ABSTRACT
The development of more effective vaccines against Mycobacterium tuberculosis has become a world priority. Previously, we have shown that a recombinant BCG expressing the LTAK63 adjuvant (rBCG-LTAK63) displayed higher protection than BCG against tuberculosis challenge in mice. In order to elucidate the immune effector mechanisms induced by rBCG-LTAK63, we evaluated the immune response before and after challenge. The potential to induce an innate immune response was investigated by intraperitoneal immunization with BCG or rBCG-LTAK63: both displayed increased cellular infiltration in the peritoneum with high numbers of neutrophils at 24 h and macrophages at 7 d. The rBCG-LTAK63-immunized mice displayed increased production of Nitric Oxide at 24 h and Hydrogen Peroxide at 7 d. The number of lymphocytes was higher in the rBCG-LTAK63 group when compared to BCG. Immunophenotyping of lymphocytes showed that rBCG-LTAK63 immunization increased CD4+ and CD8+ T cells. An increased long-term Th1/Th17 cytokine profile was observed 90 d after subcutaneous immunization with rBCG-LTAK63. The evaluation of immune responses at 15 d after challenge showed that rBCG-LTAK63-immunized mice displayed increased TNF-α-secreting CD4+ T cells and multifunctional IL-2+ TNF-α+ CD4+ T cells as compared to BCG-immunized mice. Our results suggest that immunization with rBCG-LTAK63 induces enhanced innate and long-term immune responses as compared to BCG. These results can be correlated with the superior protection induced against TB.
KEYWORDS: Tuberculosis, vaccine, recombinant BCG, innate immunity, long-term immunity
1. Introduction
Tuberculosis (TB) is one of the top 10 leading causes of mortality worldwide and is the most important cause of death due to a single infectious disease. Recent data indicate that Mycobacterium tuberculosis (Mtb) was responsible for at least 1.3 million deaths and 10 million cases in 2017.1 Moreover, there is a rising frequency of multi-, extensively-, and even totally drug-resistant Mtb strains posing the threat of virtually untreatable disease.2 The only available vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), has shown variable results of protective efficacy. BCG vaccination has been shown to prevent tuberculous meningitis and miliary TB in infants.3 However, protection against pulmonary TB in adults is extremely variable, as shown by a meta-analysis that revealed protective efficacies ranging from 0% to 80%.4 Therefore, the development of new therapeutic strategies and more effective vaccines has become a world priority in order to control spread of the infection. New vaccination strategies being investigated against TB include recombinant BCG (rBCG) or attenuated Mtb strains to replace BCG, as well as subunit or non-replicating viral vector-based vaccines as a boost for BCG, and others.5,6
These vaccine strategies were developed to improve the immune responses and protection against Mtb as compared to BCG. There are several rBCG strains overexpressing immunodominant Mtb antigens, such as Ag85A,7 Ag85C,8 ESAT-69 and Hsp-X,10 to enhance the antigen-specific Th1 immune response against Mtb. Another promising strategy generated rBCG ΔureC hly+ (VPM1002), a phagosome escape mutant.11 These rBCG strains have shown increased immunogenicity and/or enhanced levels of protection against Mtb challenge in animal models. Few vaccine candidates have progressed to clinical trials, supporting increased research in vaccine development against TB.12
Although studies on the induction of protective immune responses for TB have advanced, effector mechanisms required for protection have not yet been elucidated. The lack of robust or reliable immune correlates of protection for TB, validated models and translational studies between animal and human models have delayed the development of new vaccine strategies.13 In this sense, elucidation of protective mechanisms would allow more efficient evaluation of vaccine candidates at an early preclinical stage of development and provide a relevant measure of immunogenicity for phase I trials, guiding progression into efficacy trials.14,15
Particularly, the innate immune response is considered crucial for the development of an efficient adaptive immune response.16 Animal studies have revealed the role of adaptive immune responses mediated by CD4+ T cells producing IFN-γ or TNF-α and polyfunctional T cells producing INF-γ, TNF-α and IL-2 in the protection against Mtb infection. This has guided vaccine development during decades.17 The importance of T cell subpopulations in long-term immune responses for TB has been demonstrated, including a possible role for IL-17 in protection.18
We have previously described the development of rBCG strains expressing the nontoxic mutant of E. coli heat labile enterotoxin (LT) as adjuvant. One of our constructs, the rBCG-LTAK63lo strain, here called rBCG-LTAK63, induced higher immunogenicity against mycobacteria in the spleens and lungs of immunized mice. Furthermore, this rBCG strain induced superior protection in different intratracheal Mtb challenge models as compared to native BCG.19 The adjuvant properties of LT have been extensively studied, inducing increased immunogenicity and protective efficacy in several models.20–22 It is reported that LT can induce enhanced production of inflammatory cytokines and chemokines and transient recruitment of immune cells to the site of immunization. It influences dendritic cell maturation, antigen presentation, T cell activation and promotes the induction of antigen-specific cytotoxic T lymphocyte (CTL) responses in mouse models. The genetically detoxified mutant, LTK63, displays different adjuvant properties.23–25 The adjuvant properties of LTAK63 expressed in BCG are under investigation.
We here report increased innate and long-term immune responses induced by rBCG-LTAK63 immunization. Furthermore, the immune responses induced 15 d after challenge were clearly different than those induced by BCG.
2. Materials and methods
2.1. Bacterial strains
The Mycobacterium bovis BCG Moreau strain (Instituto Butantan) was used to generate the recombinant BCG strain; M. tuberculosis H37Rv was used in the challenge experiments and to generate the Mtb extract used in the immunology experiments, as previously described.19 BCG, rBCG-LTAK63 and M. tuberculosis H37Rv strains were grown in Middlebrook 7H9 medium (MB7H9) (Difco, Detroit, MI) supplemented with oleic-albumin-dextrose-catalase broth (OADC) (BBL, Cockeysville, MD) and 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO) (MB7H9/OADC/Tw), containing or not 20 μg/mL kanamycin and incubated at 37ºC with 5% CO2 until cultures reached an optical density (OD) of ~0.8. Bacteria were harvested by centrifugation at 2.800 x g, washed twice with distilled water and resuspended in 10% Glycerol. The mycobacterial preparations were maintained at −80ºC until used. Colony-forming units (CFU) were determined 48 h after freezing at −80°C.
2.2. Animals and immunization
All animal experiments were performed according to Brazilian and international guidelines on animal experimentation and approved by the Ethics Committee of Instituto Butantan, São Paulo-SP (CEUAIB), (Permit number 1202/14). Five to seven-week-old female BALB/c mice were obtained from the University of São Paulo, SP, Brazil. For evaluation of innate immune responses, BCG or rBCG-LTAK63 (1x106 CFU/500 μL) preparations were administered intraperitoneally (i.p.). For evaluation of long-term immune responses and immune response after challenge, mice were immunized subcutaneously (s.c.) with 1 × 106 CFU/100 µL of BCG or rBCG-LTAK63 (Figure 1). Control animals received saline.
Figure 1.
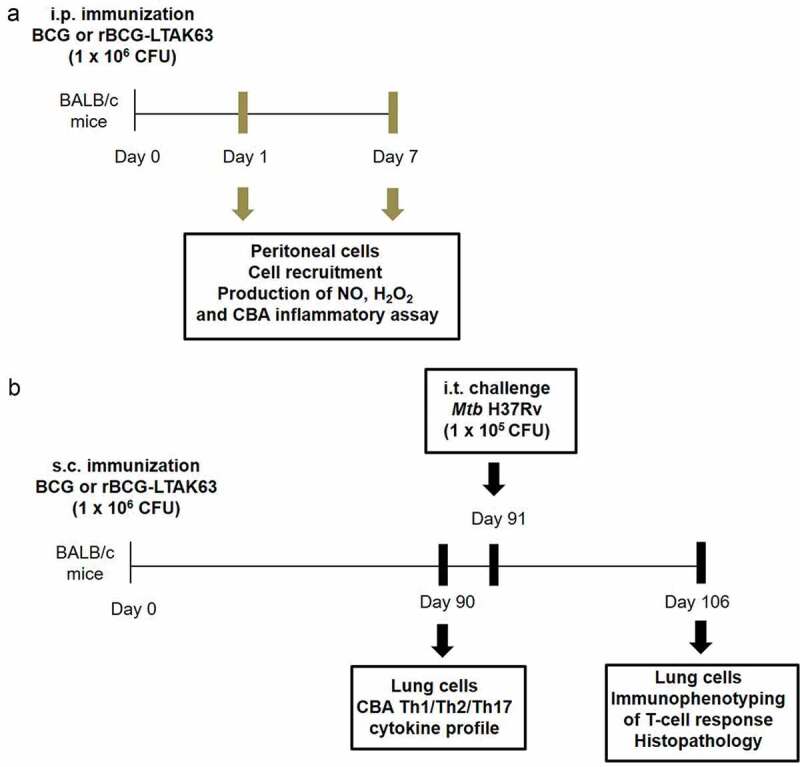
Animals, immunization and challenge schedule.
(a) BALB/c mice were immunized intraperitoneally (i.p.) with BCG or rBCG-LTAK63 (1 x 106 CFU). At 24 h and 7 d, peritoneal cells were recovered for the evaluation of cell recruitment, production of Nitric Oxide (NO), Hydrogen peroxide (H2O2) and inflammatory cytokines (without in vitro stimulation). (b) BALB/c mice were immunized subcutaneously (s.c.) with BCG or rBCG-LTAK63 (1 x 106 CFU). At 90 d after immunization, recovered lung cells were in vitro stimulated with Mtb extract for evaluation of Th1/Th2/Th17 cytokine profile by CBA in the culture supernatant. At day 91 after immunization, the animals were challenged with Mtb H37Rv (1 x 105 CFU) and 15 d after challenge (day 106), recovered lung cells were in vitro stimulated with Mtb extract and analyzed by immunophenotyping for determination of T-cell response (intracellular cytokine staining). Lungs were collected for histopathological analysis.
2.3. Peritoneum cells, differential cell count and flow cytometry
The BCG or rBCG-LTAK63 preparations were administered i.p. to BALB/c mice and the recruited cells were obtained from the peritoneal cavity after 24 h and 7 dby injection of 5 mL of PBS. The total cell number was obtained by staining with crystal violet and counting in a Neubauer chamber. Later, 200 μL of peritoneal cells was placed on a slide and centrifuged at 200 x g at 4°C for 7 min using a cell cytospin apparatus (StatSpin CytoFuge 2, Beckman Colter, Inc). The slides were dried and cells were stained using the Instant Prov staining Kit, according to the manufacturer’s instructions, which allows identification of the different cell types by morphology. The number of neutrophils, macrophages and lymphocytes were obtained by differential cell counting through microscopic analysis and confirmed by immunophenotyping. The antibodies used were CD11b (PerCP-Cy5), CD11c (PE-Cy7), F4/80 (BV421), Ly6G/C (APC-Cy7), MHCII (PE), CD3 (APC-Cy7). For immunophenotyping of the T-cell population, the antibodies used were CD3 (APC-Cy7), CD4 (PE-Cy5), CD8 (PE-Cy7) and CD69 (FITC). The following gating strategy was used: after exclusion of doublets (FSC-H vs FSC-A), the cell population was gated based on size and granularity (SSC-A vs FSC-A) and then gated on distinct populations, macrophages (CD11bpos, F4/80pos, MHCIIpos), neutrophils (CD11bpos, MHCIIneg, CD11cneg, Ly6G/Cpos), T lymphocytes (CD3 pos), CD4 T (CD3 pos, CD4 pos) or CD8 T (CD3 pos, CD8 pos).
2.4. Measurement of NO, H2O2 and inflammatory cytokines
For analysis of Hydrogen peroxide (H2O2) production, peritoneal cells were cultured in Phenol Red solution for 1 h at 37°C with 5% CO2. For analysis of Nitric oxide (NO) and inflammatory cytokine production, peritoneal cells were cultured in RPMI 1640 medium (Life Technologies-Invitrogen) with 10% FBS (Fetal Bovine Serum) for 48 h at 37°C with 5% CO2 (without ex vivo stimulation). H2O2 production was quantified using the method described by Pick and Mizel.26 For H2O2 production, absorbance was measured at 620 nm using a microplate reader (Bio Tek), and concentration was determined using a hydrogen peroxide standard curve (5–40 μM in triplicate). NO production was quantified in cell culture supernatants by the Griess reaction,27 measuring absorbance at 550 nm using a microplate reader (Bio Tek); the concentration was determined using a sodium nitrite standard curve (0–100 μM in triplicate). Cytokine levels were determined using the Cytometric Bead Array (CBA) Mouse Inflammatory Cytokine Kit (BD Biosciences, San Diego, CA).
2.5. Mtb challenge and lung cells isolation for flow cytometry analysis
BALB/c mice were immunized s.c. with a single dose of BCG or rBCG-LTAK63 (1x106 CFU/100 µL) and challenged after 90 dby the intratracheal (i.t) route with M. tuberculosis H37Rv (1 × 105 CFU/100 µL) (Figure 1), as a dose previously established in the laboratory.19,28 Lung cells from BALB/c mice were isolated 3 months after immunization and 15 after challenge as previously described.19 At 90 d after immunization, isolated lung cells were stimulated with 5.0 µg/mL Mtb extract and the Th1/Th2/Th17 cytokine profile was evaluated using CBA Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences, San Diego, CA). Fifteen days after challenge, at day 106, isolated lung cells were stimulated with 5.0 µg/mL Mtb extract for 6 h in the presence of brefeldin A (5.0 µg/mL), for intracellular cytokine staining. The following antibodies were used: CD3 (APC-Cy7), CD4 (PE-Cy5), IL-2 (APC), IFN-γ (FITC), TNF-α (PE) or IL-17 (PE) (BD Bioscience). Cells were analyzed using FACSCanto II and FACSDiva software (BD Biosciences).
2.6. Histopathology
At 15 d after i.t. challenge with Mtb H37Rv, the right caudal lung lobe was perfused with 10% formaldehyde-buffered saline. Serial 4–5 µm sections were stained with HE dye to analyze the tissue alterations. The samples were examined with a Leica microscope (Germany) and images were captured with a Coolpix P995 Nikon camera (Japan). The reduction in the percentage of pulmonary intra-alveolar space was determined as described elsewhere.29 Five random images of each lung HE-stained section (100× magnification) were analyzed using the ImageJ software (National Institutes of Health, USA).
2.7. Statistical analysis
GraphPad Prism (GraphPad Software, Inc.) was used for statistical analysis. The significance of differences among groups was calculated by one-way ANOVA or Student’s t-test as described in the figure legends. Differences between mean values were considered significant when P < .05.
3. Results
3.1. rBCG-LTAK63 increases NO production 24 h after immunization
In order to investigate the potential role of innate immune responses induced by rBCG-LTAK63, mice were immunized i.p. and immigrant cells were isolated from the peritoneal cavity 24 h or 7 d after immunization. The intraperitoneal route is classically used to evaluate innate and/or inflammatory responses since the peritoneal cavity is composed mainly of macrophages.30 The total number of cells increased rapidly in the peritoneum at 24 h after administration of either BCG or rBCG-LTAK63 (Figure 2(a)). The cell population at this timepoint was mainly composed by peritoneal neutrophils, as determined by differential cell counting with Instant Prov staining kit and confirmed by immunophenotyping (Figure 2(a)). No difference was observed in the number of macrophages or lymphocytes.
Figure 2.
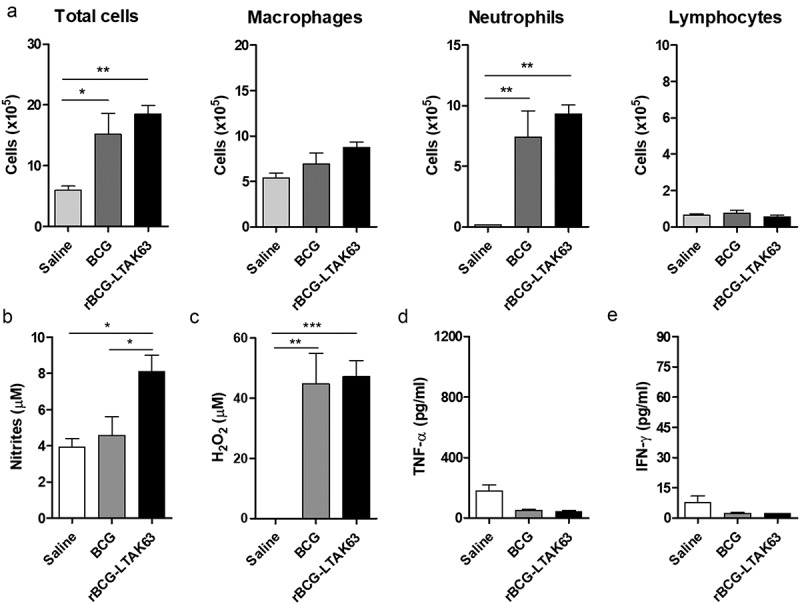
rBCG-LTAK63 increases NO production at 24 h after intraperitoneal immunization.
(a) Analysis of cell population was performed in the peritoneal fluid of BALB/c mice 24 h after intraperitoneal immunization with BCG or rBCG-LTAK63 (1 x 106 CFU). At 24 h after immunization, the production of (b) NO and (c) H2O2 was evaluated in the cells isolated from the peritoneum. The production of (d) TNF-α and (e) IFN-γ was evaluated by the CBA inflammatory assay (non-stimulated ex vivo). The control group received Saline. The results represent one of two independent experiments with similar results (n = 4–5 animals per group). Statistical significance was determined by one-way ANOVA. Significant differences were observed for indicated groups (* P < .05; ** P < .01; *** P < .001).
The production of oxygen/nitrogen reactive species indicates macrophage activation and is important for killing of M. tuberculosis.31 Thus, we evaluated the production of oxygen/nitrogen reactive species and inflammatory cytokines by cells isolated from the peritoneum of immunized mice. An increased production of NO was observed 24 h after immunization with rBCG-LTAK63 as compared to the BCG group (Figure 2(b)). Higher H2O2 concentration was observed in animals immunized with BCG or rBCG-LTAK63 (Figure 2(c)). There was no difference in the levels of the inflammatory cytokines, IFN-γ and TNF-α at 24 h (Figure 2(d, e)).
3.2. rBCG-LTAK63 increases lymphocyte recruitment 7 d after immunization
The total number of cells remained increased in the peritoneum at 7 d after administration of either BCG or rBCG-LTAK63 (Figure 3(a)). The number of neutrophils is still increased, although lower than at 24 h. There was also an increase in the number of macrophages. Most interesting, the number of lymphocytes induced by rBCG-LTAK63 at 7 d was higher than that induced by BCG (Figure 3(a)). Although NO production was not maintained (data not shown), persistent production of H2O2 was observed only in the rBCG-LTAK63 group (Figure 3(b)). At 7 d, high concentrations of IFN-γ were detected in both immunized groups; while higher levels of TNF-α were observed only in the rBCG-LTAK63 group (Figure 3(c,d)).
Figure 3.
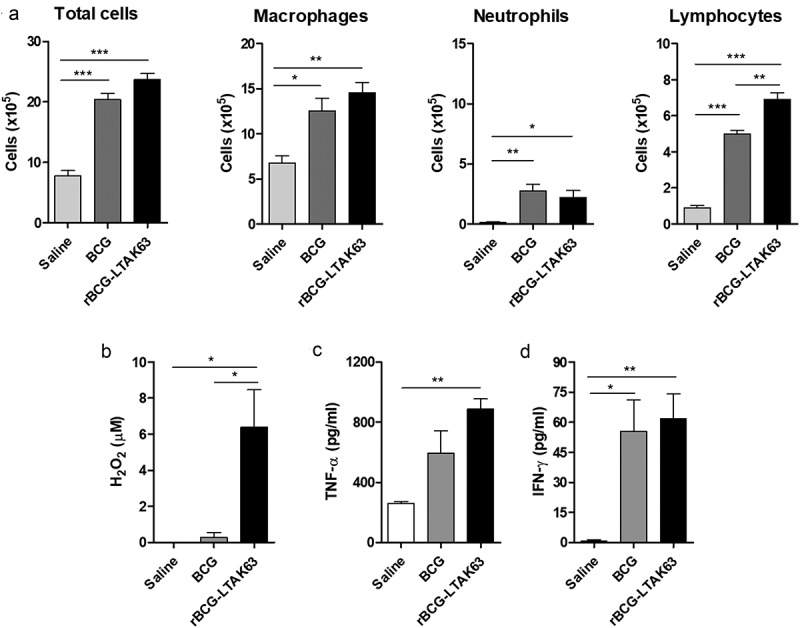
rBCG-LTAK63 increases lymphocyte recruitment at 7 d after intraperitoneal immunization.
(a) Analysis of cell population was performed in the peritoneal fluid of BALB/c mice 7 d after intraperitoneal immunization with BCG or rBCG-LTAK63 (1 x 106 CFU). At 7 d after immunization, the production of (b) H2O2 was evaluated in the cells isolated from the peritoneum. The production of (c) TNF-α and (d) IFN-γ was evaluated by the CBA inflammatory assay (non-stimulated ex vivo). The control group received Saline. The results represent one of two independent experiments with similar results (n = 4–5 animals per group). Statistical significance was determined by one-way ANOVA. Significant differences were observed for indicated groups (* P < .05; ** P < .01; *** P < .001).
3.3. rBCG-LTAK63 increases CD4+ and CD8+ T cell recruitment 7 d after immunization
The higher number of lymphocytes at 7 d led us to investigate which type of T cell population was being induced. Increased CD8+ T cells were observed only in the rBCG-LTAK63-immunized animals (Figure 4(b)), while either BCG or rBCG-LTAK63 showed increased CD4+ T cells when compared to the Saline control. Furthermore, higher numbers of CD4+ T cells were induced in rBCG-LTAK63-immunized animals when compared to BCG (Figure 4(a)).
Figure 4.
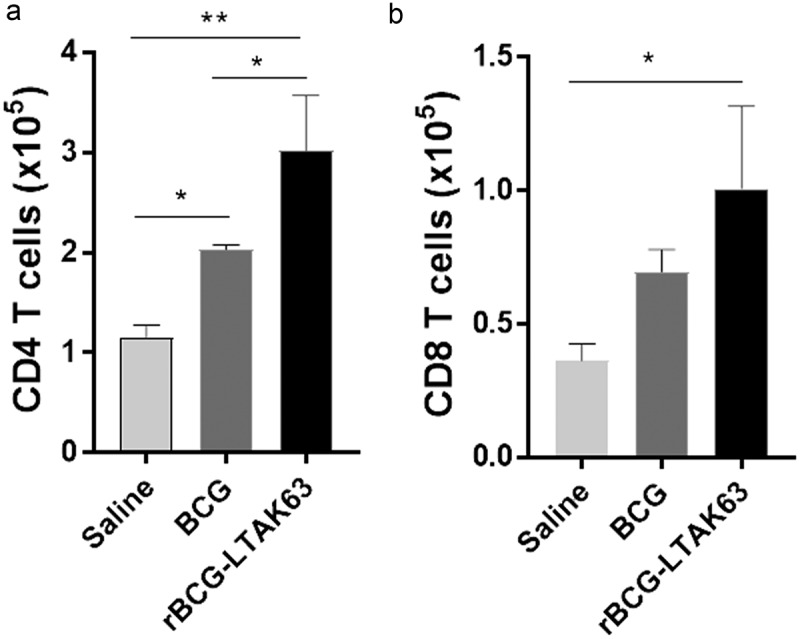
rBCG-LTAK63 increased CD4 and CD8 T cell recruitment at 7 d after intraperitoneal immunization.
Immunophenotyping of (a) CD4 T cell population and (b) CD8 T cell population in the peritoneal fluid of BALB/c mice 7 d after i.p. immunization with BCG or rBCG-LTAK63 (1 x 106 CFU). The control group received Saline. The results represent one of two independent experiments with similar results (n = 4 animals per group). Statistical significance was determined by one-way ANOVA. Significant differences were observed for indicated groups (* P < .05; ** P < .01).
3.4. Increased long-term Th1 and Th17 cytokine profile induced in the lungs by rBCG-LTAK63 immunization
We analyzed the long-term immune responses induced in the lungs of BALB/c mice 90 d after s.c. immunization with BCG or rBCG-LTAK63. Cells isolated from the lungs of rBCG-LTAK63-immunized mice and stimulated with Mtb extract showed significantly higher production of IFN-γ, TNF-α, IL-6, IL-17 and IL-10, as compared to BCG (Figure 5). Significant IL-2 production was detected in animals immunized with both BCG and rBCG-LTAK63, and there was a tendency for higher production of IFN-γ, TNF-α and IL-17 in the BCG group as compared to Saline, although not statistically significant. Thus, immunization with BCG induced a long-term type 1 response and rBCG-LTAK63 induced increased long-term concomitant type 1 and type 17 cytokine responses in the lungs of immunized mice.
Figure 5.
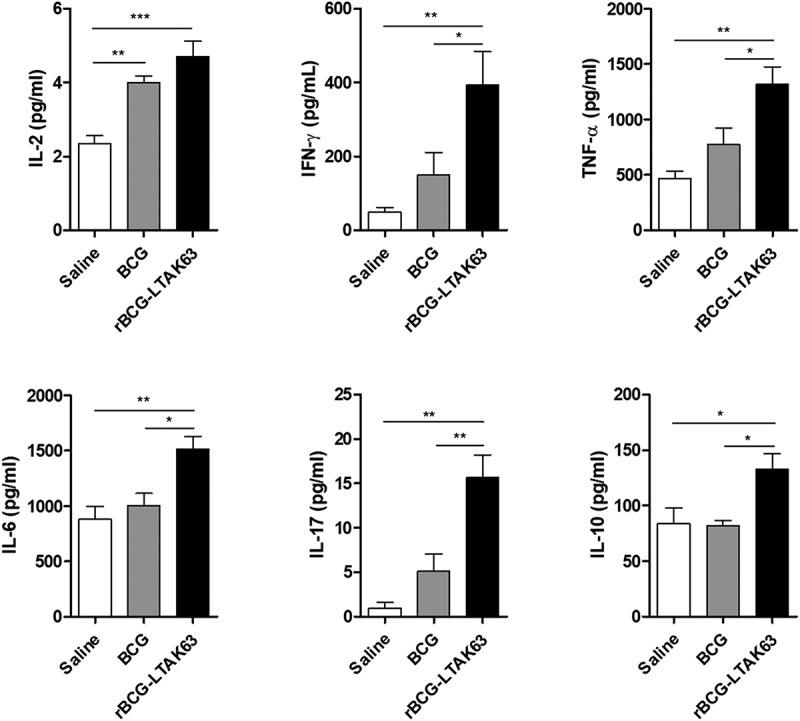
Increased immune response induced in the lungs of BALB/c mice immunized with rBCG-LTAK63 after 3 months.
Cytokine production was analyzed in the lungs of BALB/c mice 3 months after s.c. immunization with BCG or rBCG-LTAK63 (1 x 106 CFU). A total of 2 × 105 cells was stimulated with Mtb extract for 24 h or 48 h and the supernatants were analyzed by multiplex cytokine assays (IL-2, IL-17 and IL10 at 24 h; IFN-γ, TNF-α and IL-6 at 48 h). The results represent one of two independent experiments with similar results (n = 4–5 animals per group). Statistical significance was determined by one-way ANOVA. Significant differences were observed for the indicated groups (* P < .05; ** P < .01; *** P < .001).
3.5. Increased CD4+ T cells producing TNF-α in rBCG-LTAK63-immunized animals 15 d after infection with Mtb strain
In order to evaluate the immune responses in rBCG-LTAK63-immunized BALB/c mice 15 d after Mtb challenge, we measured the intracellular CD4+ T cells cytokine production. Higher TNF-α-secreting CD4+ T cells were detected in the rBCG-LTAK63 group as compared to the BCG group (Figure 6). Furthermore, the multifunctional IL-2+ TNF-α+ CD4+ T cells were significantly increased in the rBCG-LTAK63 group as compared to the BCG group (Figure 6).
Figure 6.
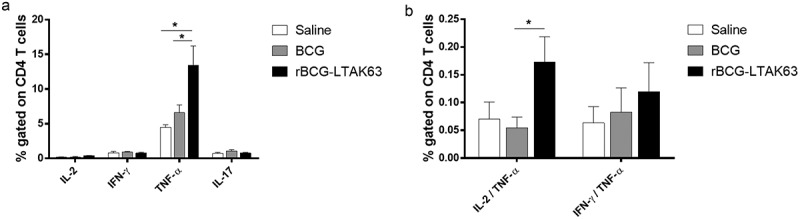
Challenge with Mtb strain increased CD4 T cells producing TNF-α in rBCG-LTAK63-immunized animals.
BALB/c mice immunized with BCG or rBCG-LTAK63 (1 x 106 CFU) were challenged intratracheally with 1 × 106 CFU Mtb H37Rv. Intracellular cytokine production was evaluated 15 d after challenge. Analysis of lung cells in culture stimulated with 5.0 µg/mL Mtb extract for 6 h and in the presence of brefeldin A by multicolor flow cytometry. The results represent one of two independent experiments with similar results (n = 4–5 animals per group). Statistical significance was determined by one-way ANOVA. Bars are mean ± SEM. Significant differences were observed for the indicated groups (* P < .05).
In order to investigate if the higher levels of TNF-α would be harmful to rBCG-LTAK63-immunized mice, we analyzed weight variation before and after challenge and the histopathological effects. The weight did not vary significantly among the groups before challenge. Mice immunized with rBCG-LTAK63 did not show weight loss after challenge, contrary to the BCG or Saline groups (Figure 7(a)). Histopathological analysis of the lungs of mice immunized with rBCG-LTAK63 at 15 d after challenge showed more preserved lung tissue as compared to the BCG group, determined by the higher alveolar area (Figure 7(b)).
Figure 7.
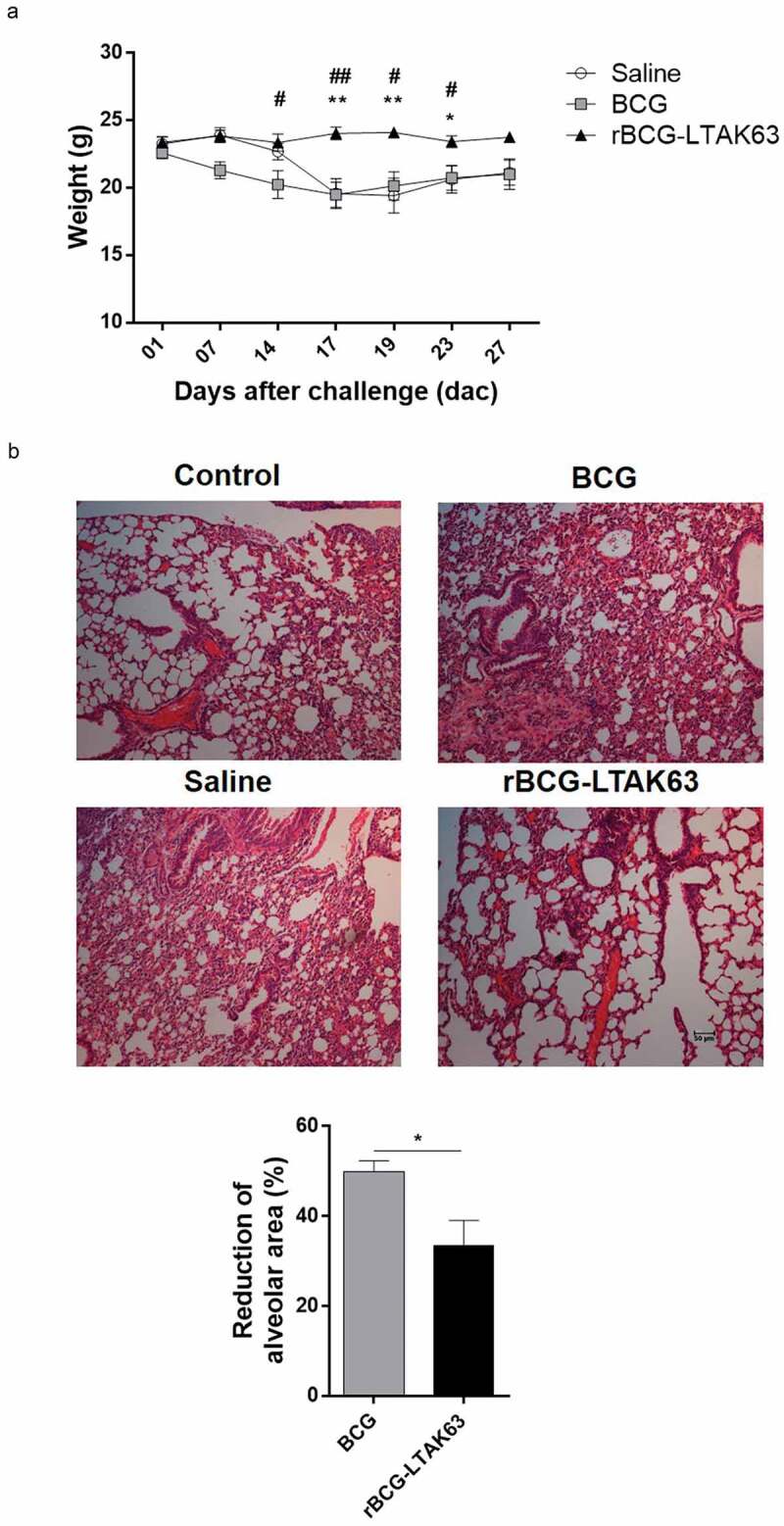
Kinetics of weight variation and histopathological analysis after challenge.
(a) BALB/c mice immunized with BCG or rBCG-LTAK63 (1 x 106 CFU) 90 d before intratracheal challenge with Mtb H37Rv (1 x 105 CFU) and the kinetics of weight variation was followed. (b) On day 15 after challenge, lungs from BALB/c immunized mice or those receiving Saline were fixed and sections were stained with HE prior to histopathological analysis. Non-challenged mice were used as Control. Images show representative lung sections (100 x magnification; bars scales correspond to 50 µm). Morphometrics of lung sections is shown. The results represent one of two independent experiments with similar results (n = 6–8 animals per group). Bars are mean ± SEM. Statistical significance was determined by one-way ANOVA in (a) and by Student’s t-test in (b).
4. Discussion
The importance of an efficient innate immune response in the development of a vaccine against TB is well established. It is known that the delay to trigger the immune response to eliminate Mtb contributes to disease progression.32 In order to evaluate the potential innate immune responses induced by the novel vaccine candidate, rBCG-LTAK63, we used the established intraperitoneal immunization model,33 since the peritoneal cell population is composed mostly of macrophages30 . Several studies show that macrophages, which are the main target of Mtb, play a crucial role in the host innate anti-mycobacterial defense and have many pathways to eliminate Mtb.34 The rBCG-LTAK63 strain was able to promote an efficient recruitment of cells, with increased neutrophils at 24 h. Macrophages, neutrophils and lymphocytes were increased at 7 d after immunization with both strains and the increase in lymphocytes was higher than that induced by BCG. Our results demonstrated superior production of NO (at 24 h) and H2O2 (at 7 d) in rBCG-LTAK63 – immunized mice as compared to BCG, indicating increased activation of inflammatory cells. Therefore, rBCG-LTAK63 induced higher cell activation at 24 h and both recruitment and activation at 7 d. It is known that activated macrophages produce NO and H2O2 during the oxidative burst, which can kill Mtb,35 constituting an important part of the defense mechanisms against Mtb.31 However, the role of NO is not restricted to killing of the mycobacterium by activated macrophages; in mouse bone marrow-derived macrophages, NO generated by NOS2 was found to elicit host cell apoptosis and thereby restrict the growth of intracellular Mtb.36 NO can act directly on the differentiation of Th1, Th2 or Th17 cells,37 therefore affecting adaptive immune responses. In an ex-vivo model system of Mtb infection using lymphocytes and macrophages, it was shown that intracellular Mtb growth was affected by CD4+ T cells in the presence of NO-dependent macrophages.38
The rBCG-LTAK63 strain was able to promote an increase in the number of lymphocytes at 7 d after i.p. immunization. It is possible to hypothesize that the early activation of the immune response, demonstrated by production of NO at 24 h, could be involved in the induction of higher numbers of lymphocytes observed on the 7th day after administration of rBCG-LTAK63. Immunophenotyping of these cells showed that CD4+ T cells are a large proportion of the cell population induced by both strains. Only the rBCG-LTAK63 group presented increased levels of CD8+ T cells when compared to the Saline group. Clearance of the bacteria by macrophages is in part dependent on activation of macrophages, CD4+ T cells, CD8+ T cells and NK cells.39 The induction of CD4+CD69+ T cells in BCG and rBCG-LTAK63-immunized animals were comparable (Supplementary Figure 1). It will be important to further characterize the activation of T cells.
It is known that the delayed T-cell responses during the first d after Mtb infection provide a critical window of opportunity in which the bacteria can establish infection.40 Lymphocytes are essential to initiate a specific response against Mtb, increasing the production of IFN-γ and TNF-α.41 We also observed production of high levels of inflammatory cytokines, such as IFN-γ and TNF-α, at 7 d. It will be important to further characterize the mechanisms of macrophage and T-cell activation by rBCG-LTAK63 and BCG in other experimental models.
Investigation of the long-term immune responses following immunization with rBCG-LTAK63 showed increased production of type 1 cytokines, such as IFN-γ and TNF-α, in the lungs of immunized mice when compared to BCG. Although there is some discussion on the role of IFN-γ and TNF-α in the protection against Mtb, these cytokines are considered important agents for control of the infection,42 especially when they are produced by CD4+ T cells.43 Higher production of IL-17 and IL-6 was also observed in the lung cells of mice immunized with rBCG-LTAK63. More recently, a role for Th17 response has been shown to be important for the control of Mtb infection. Different cell types are able to respond to the IL-17 cytokine, such as macrophages, lymphocytes and dendritic cells. IL-17 induces the expression of pro-inflammatory cytokines, such as IL-6, IL-8 and antimicrobial proteins.33,44,45 On the other hand, increased IL-10 production was observed in cells isolated from the lungs of mice immunized with rBCG-LTAK63 when compared to the BCG group. IL-10 is considered a regulatory cytokine, being important for an adequate balance between inflammatory and immunopathological responses. IL-10 can be produced by a variety of cells, including regulatory T cells. However, it is probably being produced in response to the Th1 cytokine production.46,47
We investigated the T cell response elicited by immunization with BCG or rBCG-LTAK63 after the challenge with Mtb. At 15 d after challenge, higher TNF-α-secreting CD4+ T cells were observed in the lungs of rBCG-LTAK63-immunized mice when compared to BCG, in agreement with our previous observations before challenge.19 This increased production of TNF-α could be important to reduce the dissemination of Mtb and promote higher levels of protection later, since it is essential for the effective control of mycobacterial infection in mice and humans.42 TNF-α can be produced by several cell types. However, there is evidence that T cell-derived TNF (α or β) is necessary to sustain control of Mtb infection.48 In our results, the TNF-α-secreting CD4+ T cells were evaluated by intracellular cytokine staining (ICS) using monoclonal antibodies, which should be more specific. TNF-α is required not only for bactericidal mechanisms, but also for downregulation of inflammation during infection.49 In order to investigate if the increased levels of TNF-α could also lead to undesirable pathological effects,50 we followed mouse weight before and after challenge, and lung histopathology. The results indicate that the levels of TNF-α induced by rBCG-LTAK63 in this model did not cause weight loss or lung pathology.
The lack of validated and translational models for evaluation of protective immunity has precluded establishment of correlates of protection for TB.13 Our data revealed important aspects of the immune responses induced by this novel vaccine candidate correlating with protection in the current models. We will only be able to estimate the real relevance of this vaccine in models that are closer to humans.
The Heat-labile E. coli enterotoxin and its derivatives have a broad spectrum of adjuvant properties, which can improve the innate and adaptive immune responses of co-administered antigens.22,51 The rBCG-LTAK63 construct takes advantage of these properties inducing a variety of immune mechanisms, many of which are clearly different from BCG. We hypothesize that the expression of LTAK63 in BCG is able to induce an increased innate immune response involving mainly active macrophages, with early production of NO, and increased recruitment of CD4+ and CD8+ T cells. This potential to activate an increased initial immune response would be a key element to drive the Th1 and Th17 long-term immune response and the early production of TNF-α and IL-2/TNF-α by CD4+ T cells after challenge. On a whole, a concerted action of different immune responses is important to hinder the dissemination of Mtb and increase protection levels in rBCG-LTAK63-immunized animals.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [2017/24832-6] and [2014/01271-0].
Acknowledgments
C.C.S., D.R., A.I.K., L.C.C.L. and I.P.N. contributed to the design of the experiments, analyzed and interpreted data. C.C.S., D.R and I.P.N. performed the experiments on the evaluation of immune response. C.C.S., D.R., A.I.K., L.C.C.L. and I.P.N. wrote the paper. We would like to thank Caio César Barbosa Bomfim for his assistance in the histopathological analysis.
Disclosure of potential conflicts of interest
I.P.N and L.C.C.L have a patent application involving the rBCG-LTKA63 use as Mtb vaccines. The other authors C.C.S, D.R and A.I.K. declare no competing financial interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Nieuwenhuizen NE, Kaufmann SHE.. Next-generation vaccines based on bacille calmette-guerin. Front Immunol. 2018;9:121. doi: 10.3389/fimmu.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith T, Wolff KA, Nguyen L. Molecular biology of drug resistance in mycobacterium tuberculosis. Curr Top Microbiol Immunol. 2013;374:53–80. doi: 10.1007/82_2012_279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Barreto ML, Cunha SS, Pereira SM, Genser B, Hijjar MA, Yury Ichihara M, de Brito SC, Dourado I, Cruz A, Santa’Ana C, et al. Neonatal BCG protection against tuberculosis lasts for 20 years in Brazil. Int J Tuberc Lung Dis. 2005;9:1171–73. [PubMed] [Google Scholar]
- 5.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–62. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 6.Pitt JM, Blankley S, McShane H, O’Garra A. Vaccination against tuberculosis: how can we better BCG? Microb Pathog. 2013;58:2–16. doi: 10.1016/j.micpath.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Sugawara I, Udagawa T, Taniyama T. Protective efficacy of recombinant (Ag85A) BCG Tokyo with Ag85A peptide boosting against Mycobacterium tuberculosis-infected guinea pigs in comparison with that of DNA vaccine encoding Ag85A. Tuberculosis (Edinb). 2007;87:94–101. doi: 10.1016/j.tube.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Jain R, Dey B, Dhar N, Rao V, Singh R, Gupta UD, Katoch VM, Ramanathan VD, Tyagi AK. Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS One. 2008;3:e3869. doi: 10.1371/journal.pone.0003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–39. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Chen L, Chen Z, Zhang Y, Zhou Z, Lu J, Fu R, Wang C, Fang Z, Fan X. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine. 2010;28:5237–44. doi: 10.1016/j.vaccine.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 11.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, et al. Increased vaccine efficacy against tuberculosis of recombinant mycobacterium bovis bacille calmette-guérin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–79. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grode L, Ganoza CA, Brohm C, Weiner J 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31:1340–48. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 13.Weiner J 3rd, Kaufmann SH. Recent advances towards tuberculosis control: vaccines and biomarkers. J Intern Med. 2014;275:467–80. doi: 10.1111/joim.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet. 2010;375:2110–19. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- 15.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc London Ser B Biol Sci. 2011;366:2782–89. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Crevel R, Ottenhoff TH. van der Meer JW. Innate immunity to mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15:294–309. doi: 10.1128/cmr.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freches D, Korf H, Denis O, Havaux X, Huygen K, Romano M. Mice genetically inactivated in interleukin-17A receptor are defective in long-term control of mycobacterium tuberculosis infection. Immunology. 2013;140:220–31. doi: 10.1111/imm.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimento IP, Rodriguez D, Santos CC, Amaral EP, Rofatto HK, Junqueira-Kipnis AP, Gonçalves EDC, D’Império-Lima MR, Hirata MH, Silva CL, et al. Recombinant BCG Expressing LTAK63 adjuvant induces superior protection against mycobacterium tuberculosis. Sci Rep. 2017;7:2109. doi: 10.1038/s41598-017-02003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partidos CD, Pizza M, Rappuoli R, Steward MW. The adjuvant effect of a non-toxic mutant of heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology. 1996;89:483–87. doi: 10.1046/j.1365-2567.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti M, Rossi M, Giannelli V, Giuliani MM, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 22.Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–32. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, Mills KH, Rappuoli R, Del Giudice G. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534–41. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 24.Douce G, Giannelli V, Pizza M, Lewis D, Everest P, Rappuoli R, Dougan G. Genetically detoxified mutants of heat-labile toxin from Escherichia coli are able to act as oral adjuvants. Infect Immun. 1999;67:4400–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Da Hora VP, Conceicao FR, Dellagostin OA, Doolan DL. Non-toxic derivatives of LT as potent adjuvants. Vaccine. 2011;29:1538–44. doi: 10.1016/j.vaccine.2010.11.091. [DOI] [PubMed] [Google Scholar]
- 26.Pick E, Charon J, Mizel D. A rapid densitometric microassay for nitroblue tetrazolium reduction and application of the microassay to macrophages. J Reticuloendothel Soc. 1981;30:581–93. [PubMed] [Google Scholar]
- 27.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–57. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morais Fonseca D, Rosada RS, E Paula MO, Wowk PF, Franco LH, Soares EG, Silva CL, Deperon Bonato VL. Experimental tuberculosis: designing a better model to test vaccines against tuberculosis. Tuberculosis (Edinb). 2010;90:135–42. doi: 10.1016/j.tube.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Amaral EP, Ribeiro SCM, Lanes VR, Almeida FM, de Andrade MRM, Bomfim CCB, Salles ÉM, Bortoluci KR, Coutinho-Silva R, Hirata MH, et al. Pulmonary infection with hypervirulent Mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog. 2014;10:e1004188. doi: 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassado Ados A, D’Imperio Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Front Immunol. 2015;6:225. doi: 10.3389/fimmu.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 32.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–91. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 33.Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis. 2011;204:1573–84. doi: 10.1093/infdis/jir592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrero E, Biswas P, Vettoretto K, Ferrarini M, Uguccioni M, Piali L, Leone BE, Moser B, Rugarli C, Pardi R. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leucocyte subpopulations: focus on gammadelta cells. Immunology. 2003;108:365–74. doi: 10.1046/j.1365-2567.2003.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodnar KA, Serbina NV, Flynn JL. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69:800–09. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–78. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Cowley SC, Elkins KL. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. 2003;171:4689–99. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 39.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winslow GM, Cooper A, Reiley W, Chatterjee M, Woodland DL. Early T-cell responses in tuberculosis immunity. Immunol Rev. 2008;225:284–99. doi: 10.1111/j.1600-065X.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and chemokines in mycobacterium tuberculosis infection. Microbiol Spectr. 2016:4. doi: 10.1128/microbiolspec.TBTB2-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavalcanti YV, Brelaz MC, Neves JK, Ferraz JC, Pereira VR. Role of TNF-Alpha, IFN-Gamma, and IL-10 in the development of pulmonary tuberculosis. Pulm Med. 2012;2012:745483. doi: 10.1155/2012/745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green AM, Difazio R, Flynn JL. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol. 2013;190:270–77. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyadova IV, Panteleev AV. Th1 and Th17 cells in tuberculosis: protection, pathology, and biomarkers. Mediators Inflamm. 2015;2015:854507. doi: 10.1155/2015/854507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 46.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 47.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Allie N, Grivennikov SI, Keeton R, Hsu N-J, Bourigault M-L, Court N, Fremond C, Yeremeev V, Shebzukhov Y, Ryffel B, et al. Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection. Sci Rep. 2013;3:1809. doi: 10.1038/srep01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, Tsang E, Tsai MM, Flynn JL, Chan J. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–55. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1165–70. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 51.Tritto E, Muzzi A, Pesce I, Monaci E, Nuti S, Galli G, Wack A, Rappuoli R, Hussell T, De Gregorio E. The acquired immune response to the mucosal adjuvant LTK63 imprints the mouse lung with a protective signature. J Immunol. 2007;179:5346–57. doi: 10.4049/jimmunol.179.8.5346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


