals (people who have no medical condition or other indications) could be seriously affected by vaccination. Therefore, in order to ensure the safety and effectiveness of vaccination, factors such as age, sex, pre-existing antibodies, and genetic polymorphisms need to be considered while designing precision immunization regimes for healthy individuals. The definition of special individuals is relative to that of general healthy individuals; they can be classified as pregnant women, cancer patients, people living with human immunodeficiency virus (HIV) and asplenic patients, etc., according to their special condition or illness which may lead to changes in immunological responses or risk tolerance. Considering the different types of vaccines to be administered, the optimal immunization regimen could be adjusted with vaccination timing, dosage, and doses, interval between vaccine doses, or formulation with or without adjuvants.
Figure 1.
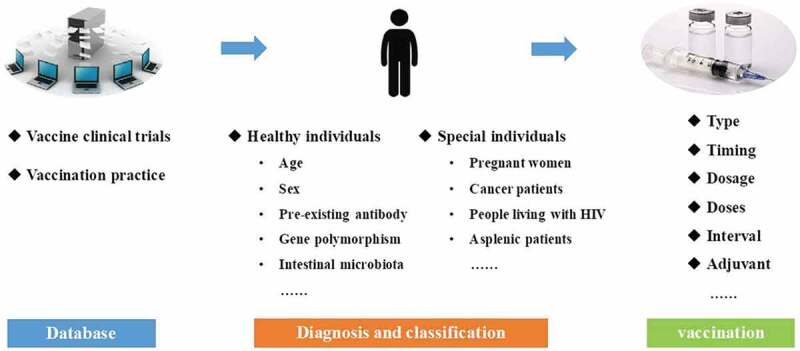
The specific implementation process of precision immunization.
Here, we will elaborate on the critical factors and correlative evidence in the implementation process of precision immunization according to the above classification.
Healthy individuals
Age
Age is an important factor, which could have a major effect on the immune response. Over the course of one’s life, immunity increases from childhood to adulthood and then weakens with age, exhibiting a saddle-type change which is low at both ends. The humoral and cellular immune responses of infants and the elderly are often found to be different from those of adults.7,8
The immune system in newborns and young infants is functionally immature at birth. The immaturity of the immune system often causes unsatisfying antibody responses in neonates with an overall delayed onset, reaching lower peak levels, and exhibiting a shorter duration following vaccination.9 Moreover, neonates have low levels of several components of the complement system, diminished Th1 effector capacity, and a limited ability to generate memory cells.10,11 Therefore, infants and young children are usually more vulnerable to infections.
Sometimes, increasing vaccine doses or adding adjuvant could be feasible methods to solve the problem of poor immune response and antibody persistence.12 However, in some cases, a new generation of vaccines may be needed to cope with the issue. For example, capsule polysaccharide vaccines including Haemophilus influenzae type b (Hib) vaccine, meningococcal vaccine, and pneumococcal vaccine are highly immunogenic in older children and adults, but not in young children under 2 years of age. A novel conjugate vaccine consisting of the capsular polysaccharide of Hib covalently linked to a protein vector was evaluated in 114000 infants in a randomized trial, which was found to be safe and highly effective in infants and young children.13 For low birth weight or preterm infants, we often assume that their immune systems are not functional and inferior to those of full-term infants. Usually, we vaccinate the preterm infants or full-term infants with the same regimen. However, hepatitis B vaccination in preterm infants is an exception. For preterm infants weighing less than 2000 g born to HBsAg-positive mothers, the first dose of hepatitis B vaccine should be administered as soon as possible after birth, ideally within 24 h. However, the birth dose should not count as part of the primary three-dose series, and the three doses of the standard primary series should be given according to the national immunization schedule at appropriate time, such as when the infant reaches 2000 g.14
Currently, most developed countries recommend four kinds of vaccines for the elderly, aged ≥65 years: trivalent inactivated influenza vaccine (TIV), herpes zoster vaccine, pneumococcal vaccine, and a vaccine combining tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.15 The crucial problem connected with vaccination in the elderly is their lower ability to mount an effective immune response to vaccination and a shorter duration of antibody persistence,16 as compared to that in younger individuals. Immunosenescence is the main reason for lower immunogenicity and effectiveness of vaccines in the elderly, featured by impaired ability to respond to new antigens and unsustained memory responses.17
One way to enhance the immune response of the elderly is to increase the dosage of vaccine antigens. For example, high-dose TIV (60 μg of each strain) is found to be safe and tolerant in the elderly over 65 years of age, and it can induce a stronger immune response than the standard-dose vaccine (15 μg).18,19 The U.S. Food and Drug Administration licensed the high-dose TIV for use among persons aged ≥65 years.20 Besides, adding adjuvants to vaccines is another way to improve the immune response in the elderly. The MF59 adjuvanted TIV and AS03 adjuvanted TIV have been found to significantly increase the immune response and efficacy of TIV in the elderly.21–24 Furthermore, the AS01B adjuvanted zoster vaccine had significantly reduced the risks of herpes zoster and postherpetic neuralgia among adults aged 50 to 70 years and above.25,26 A recent meta-analysis showed the herpes zoster adjuvant recombinant subunit vaccine was statistically superior to both the live attenuated vaccine (vaccine efficacy 85%, 95% credible interval 31% to 98%) and placebo (94%, 79%, to 98%).27 Subunit vaccines are usually safer but less immunogenic, and therefore need to be combined with an adjuvant to enhance the immune response.
Sex
The biological differences associated with the gender of individuals are a major source of variation of immune responses to vaccination, although it is often ignored. In a comprehensive review of the literature, sex-difference in immune response was reported in 14 different vaccines.28 Sex-difference was found to be antigen dependent, with females having greater antibody response than males for the following vaccines: influenza, hepatitis A, hepatitis B, rubella, measles, diphtheria, tetanus, brucella, and rabies. Conversely, males showed a greater antibody response than females for the following vaccines: influenza, pneumococcal polysaccharide, diphtheria, measles, yellow fever, meningococcal A, meningococcal C, Venezuelan equine encephalitis, and rabies. Furthermore, two phase 3, double-blind, randomized trials conducted in Australia, Canada, Italy, and the United States found that a herpes simplex virus (HSV) type 2 subunit vaccine has efficacy in women who are seronegative at baseline for both HSV type 1 and HSV type 2 at baseline but not in those who are seropositive for HSV type 1 and seronegative for HSV type 2 at baseline. However, it had no efficacy in men, regardless of their HSV serologic status.29 In addition, studies found that sex affects the adverse effects of vaccination as well, including fever, pain, and inflammation.30 However, it is worth noting that gender differences in adverse reactions to vaccines may also be a result of reporting bias.
Pre-existing antibody
Before vaccination, there are pre-existing antibodies to certain pathogens in the human body. Pre-existing antibodies can be acquired through passive immunization, such as immunoglobulin injection, placental transport or breast-feeding, or active immunization, such as infection, or vaccination. The effect of pre-existing antibodies on vaccine effectiveness is enormous. In June 2018, an extended analysis reported that the tetravalent dengue vaccine was protective in persons who had exposure to dengue before vaccination, but increased the risk of dengue in those who had not been exposed.31 Therefore, when conducting clinical evaluation for vaccines, stratification analysis based on the presence of pre-existing antibodies is usually needed. To assess the efficacy of inactivated vaccines, live attenuated vaccines and protein vaccines, it is necessary to detect the antibody levels of the subjects to the pathogen contained in the vaccine, before vaccination. Assessment of vectored vaccines is more complicated since both the antibody against the vector and the target antibodies to the pathogen could affect the vaccines’ efficacy. Pre-existing antibodies to the vector may neutralize the virus which acts as a vector in the vaccine, thereby preventing the vaccine antigen from being expressed effectively, ultimately reducing the immune response. The results of the first phase I clinical trial of a recombinant adenovirus type-5 vector-based Ebola vaccine in China suggested that pre-existing antibodies against adenovirus vector could significantly weaken the specific humoral and cellular immunity to Ebola glycoprotein, and reduce the persistence of vaccine-induced immunity. However, the antibodies against adenovirus increased significantly.32
Several immunological mechanisms may explain the effect of pre-existing antibodies on vaccine effectiveness, including original antigenic sin vs. de novo immune responses, immune interference, and antibody-dependent enhancement. Original antigenic sin refers to the propensity of the body’s immune system to preferentially utilize immunological memory based on a previous infection when a second slightly different version of that foreign entity is encountered.33 This leaves the immune system trapped by the first response it has made to each antigen, and is unable to mount potentially more effective responses during subsequent infections. Original antigenic sin is believed to play a role in influenza virus infections and impacts the effectiveness of influenza vaccines. Researchers found decreased antibody production in patients who had been vaccinated with the seasonal H1N1 vaccine in the previous 3 months due to cross-reactivity of the previously developed monotypic antibodies.34 Contrary to original antigenic sin, if pre-existing immunity does not impair the immune response to the subsequent novel antigen, it is considered to be a de novo immune response.35 Another mechanism is immune interference, which provides an important basis for vaccination sequence. The result of a clinical trial showed that the rates of antibody response in participants with two sequential vaccinations with equine encephalitis vaccines were significantly different.36 For a number of viral pathogens, under certain conditions, antibodies provide an attractive means of enhanced virus entry and replication in a number of cell types, which is known as antibody-dependent enhancement;37 the dengue vaccine mentioned above is an example of this mechanism. These cases indicate that it may be possible to develop personalized immunization strategies for individuals by measuring their pre-existing antibodies or even based on their vaccination history.
Gene polymorphism
Host genetic polymorphisms may modulate the immune response in multiple ways on different scales. For example, despite the effectiveness of the hepatitis B vaccine (HBV), 5–10% of immunocompetent subjects failed to respond after HBV vaccination; genetic polymorphism is one of the potential explanations.38 A large population-based study assessing associations between human lymphocyte antigen (HLA) class I genes and immune responses to rubella virus found HLA-B*3503 and HLA-Cw*1502 were positively associated with lymphoproliferative responses to rubella virus antigens, whereas the HLA-B*3901 allele was negatively associated.39 Another cohort study reported a genetic association between humoral antibody level after vaccination with the measles vaccine and the HLA class II genes.40 The results showed HLA-DRB1∗03 and HLA-DPA1∗0201 were significantly associated with measles vaccine seronegativity, while DQA1∗0201, DQB1∗0201, and DQA1∗0501 provided suggestive evidence of association with seronegativity. Furthermore, some studies revealed that non-HLA genes such as cytokine gene,41,42 cellular receptor gene,43 and toll-like receptor gene44 are also associated with vaccine immune responses. Over the last few years, it has been proposed frequently that genetic information might be used to predict vaccine effectiveness and might help to develop more effective, individualized immunization strategies.45 Till now, there are no clinical studies aimed at improving vaccine immune responses based on host genetic polymorphisms; however, such studies may be proposed in the near future.
Intestinal microbiota
The intestinal microbiota is an underappreciated but very important factor associated with varying efficacy of oral vaccines. The efficacy of oral rotavirus vaccine (RVV) against severe rotavirus gastroenteritis is as high as 85–98% in developed countries,46–49 but only 39.3–61.2% in developing countries.50,51 A retrospective nested case-control study comparing pre-vaccination, fecal microbiome compositions between the matched RVV responders and non-responders, found that the overall microbiome composition was significantly different.52 RVV response correlated with an increased abundance of Streptococcus bovis and a decreased abundance of the Bacteroidetes phylum. In another similar study from Pakistan, RVV response was found to be correlated with a higher relative abundance of bacteria belonging to the Clostridium cluster XI and Proteobacteria, including bacteria related to Serratia and Escherichia coli.53
In addition, the effect of intestinal microbiota on vaccine response was also found in oral typhoid vaccine54 and oral polio vaccine.55 Based on the above evidences, the intestinal microbiota may have an impact on oral vaccine response, though this conclusion needs further confirmation.
Special individuals with medical condition and other indications
Pregnant women
Generally, women with high-risk conditions should be vaccinated before pregnancy, because physiological changes during pregnancy lead to an elevated risk of infectious diseases. However, some vaccines recommended during pregnancy can benefit both pregnant women and infants.56 A randomized study showed that maternal immunization with inactivated influenza vaccine resulted in a reduction of 63% in laboratory-confirmed influenza illness in infants up to 6 months of age and reductions of 29% and 36% in rates of respiratory illness with fever in infants and mothers, respectively.57 A large observational cohort study showed that there was a 41% reduction in the risk of laboratory-confirmed influenza virus infection for infants born to influenza-vaccinated women compared with infants born to unvaccinated mothers.58
In addition to preventing infections in pregnant women and infants, maternal immunization impacts birth outcomes. In a randomized, controlled trial in Bangladesh, maternal immunization was associated with a lower proportion of infants who were small for gestational age and an increase in mean birth weight. Similarly, a large randomized, controlled trial in Nepal demonstrated a 15% reduction in low birth weight among newborns of mothers who received influenza vaccination in pregnancy, compared to newborns of unvaccinated women. Besides, observational studies found that the influenza vaccination was associated with a decrease in the stillbirth rate.59,60
As a rule, inactivated influenza vaccine and the tetanus, diphtheria, and acellular pertussis vaccines are recommended for pregnant women.61 High-risk susceptible pregnant women may be vaccinated with inactivated hepatitis A vaccine,62 recombinant HBV,63 meningococcal serogroups A, C, W, Y vaccine (MenACWY),64 and pneumococcal polysaccharide vaccine (PPV)65 after weighing the potential risks and benefits. Women, who inadvertently receive human papillomavirus vaccines while pregnant, are required to delay the remaining doses until after pregnancy.66
Overall, inactivated vaccines are fairly safe in pregnancy, while live vaccines are generally avoided. Smallpox vaccination during pregnancy has resulted in fetal infection with vaccinia virus present in the vaccine.67 However, for many live vaccines, maternal immunization poses a theoretical rather than a documented risk, which needs further evidence.
Cancer patients
Due to the malignancy itself or the immunosuppressive treatment administered, patients with cancer in general are more susceptible to vaccine-preventable infections, such as influenza and invasive pneumonia.68 Moreover, the compromised immune system would mount a less satisfying immune response to the vaccination, if the routine immunization schedule recommended for healthy individuals is followed. There are two commonly used methods to improve the effect of vaccination in people with cancer: one is to increase vaccine dosage, and the other is to increase the number of doses. A randomized, open-label, prospective study in India illustrated that compared with cancer patients who were vaccinated with hepatitis B vaccine (20 μg) at 0, 1, and 6 months, those receiving double-dose (40 μg) hepatitis B vaccine at 0, 1, and 3 weeks before chemotherapy and additional three double doses post chemotherapy, showed increased seroprotection level by 26%.69
Besides, the safety and tolerance of the vaccine in cancer patients might be significantly different from that in healthy individuals, which is a critical issue that needs to be addressed, especially for live vaccines. A study in Hong Kong found that one of the 17 children (subjects) developed possibly vaccine-related chickenpox with self-limiting hepatitis at 5 weeks following varicella vaccination.70 In January 2017, a 71-year-old immunocompromised man with chronic lymphocytic leukemia presented with a bilateral vesicular facial rash 22 days after receiving live attenuated zoster vaccine, following which, he succumbed to secondary varicella zoster virus infection.71 Currently, as a safety measure, it is recommended that patients with cancer should be vaccinated with inactivated vaccines. Further, the use of live attenuated vaccines and any vaccination during immunosuppressive therapy must be avoided in such individuals.
In addition to vaccinating cancer patients with prophylactic vaccines against the increased risk of infections, precision immunization with therapeutic cancer vaccines are essential for achieving therapeutic effect in cancer patients. However, due to immune tolerance and the risk of inducing autoimmune diseases, traditional therapeutic vaccines targeting tumor-associated antigens are difficult to be promoted on a large scale in clinics. Neoantigens, which are absent in normal cells and originate from tumor somatic mutations, represent promising targets for personalized cancer vaccine strategy.72 Based on gene sequencing, neoantigen vaccine is a customized vaccine that targets different mutation sites in each cancer patient. In July 2017, Boston cancer institute evaluated a peptide vaccine targeting up to 20 neoantigens in six patients with previously untreated high-risk melanoma after surgical resection with curative intent.73 At a median follow-up of 25 months after vaccination, four patients remained without disease recurrence. The other two patients had disease recurrence after the last vaccination. Subsequently, both patients underwent treatment with the anti-PD-1 antibody and achieved complete radiographic responses that are ongoing. Another research in Germany indicated that neo-epitope vaccines may prevent recurrent disease in melanoma patients, and the response to vaccination may be improved in combination with PD-1 blockade therapy.74 These research initiatives indicate that neoantigen vaccines can be exploited, thereby opening a path to personalized immunotherapy for patients with cancer.
People living with HIV
Some vaccine-preventable diseases, such as pneumonia, influenza, hepatitis, meningitis and herpes zoster, play an important role in accelerating the natural history of HIV infection, ultimately leading to death.75 The challenge of vaccination in individuals living with HIV is incomplete immune response due to the impaired immune system. The most typical features of this are lower specific antibody levels after vaccination and rapid decline of antibody titer. However, it is feasible to improve the immune response of people living with HIV by modifying the immunization schedule. A randomized trial conducted at 33 centers in France indicated that in adults with HIV-1, both the four intramuscular double-dose regimen (40 μg, at weeks 0, 4, 8, and 24) and the four intradermal low-dose regimen (4 μg, at weeks 0, 4, 8, and 24) improved serological response compared with the standard hepatitis B vaccine regimen (20 μg, at weeks 0, 4, and 24) significantly, from 65% to 82% and 77%76 respectively. Another research found that the antibody responses of HIV-infected children on highly active antiretroviral therapy were comparable to those of healthy infants when a regime comprising two doses of pneumococcal conjugate vaccine (PCV) and one dose of PPV at 8-week intervals77 was administered.
Moreover, HIV-infected people who were not severely immunocompromised could be vaccinated with some live attenuated vaccines, such as measles, mumps, and rubella vaccine,78,79 varicella vaccine80 and live attenuated herpes zoster vaccine.81 These findings suggest that the precise immunization of HIV patients should be determined according to their immune status.
Asplenic patients
Individuals with functional or anatomical asplenia are at increased risk of infection caused by encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis and Hib.82 However, PPV is of limited immunogenicity in asplenic patients.83 The development of PCV led to recommendations of combined PCV/PPV23 schedules for asplenic patients, with PPV23 used to increase serotype coverage.84 A randomized cross-over clinical trial demonstrated that PCV vaccine before PPV can be more effective in asplenic patients as a booster dose.85 Therefore, the Advisory Committee on Immunization Practices (ACIP) suggests asplenic patients aged ≥2 years should receive a dose of PCV13 first, followed by a dose of PPSV23 at least 8 weeks later. In addition, a second PPSV23 dose is recommended 5 years after the first PPSV23 dose.86,87 For Hib vaccine, several studies showed that the majority of asplenic patients mounted sufficient response after one dose of vaccination.88,89 In 2019, an immunization schedule approved by the ACIP recommends asplenic patients aged 2 years or older to receive two doses of MenACWY at least 8 weeks apart,3 and those aged 19 years or older to be administered two doses of MenACWY at least 8 weeks apart and must be revaccinated with one dose every 5 years.4
Other special individuals
In addition to the above classification of special individuals, there are other special individuals, such as solid organ transplant (SOT) or hematopoietic stem cell transplant (HSCT) recipients and hemodialysis patients, in whom vaccinations remain relatively underutilized. The vaccination practices in SOT and HSCT recipients have been discussed in a review published in 2017.90 The results showed that most live attenuated vaccines continue to be contraindicated post-transplantation, but there are emerging safety profiles and efficacy data to support the use of specific live attenuated vaccines.
For hemodialysis patients, the attempts to overcome their impaired immune response through vaccinations have produced mixed results. Increased dosage and AS04 adjuvanted HBV were found to be effective to improve the response rate in these subjects.91–93 However, an additional booster dose of the influenza vaccine did not effectively enhance immune response in hemodialysis patients.94
Considerations for the future
Precision medicine has gained increasing popularity over recent years. The Precision Medicine Initiative defines it as an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person.95 Due to the population-based nature of traditional vaccination, the benefits of vaccination have been conventionally evaluated by the benefits of population. Nonetheless, vaccines are given to some individuals who might not benefit from the vaccination, or are at an increased risk due to specific health issues, such as the use of live attenuated vaccines in immunocompromised individuals.96 Therefore, it is necessary to emphasize the need of applying precision medicine strategy to individuals and moving away from the current ‘one size fits all approach’ of vaccination. Assessing the benefit/risk profile of the vaccination and the specific health condition of the individuals (which is one of the critical parts of precision immunization) would help determine whether these individuals need to be vaccinated. For example, yellow fever vaccines are generally not recommended in pregnancy, and pregnant women should avoid traveling to areas with endemic yellow fever; however, if the travel is unavoidable, vaccination should be considered.97 Furthermore, the implementation of precision immunization can both improve the effectiveness and minimize the adverse reactions of vaccination in individuals by adjusting immunization strategies (schedule, dosage, co-administration).
At present, precision immunization is still in the early stages of development. Reports on vaccine clinical trials focusing on special individuals are relatively limited and often, a single factor or underlining condition of the recipients is considered. In a review exploring factors affecting vaccine immunogenicity and safety, the authors also stressed on the importance of taking all procedural and technical factors and demographic parameters, which potentially influence the performance of a vaccine, into account when designing a clinical trial. In addition, controls should be included when randomizing subjects into a comparative study and while analyzing the results.98 In healthy individuals, routine immunization is relatively simple, but precise immunization requires consideration of the comprehensive effects of age, gender, pre-existing antibodies, gene polymorphisms, and intestinal microbiota on vaccine effectiveness. In special individuals, the aforementioned factors should also be combined with their medical conditions. In addition, the above factors may be interactive rather than independent, which makes precision immunization more challenging to achieve.
Precision immunization is a systematic project, which can only be achieved by the joint efforts of all parties. The government should pay attention to the risk of vaccine-preventable diseases and the related heavy disease burden faced by a large number of special populations, and then promote relevant policies and regulations for vaccination of special populations. Research institutions ought to channelize their efforts toward investigating feasible strategies for precision immunization in special individuals, and provide scientific basis for medical personnel to propel precision immunization. Health workers should encourage high-risk individuals and their families to be vaccinated properly, thereby prolonging their survival and improving their life of quality. For vaccine research and development enterprises, more efforts should be focused on the development of new generation vaccines, which could be used for those with underlying medical conditions. For example, some live attenuated vaccines cannot be used in immunocompromised individuals, such as cancer patients; therefore, it is necessary to modify the relevant vaccines. The development of subunit vaccines may overcome the safety problems associated with live attenuated vaccines in immunocompromised persons.
In the future, there are still many problems to be solved in the process of promoting precision immunization. The establishment of a database for precision immunization can be achieved by collating the existing data from vaccine clinical trials and the massive real-world evidence from the practice of vaccination. However, this database needs to be continuously perfected and updated with collected data and evidence, in order for it be equipped to provide better assistance to precision immunization. However, most of this type of data is anecdotal at best and does not include much information at the individual level. Clinical trials and controlled comprehensive post-marketing surveillance studies will be needed to collect useful information on individuals (e.g., HLA typing, concurrent infections, underlying medical conditions, etc.). Currently, HLA typing is relatively expensive, and it will take decades or even hundreds of years to build a database of all human HLA typing. In addition, since the current clinical practice is still mainly focused on treatment, it will be a difficult goal to determine the co-infection and underlying health conditions, both technically and practically. These will depend on more advanced diagnostic technology and changing human concepts. Besides, the physical status of individuals is dynamic; for individuals with impaired immune function, such as HIV-infected persons and cancer patients, the best strategy to get the desired results after vaccination is to carry out related immunological detections before vaccination.99 Nevertheless, since immunoassay technology is relatively time-consuming and expensive, it is difficult to be popularized at the grass-root level, especially in the developing countries. Specific costs and supporting facilities are difficult to determine without large-scale investigation and practice; therefore, the investment cost of precision immunization cannot be precisely estimated.
The advancement of precision immunization is full of challenges; nevertheless, its prospects are bright. In the future, precision immunization will eventually become a new trend in human vaccination.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Roush SW, Murphy TV.. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. Jama. 2007;298(18):2155–63. doi: 10.1001/jama.298.18.2155. PMID: 18000199. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado YA. Current controversies in vaccination: vaccine safety. Jama J Am Med Assoc. 2002;288(24):3155–58. doi: 10.1001/jama.288.24.3155. PMID: 12495396. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . Recommended child and adolescent immunization schedule for ages 18 years or younger, United States. 2019. [accessed 2019 February 5]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
- 4.Centers for Disease Control and Prevention . Recommended adult immunization schedule for ages 19 years or older, United States. 2019. [accessed 2019 February 5]. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
- 5.GOV.UK . Complete routine immunisation schedule [accessed 2019 July 12]. https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule
- 6.Australian Government Dapartment of Health . The Australian immunisation handbook [accessed 2019 July 12]. https://immunisationhandbook.health.gov.au [Google Scholar]
- 7.Ota MOC, Vekemans J, Schlegel-Haueter SE, Fielding, K., Whittle, H., Lambert, P H., McAdam, Keith P W J., Siegrist, C A., Marchant, A.. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine. 2004;22(3):511–19. doi: 10.1016/j.vaccine.2003.07.020. PMID: 14670334. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger B, Herndlerbrandstetter D, Schwanninger A, Weiskopf, D., Grubeck-Loebenstein, B., Linnik, J E., Egli, A.. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46(7):1078–84. doi: 10.1086/529197. PMID: 18444828. [DOI] [PubMed] [Google Scholar]
- 9.Patricia P, Camila Q, Ana Lúcia SL, Zago C.A. and Carneiro-Sampaio M.. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2011;2012(3):1–13. doi: 10.1155/2012/985646. PMID: 22235228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schelonka RL, Infante AJ. Neonatal immunology. Semin Perinatol. 1998;22(1):2–14. PMID: 9523395. [DOI] [PubMed] [Google Scholar]
- 11.Becky A, Claude L, Stuart MC. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–64. doi: 10.1038/nri1394. PMID: 15229474. [DOI] [PubMed] [Google Scholar]
- 12.Excler JL. Potentials and limitations of protein vaccines in infants. Vaccine. 1998;16(14–15):1439–43. doi: 10.1016/S0264-410X(98)00105-4. PMID: 9711785. [DOI] [PubMed] [Google Scholar]
- 13.Eskola J, Kayhty H, Takala AK, Peltola, H, Rönnberg, P R., Kela, E, Pekkanen, E, McVerry, P H., Mäkelä, P H.. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990;323(20):1381–87. doi: 10.1056/NEJM199011153232004. PMID: 2233904. [DOI] [PubMed] [Google Scholar]
- 14.Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccin. 2015;11(11):2556–63. doi: 10.1080/21645515.2015.1074358. PMID: 26291883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goronzy JRJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428–36. doi: 10.1038/ni.2588. PMID: 23598398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–69. doi: 10.1016/j.vaccine.2005.08.105. PMID: 16213065. [DOI] [PubMed] [Google Scholar]
- 17.Triglav TK, Poljak M. Vaccination indications and limits in the elderly. Acta Dermatovenerol Alp Pannonica Adriat. 2013;22(3):65–70. doi: 10.2478/v10162-012-0037-9. PMID: 24089135. [DOI] [PubMed] [Google Scholar]
- 18.Diazgranados CA, Dunning AJ, Jordanov E, Landolfi, V., Denis, M., Talbot, H K.. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine. 2013;31(6):861–66. doi: 10.1016/j.vaccine.2012.12.013. PMID: 23261045. [DOI] [PubMed] [Google Scholar]
- 19.Falsey AR, Treanor JJ, Tornieporth N, Capellan, J., Gorse, G J.. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200(2):172–80. doi: 10.1086/599790. PMID: 19508159. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (fluzone high-dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59(16):485–86. PMID: 20431524. [PubMed] [Google Scholar]
- 21.Mannino S, Villa M, Apolone G, Weiss, N S., Groth, N., Aquino, I., Boldori, L., Caramaschi, F., Gattinoni, A., Malchiodi, G., et.al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in Northern Italy. Am J Epidemiol. 2012;176(6):527–33. doi: 10.1093/aje/kws313. PMID: 22940713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey SE, Reyes MR, Reynales H, Bermal, N N., Nicolay, U., Narasimhan, V., Forleo-Neto, E., Arora, A K.. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine. 2014;32(39):5027–34. doi: 10.1016/j.vaccine.2014.07.013. PMID: 25045825. [DOI] [PubMed] [Google Scholar]
- 23.Mcelhaney JE, Beran J, Devaster JM, Esen, M., Launay, O., Leroux-Roels, G., Ruiz-Palacios, G M., van Essen, G A., Caplanusi, A., Claeys, C., et.al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13(6):485–96. doi: 10.1016/S1473-3099(13)70046-X. PMID: 23518156. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Palacios GM, Leroux-Roels G, Beran J, Devaster JM, Esen M, Launay O, Mcelhaney JE, van Essen GA, Benoit A, et al. Immunogenicity of AS03-adjuvanted and non-adjuvanted trivalent inactivated influenza vaccines in elderly adults: a phase 3, randomized trial and post-hoc correlate of protection analysis. Hum Vaccin Immunother. 2016;12(12):3043–55. doi: 10.1080/21645515.2016.1219809. PMID: 27690762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himal L, Cunningham AL, Olivier G, Chlibek, R., Diez-Domingo, J., Hwang, S-J., Levin, M J., McElhaney, J E., Poder, A., Puig-Barberà, J., et.al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. PMID: 25916341. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díezdomingo J, Godeaux O, Levin MJ, Mcelhaney JE, Puigbarberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi: 10.1056/NEJMoa1603800. PMID: 27626517. [DOI] [PubMed] [Google Scholar]
- 27.Tricco AC, Zarin W, Cardoso R,Veroniki AA, Khan PA, Nincic V, Ghassemi M, Warren R, Sharpe JP, Page AV, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ. 2018;363:k4029. doi: 10.1136/bmj.k4029. PMID: 30361202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29):3551–55. doi: 10.1016/j.vaccine.2008.04.054. PMID: 18524433. [DOI] [PubMed] [Google Scholar]
- 29.Stanberry LR, Spruance SL, Cunningham AL, Bernstein, D I., Mindel, A., Sacks, S., Tyring, S., Aoki, F Y., Slaoui, M., Denis, M., Vandepapeliere, P., Dubin, G.. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–61. doi: 10.1056/NEJMoa011915. PMID: 12444179. [DOI] [PubMed] [Google Scholar]
- 30.Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5(7):441–49. doi: 10.4161/hv.8476. PMID: 19377279. [DOI] [PubMed] [Google Scholar]
- 31.Sridhar S, Luedtke A, Langevin E, Zhu, M., Bonaparte, M., Machabert, T., Savarino, S., Zambrano, B., Moureau, A., Khromava, A., et.al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–40. doi: 10.1056/NEJMoa1800820. PMID: 29897841. [DOI] [PubMed] [Google Scholar]
- 32.Li JX, Hou LH, Meng FY, Wu, S-P., Hu, Y-M., Liang, Qi, Chu, K., Zhang, Z., Xu, J-J., Tang, R., et.al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob Health. 2017;5(3):e324–e334. doi: 10.1016/S2214-109X(16)30367-9. PMID: 28017642. [DOI] [PubMed] [Google Scholar]
- 33.Monto AS, Malosh RE, Petrie JG, Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis. 2017;215(12):1782–88. doi: 10.1093/infdis/jix173. PMID:28398521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vatti A, Monsalve DM, Pacheco Y, Chang, C., Anaya, J-M., Gershwin, M E.. Original antigenic sin: a comprehensive review. J Autoimmun. 2017;83:12–21. doi: 10.1016/j.jaut.2017.04.008. PMID: 28479213. [DOI] [PubMed] [Google Scholar]
- 35.Kirsi T, Leena H, Timo V, Blazevic, V.. Pre-existing immunity to norovirus GII-4 virus-like particles does not impair de novo immune responses to norovirus GII-12 genotype. Viral Immunol. 2013;26(2):167–70. doi: 10.1089/vim.2012.0082. PMID: 23438469. [DOI] [PubMed] [Google Scholar]
- 36.Pittman PR, Liu C-T, Cannon TL, Mangiafico, J A., Gibbs, P H.. Immune interference after sequential alphavirus vaccine vaccinations. Vaccine. 2009;27(36):4879–82. doi: 10.1016/j.vaccine.2009.02.090. PMID: 19576665. [DOI] [PubMed] [Google Scholar]
- 37.Adam T, Suan-Sin F, Roberto B, Dinh, L V., King, Nicholas J C., Mahalingam, S.. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 2015;268(1):340–64. doi: 10.1111/imr.12367. PMID: 26497532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saco TV, Strauss AT, Ledford DK. Hepatitis B vaccine non-responders: possible mechanisms and solutions. Ann Allergy Asthma Immunol. 2018;121(3):320–27. doi: 10.1016/j.anai.2018.03.017. PMID: 29567355. [DOI] [PubMed] [Google Scholar]
- 39.Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Poland GA. The contribution of HLA class I antigens in immune status following two doses of rubella vaccination. Hum Immunol. 2004;65(12):1506–15. doi: 10.1016/j.humimm.2004.07.001. PMID: 15603879. [DOI] [PubMed] [Google Scholar]
- 40.Poland GA, Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Schaid DJ. Identification of an association between HLA class II alleles and low antibody levels after measles immunization. Vaccine. 2001;20(3):430–38. doi: 10.1016/S0264-410X(01)00346-2. PMID: 11672906. [DOI] [PubMed] [Google Scholar]
- 41.Chengbin W, Jianming T, Wei S, Lobashevsky, E., Wilson, C M., Kaslow, R A.. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39(4):978–88. doi: 10.1002/hep.20142. PMID: 15057902. [DOI] [PubMed] [Google Scholar]
- 42.Roh EY, Song EY, Yoon JH, Oh, S., Young Chang, Ju, Park, H., Hyun Seo, S., Shin, S.. Effects of interleukin-4 and interleukin-12B gene polymorphisms on hepatitis B virus vaccination. Ann Hepatol. 2017;16(1):63–70. doi: 10.5604/16652681.1226816. PMID: 28051794. [DOI] [PubMed] [Google Scholar]
- 43.Ovsyannikova IG, Haralambieva IH, Vierkant RA, O’Byrne, M M., Jacobson, R M., Poland, G A.. The association of CD46, SLAM and CD209 cellular receptor gene SNPs with variations in measles vaccine-induced immune responses: a replication study and examination of novel polymorphisms. Hum Hered. 2011;72(3):206–23. doi: 10.1159/000331585. PMID: 22086389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jie C, Zhenglun L, Fengmin L, Fang, X., Liu, S., Zeng, Y., Zhu, F., Chen, X., Shen, T., Li, J., Zhuang, H.. Toll-like receptors and cytokines/cytokine receptors polymorphisms associate with non-response to hepatitis B vaccine. Vaccine. 2011;29(4):706–11. doi: 10.1016/j.vaccine.2010.11.023. PMID: 21111021. [DOI] [PubMed] [Google Scholar]
- 45.Linnik JE, Egli A. Impact of host genetic polymorphisms on vaccine induced antibody response. Hum Vaccin. 2016;12(4):907–15. doi: 10.1080/21645515.2015.1119345. PMID: 26809773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Palacios GM, Irene PS, F Raúl V, Hector A, Thomas B, Sueann Costa C, Brigitte C, Felix E, Paul G, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. PMID: 16394298. [DOI] [PubMed] [Google Scholar]
- 47.Linhares AC, Velázquez FR, Irene PS, Sáez-Llorens, X., Abate, H., Espinoza, F., López, P., Macías-Parra, M., Ortega-Barría, E., Rivera-Medina, D M., et.al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–89. doi: 10.1016/S0140-6736(08)60524-3. PMID: 18395579. [DOI] [PubMed] [Google Scholar]
- 48.Vesikari T, Karvonen A, Prymula R, Schuster, V, Tejedor, J C., Cohen, R, Meurice, F, Han, H H., Damaso, S, Bouckenooghe, A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–63. doi: 10.1016/S0140-6736(07)61744-9. PMID: 18037080. [DOI] [PubMed] [Google Scholar]
- 49.Timo V, Matson DO, Penelope D, Van Damme, P., Santosham, M., Rodriguez, Z., Dallas, M J., Heyse, J F., Goveia, M G., Black, S B., et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. PMID: 16394299. [DOI] [PubMed] [Google Scholar]
- 50.Madhi SA, Cunliffe NA, Duncan S, Witte, D., Kirsten, M., Louw, C., Ngwira, B., Victor, J C., Gillard, P H., Cheuvart, B B., et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–98. doi: 10.1056/NEJMoa0904797. PMID: 20107214. [DOI] [PubMed] [Google Scholar]
- 51.Armah GE, Sow SO, Breiman RF, Dallas, M J., Tapia, M D., Feikin, D R., Binka, F N., Steele, A D., Laserson, K F., Ansah, N A., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. PMID: 20692030. [DOI] [PubMed] [Google Scholar]
- 52.Harris VC, Armah G, Fuentes S, Korpela, K E., Parashar, U., Victor, J C., Tate, J., de Weerth, C., Giaquinto, C., Wiersinga, W J., et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infect Dis. 2017;215(1):34–41. doi: 10.1093/infdis/jiw518. PMID: 27803175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris VC, Ali A, Fuentes S, Korpela, K., Kazi, M., Tate, J., Parashar, U., Wiersinga, W J., Giaquinto, C., de Weerth, C., et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2018;9(2):93–101. doi: 10.1080/19490976.2017.1376162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eloefadrosh EA, Mcarthur MA, Seekatz AM, Drabek, E F., Rasko, D A., Sztein, M B., Fraser, C M., Gilbert, J A.. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PLoS One. 2013;8(4):e62026. doi: 10.1371/journal.pone.0062026. PMID: 23637957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huda MN, Zachery L, Kalanetra KM, Rashid, M., Ahmad, S M., Raqib, R., Qadri, F., Underwood, M A., Mills, D A., Stephensen, C B.. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134(2):e362–372. doi: 10.1542/peds.2013-3937. PMID: 25002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Li J, Wang Y, Gu W, Zhu F. Effectiveness and practical uses of 23-valent pneumococcal polysaccharide vaccine in healthy and special populations. Hum Vaccin Immunother. 2017;14(18):1–10. doi: 10.1080/21645515.2017.1409316. PMID: 29261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaman K, Eliza R, Arifeen SE, Rahman, M., Raqib, R., Wilson, E., Omer, S B., Shahid, N S., Breiman, R F., Breiman, R E., Steinhoff, M C.. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–64. doi: 10.1056/NEJMoa0708630. PMID: 18799552. [DOI] [PubMed] [Google Scholar]
- 58.Eick AA, Uyeki TM, Klimov A, Hall, H., Reid, R., Santosham, M., O’Brien, K L, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;66(2):104–11. doi: 10.1001/archpediatrics.2010.192. PMID: 20921345. [DOI] [PubMed] [Google Scholar]
- 59.Regan AK, Moore HC, De KN, Omer, S B., Shellam, G., Mak, D B., Effler, P V, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis. 2016;62(10):1221–27. doi: 10.1093/cid/ciw082. PMID: 27033634. [DOI] [PubMed] [Google Scholar]
- 60.Sheffield JS, Greer LG, Rogers VL, Roberts, S W., Lytle, H., McIntire, D D., Wendel, G D, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532–37. doi: 10.1097/AOG.0b013e318263a278. PMID: 22914461. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention . Pregnancy and vaccination Accessed 2016 July 11. https://www.cdc.gov/vaccines/pregnancy
- 62.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis a through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR–7):1–23. PMID: 16708058. [PubMed] [Google Scholar]
- 63.Mast EE, Weinbaum CM, Fiore AE, Alter, M J., Bell, B P., Finelli, L., Rodewald, L E., Douglas, J M., Janssen, R S., Ward, J W.. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR–16):1–33. PMID: 17159833. [PubMed] [Google Scholar]
- 64.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez, I R., Briere, E Z., Meissner, H C., Baker, C J., Messonnier, N E, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR–2):1–28. PMID: 23515099. [PubMed] [Google Scholar]
- 65.Kim DK, Bridges CB, Harriman KH.. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older–United States, 2015. MMWR Recomm Rep. 2015;64(4):91–92. PMID: 25654609. [PMC free article] [PubMed] [Google Scholar]
- 66.Markowitz LE, Dunne EF, Saraiya M, Lawson, H W., Chesson, H., Unger, E R, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR–2):1–23. PMID: 17380109. [PubMed] [Google Scholar]
- 67.Badell ML, Meaney-Delman D, Tuuli MG, Rasmussen, S A., Petersen, B W., Sheffield, J S., Beigi, R H., Damon, I K., Jamieson, D J. Risks associated with smallpox vaccination in pregnancy. Obstet Gynecol. 2015;125(6):1439–51. doi: 10.1097/AOG.0000000000000857. PMID: 26000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charshafian S, Liang SY. Infectious disease emergencies in patients with cancer: rapid fire. Emerg Med Clin North Am. 2018;36(3):493–516. doi: 10.1016/j.emc.2018.04.001. PMID: 30037437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sodhi JS, Raja W, Zargar SA, Showkat, A., Parveen, S., Nisar, S., Wani, M A., Javid, G., Khan, M., Aejaz, S., et al. The efficacy of accelerated, multiple, double-dose hepatitis B vaccine against hepatitis B virus infection in cancer patients receiving chemotherapy. Indian J Gastroenterol. 2015;34(5):372–79. doi: 10.1007/s12664-015-0595-y. PMID: 26531066. [DOI] [PubMed] [Google Scholar]
- 70.Leung TF, Li CK, Hung ECW, Chan, Paul K. S., Mo, C-W., Wong, Raymond P. O., Chik, K-W.. Immunogenicity of a two‐dose regime of varicella vaccine in children with cancers. Eur J Haematol. 2015;72(5):353–57. doi: 10.1111/j.1600-0609.2004.00216.x. PMID: 15059071. [DOI] [PubMed] [Google Scholar]
- 71.Alexander KE, Tong PL, Macartney K, Beresford, R., Sheppeard, V., Gupta, M.. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection ☆. Vaccine. 2018;36(27):3890–93. doi: 10.1016/j.vaccine.2018.05.078. PMID: 29807711. [DOI] [PubMed] [Google Scholar]
- 72.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. PMID: 25838375. [DOI] [PubMed] [Google Scholar]
- 73.Ott PA, Hu Z, Keskin DB, Shukla, S A., Sun, J., Bozym, D J., Zhang, W., Luoma, A., Giobbie-Hurder, A., Peter, L., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–21. doi: 10.1038/nature22991. PMID: 28678778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahin U, Derhovanessian E, Miller M, Kloke, B-P., Simon, P., Löwer, M., Bukur, V., Tadmor, A D., Luxemburger, U., Schrörs, B., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–26. doi: 10.1038/nature23003. PMID: 28678784. [DOI] [PubMed] [Google Scholar]
- 75.Low A, Gavriilidis G, Larke N, B-Lajoie, M-R., Drouin, O., Stover, J., Muhe, L., Easterbrook, P.. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(12):1595–603. doi: 10.1093/cid/ciw125. PMID: 26951573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Launay O, Van DDV, Rosenberg AR, Michel, M-L., Piroth, L., Rey, D., Colin de Verdière, N., Slama, L., Martin, K., Lortholary, O., et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. Jama J Am Med Assoc. 2011;305(14):1432–40. doi: 10.1001/jama.2011.351. PMID: 21486976. [DOI] [PubMed] [Google Scholar]
- 77.Abzug MJ, Pelton SI, Song LY, Fenton, T., Levin, M J., Nachman, S A., Borkowsky, W., Rosenblatt, H M., Marcinak, J F., Dieudonne, A., Abrams, E J.et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatric Infect Dis J. 2006;25(10):920–29. doi: 10.1097/01.inf.0000237830.33228.c3. PMID: 17006288. [DOI] [PubMed] [Google Scholar]
- 78.Scott P, Moss WJ, Gilani Z, Low, N.. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Infect Dis. 2011;204(Suppl 1(Supplement 1)):S164–178. doi: 10.1093/infdis/jir071. PMID: 21666158. [DOI] [PubMed] [Google Scholar]
- 79.Belaunzarán-Zamudio PF, García-León ML, Wong-Chew RM, Villasís-Keever, A., Cuellar-Rodríguez, J., Mosqueda-Gómez, J L., Muñoz-Trejo, T., Escobedo, K., Santos, J I., Ruiz-Palacios, G M., et al. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine. 2009;27(50):7059–64. doi: 10.1016/j.vaccine.2009.09.063. PMID: 19799846. [DOI] [PubMed] [Google Scholar]
- 80.Taweesith W, Puthanakit T, Kowitdamrong E, Bunupuradah, T., Wongngam, W., Phasomsap, C., Apornpong, T., Bouko, C., Pancharoen, C.. The immunogenicity and safety of live attenuated varicella-zoster virus vaccine in human immunodeficiency virus-infected children. Pediatric Infect Dis J. 2011;30(4):320–24. doi: 10.1097/INF.0b013e3181fe0868. PMID: 20975615. [DOI] [PubMed] [Google Scholar]
- 81.Shafran S. Live attenuated herpes zoster vaccine for HIV‐infected adults. HIV Med. 2016;17(4):305–10. doi: 10.1111/hiv.12311. PMID: 26315285. [DOI] [PubMed] [Google Scholar]
- 82.Sanjay R, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23(4):740–80. doi: 10.1128/CMR.00048-09. PMID: 20930072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Breukels MA, Zandvoort A, Dobbelsteen GP, van den Muijsenberg, A., Lodewijk, M E., Beurret, M., Klok, P A., Timens, W., Rijkers, G T.. Pneumococcal conjugate vaccines overcome splenic dependency of antibody response to pneumococcal polysaccharides. Infect Immun. 2001;69(12):7583–87. doi: 10.1128/IAI.69.12.7583-7587.2001. PMID: 11705936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Papadatou I, Orthopoulos G, Theodoridou M, Spoulou, V. Long-lasting hyporesponsivenss induced by the 23-valent pneumococcal polysaccharide vaccine (PPV23) in asplenic patients with β-thalassemia major. Vaccine. 2015;33(32):3779–83. doi: 10.1016/j.vaccine.2015.06.100. PMID: 26144903. [DOI] [PubMed] [Google Scholar]
- 85.Rezai MS, Ghaffari J, Mahdavi M, Bahari, A., Ala, S.. Conjugate and 23-valent pneumococcal polysaccharide booster vaccination in asplenic patients with thalassemia major: a randomized clinical trial study. Caspian J Intern Med. 2017;8(1):16–22. PMID: 28503278. [PMC free article] [PubMed] [Google Scholar]
- 86.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children — use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:(RR-11):1–18. PMID: 21150868. [PubMed] [Google Scholar]
- 87.Bennett NM, Whitney CG, Moore M, Pilishvili T, Dooling KL. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). Am J Transplant. 2013;13(1):232–35. doi: 10.1111/ajt.12073. PMID: 23051612. [DOI] [Google Scholar]
- 88.Meerveld-Eggink A, de Weerdt O, van Velzen-Blad H, Biesma, D H., Rijkers, G T.. Response to conjugate pneumococcal and Haemophilus influenzae type b vaccines in asplenic patients. Vaccine. 2011;29(4):675–80. doi: 10.1016/j.vaccine.2010.11.034. PMID: 21115060. [DOI] [PubMed] [Google Scholar]
- 89.Mikoluc B, Motkowski R, Käyhty H, Heropolitanska-Pliszka, E., Pietrucha, B., Bernatowska, E.. Antibody response to Haemophilus influenzae type-b conjugate vaccine in children and young adults with congenital asplenia or after undergoing splenectomy. Eur J Clin Microbiol Infect Dis. 2012;31(5):805–09. doi: 10.1007/s10096-011-1378-8. PMID: 21874399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chong PP, Avery RK. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther. 2017;39(8):1581–98. doi: 10.1016/j.clinthera.2017.07.005. PMID: 28751095. [DOI] [PubMed] [Google Scholar]
- 91.Feng Y, Shi X, Shi J, Gao, L., Liu, G., Cheng, Y., Pan, M., Li, C., Wang, J., Guo, X., et al. Immunogenicity, antibody persistence, and safety of the 60 mug hepatitis B vaccine in hemodialysis patients: a multicenter, randomized, double-blind, parallel-controlled trial. Expert Rev Vaccines. 2017;16(10):1045–52. doi: 10.1080/14760584.2017.1367667. PMID: 28803502. [DOI] [PubMed] [Google Scholar]
- 92.Kong NCT, Beran J, Kee SA, Miguel, J L., Sánchez, C, Bayas, J-M., Vilella, A, Calbo-Torrecillas, F, López de Novales, E, Srinivasa, K, Stoffel, M, et al. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int. 2008;73(7):856–62. doi: 10.1038/sj.ki.5002725. PMID: 18160963. [DOI] [PubMed] [Google Scholar]
- 93.Tong NKC, Jiri B, Swee Ann K, Miguel, J L., Sánchez, C., Bayas, J M., Vilella, A, de Juanes, J R., Arrazola, P, Calbo-Torrecillas, F., et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68(5):2298–303. doi: 10.1111/j.1523-1755.2005.00689.x. PMID: 16221232. [DOI] [PubMed] [Google Scholar]
- 94.Liao Z, Xu X, Liang Y, Xiong, Y., Chen, R., Ni, J.. Effect of a booster dose of influenza vaccine in patients with hemodialysis, peritoneal dialysis and renal transplant recipients: a systematic literature review and meta-analysis. Hum Vaccin. 2016;12(11):2909–15. doi: 10.1080/21645515.2016.1201623. PMID: 27392026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Collins FS, Harold V. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–95. doi: 10.1056/NEJMp1500523. PMID: 25635347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varghese L, Curran D, Bunge E, Vroling, H, van Kessel, F, Guignard, A, Casabona, G, Olivieri, A. Contraindication of live vaccines in immunocompromised patients: an estimate of the number of affected people in the USA and the UK. Public Health. 2017;142:46–49. doi: 10.1016/j.puhe.2016.10.013. PMID: 28057197. [DOI] [PubMed] [Google Scholar]
- 97.Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR–7):1–11. PMID: 12437192. [PubMed] [Google Scholar]
- 98.Christian H. Influence of parenteral administration routes and additional factors on vaccine safety and immunogenicity: a review of recent literature. Expert Rev Vaccines. 2014;13(3):399–415. doi: 10.1586/14760584.2014.883285. PMID: 24512188. [DOI] [PubMed] [Google Scholar]
- 99.Lindstrom V, Aittoniemi J, Salmenniemi U, Kayhty H, Huhtala H, Sinisalo M, Antibody response to the 23-valent pneumococcal polysaccharide vaccine after conjugate vaccine in patients with chronic lymphocytic leukemia. Hum Vaccin Immunother. 2019;1–4. doi: 10.1080/21645515.2019.1627160. PMID: 31216225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention . Recommended child and adolescent immunization schedule for ages 18 years or younger, United States. 2019. [accessed 2019 February 5]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
- Centers for Disease Control and Prevention . Recommended adult immunization schedule for ages 19 years or older, United States. 2019. [accessed 2019 February 5]. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html


