ABSTRACT
The long-term persistence of hepatitis B surface antibody (anti-HBs) after hepatitis B vaccination among adults was not known clearly. This study aimed to assess the immunogenicity and persistence of antibodies 8 years after hepatitis B immunization with different vaccination schedules among adults who tested negative for hepatitis B surface antigen (HBsAg), anti-HBs, and hepatitis B core antibody (anti-HBc). A total of 771 participants who received the full vaccination course (three doses) and also had a blood sample taken 1 month after the first vaccination were recruited. Of these, 529 were excluded due to the missing data of anti-HBs 8 years after the first vaccination. Vaccinations were carried out at 0–1–3, 0–1–6 and 0–1–12 month vaccination schedules, and 104, 45, and 93 participants were included, respectively. The positive seroprotection rate was 85.9% 1 month after the third vaccination, and 58.3% 8 years later (χ2 = 54.52, P < .001), while the geometric mean titer (GMT) of anti-HBs was 158.49 mIU/mL [95% confidence interval (CI): 131.83–190.55)] and 15.14 mIU/mL (95% CI: 10.96–20.42) after 1 month and 8 years, respectively. Compared with the standard 0–1–6 month vaccination schedule, the positive seroprotection rate and the GMT of the 0–1–3 month vaccination schedule had no difference. The long-term immune effect of the 0–1–3 month vaccination schedule was better than that of the 0–1–12 month vaccination schedule. No correlation was found between the GMT of anti-HBs 1 month and 8 years later.
KEYWORDS: Hepatitis B virus, vaccine, immune effect, adult, immunogenicity
Introduction
Hepatitis B vaccination is still the most economical and effective measure to prevent and control hepatitis B infection. Hepatitis B vaccination of newborns was integrated into the national immunization program of China in 1992, and newborns were encouraged to be vaccinated at their own expense. In 2002, all newborn vaccinations in China were free of charge. Following the introduction of universal hepatitis B vaccination for all infants, countries such as Malaysia,1 Thailand,2 and China have registered a marked decrease in HBsAg seroprevalence in children up to 18 years of age.
In the present study, the prevalence of HBsAg among participants aged 15–59 years was 8.12%, which confirms that HBV infection is endemic in the Chinese population. Therefore, hepatitis B vaccination in adults to prevent HBV infection is especially important.3 Three doses of 20 µg recombinant yeast hepatitis B vaccine or 20 µg Chinese hamster ovary (CHO) recombinant hepatitis B vaccine are recommended for adults. Previous studies have shown that the immune response to the hepatitis B vaccine depends on the age of the recipient, which is worse in adults than in newborns.4–6
The anti-HBs level declines progressively with time after a primary hepatitis B immunization schedule.7 Hence, many investigators have studied the positive seroprotection rate and persistence of anti-HBs after vaccination with different schedules and different kinds of hepatitis B vaccines.6,8,9 However, the duration of immune response after hepatitis B vaccination in healthy adults is not known clearly. The standard vaccination schedule for hepatitis B vaccine is 0–1–6 month, and many adults refuse to receive the second or third dose owing to occupational reasons. The time span of each dose may influence the compliance of adult vaccination, thereby modulating the effect of immunization.
This study aimed to assess the immunogenicity and persistence of antibodies 8 years after the primary hepatitis B immunization with different vaccination schedules (0–1–3, 0–1–6, and 0–1–12) among adults who tested negative for HBsAg, anti-HBs, and anti-HBc.
Materials and methods
Study procedures
This study was carried out in Wuxing County in Zhejiang Province. Participants aged 15–50 years were recruited and asked to complete a questionnaire that included information about gender, date of birth, HBV vaccination history, telephone number, and home address. All of these participates were tested for HBsAg, anti-HBs, and anti-HBc. Originally, the study recruited 771 participants whose HBsAg, anti-HBs, and anti-HBc levels were negative, and who had received the full vaccination course (three doses) and also had a blood sample taken 1 month after the first vaccination.
All participants were vaccinated with a recombinant hepatitis B vaccine (dosage 10μg, 20090521, Kangtai Biotech, Shenzhen, China). Vaccinations were carried out at the 0–1–3, 0–1–6 and 0–1–12 months vaccination schedules by different townships. Of these 771 participants, 529 participants were unavailable to follow-up, 242 had a blood sample taken in November 2018, 8 years after the first vaccination. Further, 3 mL of blood samples were collected from each participant 1 month and 8 years after the third vaccination and preserved for further testing. All participants were still anti-HBc negative 8 years later.
The exclusion criteria were as follows: organ transplantation, renal dialysis, participants with hepatitis C and acquired immune deficiency syndrome, vaccination contraindication, and previous vaccination against HBV. All study participants provided signed informed consent according to the study protocol and were willing to receive the HBV vaccine.
Laboratory testing
Frozen separated serum samples were sent to Adicon Clinical Laboratories Inc. in Hang Zhou for HBsAg, anti-HBs, and anti-HBc quantification by chemiluminescence microparticle immunoassay (CMIA). An Architect-i2000SR (Abbott, USA) analyzer was used to perform the CMIA. The following signal-to-noise ratios were considered to indicate positivity: HBsAg ≥ 0.05 IU/mL and anti-HBc levels ≥1 S/CO. A previous study suggested that anti-HBs levels of 2–9.9 mIU/mL could not be considered negative in participants with a history of vaccination or resolved infection.10 In the present study, those who had been vaccinated against HBV were excluded. Hence, the level of anti-HBs ≥10 mIU/mL was considered positive and defined as having a protective effect against HBV infection. A low response was defined as follows: 10 mIU/mL ≤ anti-HBs < 100 mIU/mL. A normal response was defined as follows: 100 mIU/mL ≤ anti-HBs <1000 mIU/mL. A high response was defined as follows: anti-HBs ≥ 1000 mIU/mL after the third dose of vaccine.11
Statistical evaluation
Statistical analyzes were performed using SPSS version 19.0 (SPSS Inc. IL, USA). Continuous variables were expressed as means ± standard deviation, and categorical variables as frequencies and proportions.
Comparisons between groups were made using the chi-square test, t test, or one-way analysis of variance. Correlations between GMTs of anti-HBs 1 month and 8 years after vaccination were assessed using the Pearson’s correlation coefficient. A P value of 0.05 or less was considered to indicate a statistically significant difference.
Results
Baseline characteristics
A total of 2100 participants aged from 15 to 50 year were initially enrolled in screening and 771 participants were finally included. A total of 1858 were excluded: 93 participants were positive for HBsAg, 260 participants were positive for anti-HBs, 801 participants were positive for anti-HBc, 90 participants were not sure about their vaccination history, 85 participants were not taken blood one month after vaccination. Finally, this study recruited 771 participants, who were negative for HBsAg, anti-HBs, and anti-HBc, and who had received the full vaccination course (three doses) and also had a blood sample taken 1 month after the first vaccination. Of these, 529 participants were excluded as their anti-HBs data 8 years after the first vaccination were missing (Figure 1).
Figure 1.
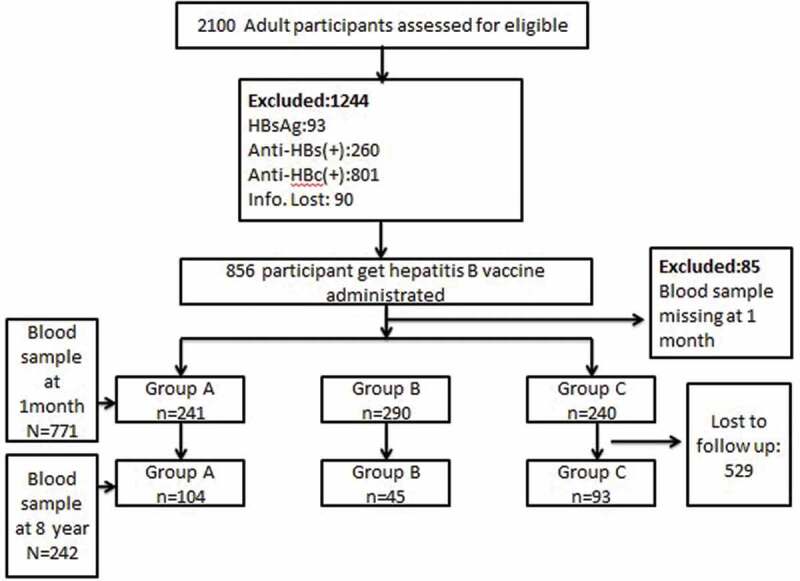
Study flow chart. Participant disposition (Group I: vaccinated at the 0–1–3 months schedule; Group II: vaccinated at the 0–1–6 months schedule; Group III: vaccinated at the 0–1–12 months).
The mean age of the participants at the time of enrollment was 32.44 ± 8.27 years (range: 15–50 years). Participants were divided into 2 groups based on whether they were in the cohort study. After 8 years follow-up, 242 participants could still be contacted, including 87 males and 155 females, with an average 32.62 ± 8.15 years. In addition, 429 participants were lost to follow-up, including 225 males and 304 females, with an average age of 32.36 ± 8.32years. The difference was not statistically significant (χ2 = 2.986, P = .084; t = 0.398, P = .691).
According to the immunization procedures, the participants in the followed cohort were divided into three groups: group I (vaccinated at 0–1–3 months, n = 241), group II (vaccinated at 0–1–6 months, n = 290), and group III (vaccinated at 0–1–12 months, n = 240). The sex and age had no significant differences among the three groups (χ2 = 0.065, P = .968; F = 1.945, P = .144). Details see Table 1.
Table 1.
Basic information about the three different schedules.
| Followed Cohort |
Lost to follow-up |
P-Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Groupa | N | n | Age (year) | Male (%) | n | Age (year) | Male (%) | Age | Male |
| Group I | 241 | 104 | 31.35 ± 7.90 | 44 (42.3) | 137 | 32.06 ± 7.90 | 53 (38.7) | 0.508 | 0.570 |
| Group II | 290 | 45 | 32.35 ± 8.15 | 13 (28.9) | 245 | 32.35 ± 8.34 | 106 (43.3) | 0.998 | 0.072 |
| Group III | 240 | 93 | 34.15 ± 8.20 | 30 (32.3) | 147 | 32.65 ± 8.23 | 66 (44.9) | 0.168 | 0.051 |
| Total | 771 | 242 | 32.62 ± 8.15 | 87 (36.0) | 529 | 32.36 ± 8.32 | 225 (42.5) | 0.691 | 0.084 |
| Parameter | F = 2.978b | χ2 = 3.351c | F = 0.175b | χ2 = 1.220c | |||||
| P value | P = .053 | P = .190 | P = .840 | P = .543 | |||||
aParticipants were assigned to one of the three groups depending on the vaccination schedule chosen. Group I: vaccinated at 0–1–3 months; group II: vaccinated at 0–1–6 months; group III: vaccinated at 0–1–12 months.
bThe variance analysis was used to compare age among the three groups.
cThe Pearson chi-square test was used to compare sex among the three groups.
Immune response to the hepatitis B vaccine 1 month and 8 years later
The positive seroprotection rate was 85.9% 1 month after the third vaccination and 58.3% 8 years later (χ2 = 54.52, P < .001). The anti-HBs GMT of participants was 158.49 mIU/mL [95% confidence interval (CI): 131.83–190.55] 1 month after the third vaccination, compared with 15.14 mIU/mL (95% CI: 10.96–20.42) 8 years later. The difference was statistically significant (t = 6.699, P < .001).
Comparison of positive seroprotection rates among different immunization procedures
According to immunization procedures, the anti-HBs positive seroprotection rate of group I, group II, and group III 1 month later was 77.6%, 86.9%, and 92.9% compared with 68.3%, 57.8%, and 47.3% 8 years later, respectively (Table 2). Significant differences were found among the three groups both 1 month (χ2 = 23.670, P < .001) and 8 years later (χ2 = 8.873, P = .012).
Table 2.
Comparison of the positive seroprotection rate 1 month and 8 years later with different schedules.
| Groupa | N | n1 | PSR1 (%) | χ2 | P | N | n2 | PSR2 (%) | χ2 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Group I | 241 | 77.6 | 23.670 | 0.000 | 104 | 71 | 68.3 | 8.873 | 0.012 | |
| Group II | 290 | 86.9c | 45 | 26 | 57.8 | |||||
| Group III | 240 | 92.9b | 93 | 44 | 47.3b |
N, Total number; n1, number of positive seroprotection 1 month after vaccination; PSR, positive seroprotection rate; n2, number of positive seroprotection 8 years later.
The chi-square test (after Bonferroni adjustment) was used for multiple comparisonsof the PSR among the three groups.
aParticipants were assigned to one of the three groups depending on the vaccination schedule chosen. Group I: vaccinated at 0–1–3 months; group II: vaccinated at 0–1–6 months; group III: vaccinated at 0–1–12 months.
bP < 0.05, compared with Group I.
cP < 0.05, compared with Group I.
After multiple comparisons (after Bonferroni adjustment), the group III and group II seroprotection rates was significantly higher than that of group I 1 month later (P < .05), and the difference was not statistically significant between group I and II (P > .05). While the group I seroprotection rate was significantly higher than that of group III 8 years later (P < .05). No statistically significant difference was found between groups I and II and groups II and III (P > .05).
Comparison of the GMTs among different immunization procedures
Significant differences in the GMTs were found among the three groups 1 month (F = 55.26, P < .001) and 8 years later (F = 3.61, P = .029). Further, there was significant difference among the three groups respectively (P < .001). The GMT of group I was significantly higher than that of group III (P = .030) 8 years later. No statistically significant differences were found between groups I and II (P = .795) and groups II and III (P = .362) 8 years later (Table 3).
Table 3.
Comparison of the GMT of anti-HBs 1 month and 8 years later with different schedules.
| Group | N | GMT1 (95% CI) (mIU/mL) | F# | P | N | GMT2 (95% CI) (mIU/mL) | F# | P |
|---|---|---|---|---|---|---|---|---|
| Group I | 241 | 51.29 (38.02–67.61) | 55.26 | <0.001 | 104 | 22.91 (14.45–36.31) | 3.61 | 0.029 |
| Group II | 290 | 165.96 (125.89–218.78)a | 45 | 16.98 (8.51–33.88) | ||||
| Group III | 240 | 478.63 (354.81–630.96)ab | 93 | 8.91 (5.25–15.49)a |
N, Total number; GMT, the geometric mean titer of anti-HBs; GMT 1, GMT 1 month later; GMT 2, GMT 8 years later.
#The one-way analysis of variance was used to compare the GMT among the three groups and the leastsignificant difference (LSD) was used for multiple comparisons.
aP < 0.05, compared with Group I.
bP < 0.05, compared with Group II
Correlations of anti-HBs GMT levels 1 month and 8 years later
The distribution of individual antibody titers revealed that overall 58.3% of participants retained protective antibody levels (>10 mIU/mL), of which 43.3% showed normal or high levels of antibodies (>100 mIU/mL) 8 years later compared with 85.86% and 59.22% 1 month after vaccination, respectively. Then, the adults in the followed cohort study were categorized according to the levels of anti-HBs at 1 month (Table 4), and the positive seroprotection rates of different levels of anti-HBs 8 years later were compared. However, no statistically significant differences were observed among the three groups (χ2 = 4.200, P = .241). The Spearman rank correlation analysis showed no correlation between the anti-HBs levels of participants 1 month and 8 years later (r = – 0.08, P = .219).
Table 4.
Correlations between anti-HBs GMTs of anti-HBs 1 month and 8 years later.
| Group | N | n (%) | χ2 | P |
|---|---|---|---|---|
| Low response | 65 | 44 (67.7) | 4.200 | 0.241 |
| Normal response | 81 | 43 (53.1) | ||
| High response | 64 | 34 (53.1) |
N, Number of different GMTs for anti-HBs 1 month after vaccination; n, number of anti-HBs >10 mIU/mL 8 years later in N.
Discussion
The participants who were vaccinated against hepatitis B were excluded in the present study. Whether adolescents or adults should receive a booster dose of hepatitis B were controversial.8,12–16 Some experts suggested that hepatitis B vaccine boosters might be required for adolescents or adults who lacked protective levels of anti-HBs following a primary series of hepatitis B vaccination in infancy or lived in middle- or high-HBV-endemic areas where opportunities for HBV exposure existed in the household and community.17 Some studies evaluated the persistence of antibodies after hepatitis B booster administration. The anti-HBs positive rate during the 5-year follow-up was 73.06%, 73.61%, 66.16%, and 55.90%, while the GMT was 35.84 mlU/mL (95% CI: 32.80–40.04), 34.40 mlU/mL (95% CI: 29.79–38.90), 27.70 mIU/mL (95% CI: 23.13–33.18), and 15.03 mIU/mL (95% CI: 12.22–18.49), respectively, in the high-response, normal-response, low-response, and no-response groups.18 In the present study, 8 years later, compared with adults who were negative for HBsAg, anti-HBs, and anti-HBc, 58.3% adults had protective levels of anti-HBs with a GMT of 15.14 mIU/mL 8 years after a primary series of hepatitis B immunization.
Similar to previous studies, the anti-HBs levels declined progressively with time after a primary hepatitis B immunization schedule. Some studies suggested that vaccine-induced immunity might persist for up to 15–20 years or even longer after a complete series,14,16 followed by a gradual decline in the anti-HBs level year by year. The anti-HBs positive seroprotection rate in the children who developed protective anti-HBs level after the primary hepatitis B vaccination series dropped from 99% (1 year after vaccination) to 83% (5 years after vaccination), 71% (7 years after vaccination), and 37% (15–17 years after vaccination).19 The studies on adults showed that the anti-HBs positive seroprotection rate was about 89.5%–95% 1 month after vaccination,6 65%–80% 1 year after vaccination, and 55%–75% 5 years after vaccination with different vaccination schedules or different kinds of vaccines.20 One recent study showed sustained immune memory and long-term protection 20–30 years after a complete primary hepatitis B vaccination course during adulthood.21 The results were similar in some ways to the viewpoint that the immune response of adults to the hepatitis B vaccine was worse than that of newborns.
The persistence of anti-HBs ≥10 mIU/mL may be related to vaccine dosage, beginning of the primary vaccine regime (at birth or later in infancy timing of the last vaccination of the primary series, that is, the time gap between the last and preceding doses), and use of plasma-derived or recombinant vaccines.22 In the present study, three different immunization procedures were chosen: 0–1–3, 0–1–6, and 0–1–12 month vaccination schedules. Some previous studies showed that the vaccination of hepatitis B with both 0–1–6 and 0–1–12 month schedules in adults resulted in better immune responses 1 month after they received three doses of vaccination.23,24 Other studies addressed this issue and similar conclusions.25 This was also similar to the findings of the present study. However, a few studies examined the long-term effect of different immunization procedures after hepatitis B vaccination in adults. A recent meta-analysis of infants confirmed that after mutual adjustment, a time gap between the last and preceding doses of the primary vaccine series of fewer than 6 months was related to a lower proportion of anti-HBs ≥10 mIU/mL.22 For infants, this might be explained by a more mature immune system in older infants. The conclusion of the present study differed from that of Katharina’s meta-analysis owing to the following reasons. First, this difference might be due to different study populations. Second, the accelerated schedule (0–1–3 months) and the standard schedule (0–1–6 months) enhanced the immune response. However, the long gap between the preceding and last doses of the 0–1–12 months vaccination schedule might serve as a booster and stimulate a high level of anti-HBs but low immune memory compared with the 0–1–3 simple immunization schedule. This novel study examined the long-term immune effect of three different schedules of hepatitis B vaccination on serologically negative adults during a follow-up period of 8 years. Therefore, the comparative results for the best schedule could not be obtained from previous studies.
It is generally assumed that the higher the anti-HBs levels after vaccination, the longer the period of protection. Xue-en Liu found that the participants with pre-boost anti-HBs levels >0.7 mIU/mL had a higher probability of achieving the immune response (anti-HBs ≥10 mIU/mL) to the booster dose of 20 ug hepatitis B vaccine compared with those with anti-HBs levels <0.7 mIU/mL (OR = 8.071, 95% CI: 2.131–30.571, P = .002).17 Koen Van Herck evaluated the persistence of antibodies 8 years after hepatitis B booster administration in healthy adult volunteers who were vaccinated according to a 0–l–2 month vaccination schedule, with a booster dose after 12 months. A strong positive correlation (r = 0.83) was found between the anti-HBs levels after 13 and 96 months.26 Unlike their study, the participants in the present study had negative HBsAg, anti-HBs, and anti-HBc levels and were never vaccinated before. No similar studies were reported to confirm the result. Hence, future studies should include more counties of Zhejiang province to validate the findings.
The present study had some shortcomings. (1) The high loss to follow-up caused by the floating population may influence the reliability and the sample size of this study. (2) The relatively small sample size in the present study might not be adequate to support the results of the inferential statistical analyses. (3) For there were no information of the participants’ status of body-mass index (BMI), smoking, drinking, chronic disease, etc, which might affect antibody response after hepatitis B vaccination.
In conclusion, adults who did not receive the hepatitis B vaccine in infancy and had negative serological markers of hepatitis B needed vaccination. The positive rates and anti-HBs levels declined progressively with time after a primary hepatitis B immunization. The anti-HBs positive seroprotection rate was 58.3%, and the GMT was 15.14 mIU/mL (95% CI: 10.96–20.42) 8 years after vaccination. Compared with the standard 0–1–6 month vaccination schedule, the anti-HBs positive seroprotection rate and the GMT of the 0–1–3 month vaccination schedule had no difference. The long-term immune effect of the 0–1–3 month vaccination schedule was better than that of the 0–1–12 month vaccination schedule. No correlation was found between the GMT of anti-HBs 1 month and 8 years later. Additional studies are needed to estimate how long the protection lasts in adults. The present study should be repeated in different cohorts to validate the findings, and the mechanism should be further studied.
Funding Statement
This study was supported by the National Scientific and Technological Major Project of China (No. 2018ZX10715014, No. 2017ZX10105001, No. 2011ZX10004-901, No. 2013ZX10004-904, and No. 2014ZX10004008.
Acknowledgments
The authors thank the Wu Xing Center for Disease Control and Prevention and other relevant personnel for their contributions to this study.
Disclosure of potential conflicts of interest
The authors declared no potential conflicts of interest.
References
- 1.Ng KP, Saw TL, Baki A, Rozainah K, Pang KW, Ramanathan M.. Impact of the expanded program of immunization against hepatitis B infection in school children in Malaysia. Med Microbiol Immunol. 2005;194:163–68. PMID: 15834754. doi: 10.1007/s00430-004-0231-4. [DOI] [PubMed] [Google Scholar]
- 2.Chongsrisawat V, Yoocharoen P, Theamboonlers A, Tharmaphornpilas P, Warinsathien P, Sinlaparatsamee S, Paupunwatana S, Chaiear K, Khwanjaipanich S, Poovorawan Y. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop Med Int Health. 2006;11:1496–502. PMID: 17002723. doi: 10.1111/j.1365-3156.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y A survey report on serum epidemiology of Hepatitis B virus in Chinese population [M].Beijing (China): People’s Medical Publishing House. 2011. April 13–15. doi: 10.1094/PDIS-01-11-0064 [DOI] [Google Scholar]
- 4.Kishino H, Takahashi K, Sawata M, Tanaka Y. Immunogenicity, safety, and tolerability of a recombinant hepatitis B vaccine manufactured by a modified process in healthy young Japanese adults. Hum Vaccin Immunother. 2018;14:1773–78. PMID: 29553862. doi: 10.1080/21645515.2018.1452578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert CL, Klopfer SO, Martin JC, Schodel FP, Bhuyan PK. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy adults >/=50 years. Hum Vaccin. 2011;7:1336–42. PMID: 22185811. doi: 10.4161/hv.7.12.18333. [DOI] [PubMed] [Google Scholar]
- 6.Ren JJ, Dai XW, Jiang ZG, Shen LZ, Chen YD, Li Q, Ren W, Liu Y, Yao J, Li L-J. Immunological effects of a 10-mug dose of domestic hepatitis B vaccine in adults. J Zhejiang Univ Sci B. 2012;13:948–54. PMID: 23125088. doi: 10.1631/jzus.B1200179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Der Meeren O, Behre U, Crasta P. Immunity to hepatitis B persists in adolescents 15–16 years of age vaccinated in infancy with three doses of hepatitis B. Vaccine Vaccine. 2016;34:2745–49. PMID: 27095043. doi: 10.1016/j.vaccine.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood S, Shah KU, Khan TM. Immune persistence after infant hepatitis-B vaccination: a systematic review and meta-analysis. Sci Rep. 2018;8:12550. PMID: 30135554. doi: 10.1038/s41598-018-30512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28:730–36. PMID: 19892043. doi: 10.1016/j.vaccine.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 10.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–96. PMID: 19785526. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 11.Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010;11:232–38. PMID: 20237496. doi: 10.1038/gene.2010.1. [DOI] [PubMed] [Google Scholar]
- 12.Li H, GJ L, Chen QY, Fang ZL, Wang XY, Tan C, Yang QL, Wang FZ, Wang F, Zhang S, et al. Long-term effectiveness of plasma-derived hepatitis B vaccine 22-28 years after immunization in a hepatitis B virus endemic rural area: is an adult booster dose needed. Epidemiol Infect. 2017;145:887–94. PMID: 28065199. doi: 10.1017/S0950268816003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine. 2010;28:623–31. PMID: 19887132. doi: 10.1016/j.vaccine.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 14.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. PMID: 21653306. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 15.Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8:e58029. PMID: 23533578. doi: 10.1371/journal.pone.0058029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35:6302–07. PMID: 28988867. doi: 10.1016/j.vaccine.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZZ, Gao YH, Lu W, Jin CD, Zeng Y, Yan L, Ding F, Li T, Liu X-E, Zhuang H. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccin Immunother. 2017;13:909–15. PMID: 27874311. doi: 10.1080/21645515.2016.1250990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao CZ. Antibody levels and immune memory persistence after primary vaccination with three doses hepatitis B vaccine among adults: a 5-year follow—up study [D]. Shandong (China): Shandong University;2016. (In Chinese). doi: 10.7666/d.Y3036812. [DOI] [Google Scholar]
- 19.Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis. 2003;187:134–38. PMID: 12508157. doi: 10.1086/345871. [DOI] [PubMed] [Google Scholar]
- 20.Chen YZ, Jiang RJ, Shen JJ. Observation on the Anti-HBs of different genetic engineering hepatitis B vaccine in adults. Chin J Vaccines Immunization. 2007;13:320–23.In Chinese [Google Scholar]
- 21.Van Damme P, Dionne M, Leroux-Roels G, Van Der Meeren O, Di PE, Salaun B, Surya Kiran P, Folschweiller N. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat . 2019;26:1066–75. PMID: 31087382. doi: 10.1111/jvh.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of Long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32:307–13. PMID: 23249904. doi: 10.1097/INF.0b013e31827bd1b0. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Yao J, Shan H, Chen Y, Jiang ZG, Ren JJ, Xu K-J, Ruan B, Yang S-G, Wang B, et al. Comparison of the effect of two different doses of recombinant hepatitis B vaccine on immunogenicity in healthy adults. Hum Vaccin Immunother. 2015;11:1108–13. PMID: 25607773. doi: 10.4161/21645515.2014.988547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao J, Li J, Chen Y, Shan H, Dai XW, Yang LN, Jiang Z-G, Ren -J-J, Xu K-J, Ruan B, et al. The response of hepatitis B vaccination on seronegative adults with different vaccination schedules. Hum Vaccin Immunother. 2015;11:1102–07. PMID: 25621975. doi: 10.4161/21645515.2014.985500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girisha KM, Kamat JR, Nataraj G. Immunological response to two hepatitis B vaccines administered in two different schedules. Indian J Pediatr. 2006;73:489–91. PMID: 16816509. doi: 10.1007/BF02759892. [DOI] [PubMed] [Google Scholar]
- 26.Van Herck K, Van Damme P, Thoelen S, Meheus A. Long-term persistence of anti-HBs after vaccination with a recombinant DNA yeast-derived hepatitis B vaccine: 8-year results. Vaccine. 1998;16:1933–35. doi: 10.1016/S0264-410X(98)00126-1. [DOI] [PubMed] [Google Scholar]


