ABSTRACT
Malaria is an illness caused by Plasmodium parasites transmitted to humans by infected mosquitoes. Of the five species that infect humans, P. falciparum exacts the highest toll in terms of human morbidity and mortality, and therefore represents a major public health threat in endemic areas. Recent advances in control efforts have reduced malaria incidence and prevalence, including rapid diagnostic testing, highly effective artemisinin combination therapy, use of insecticide-treated bednets, and indoor residual spraying. But, reductions in numbers of cases have stalled over the last few years, and incidence may have increased. As this concerning trend calls for new tools to combat the disease, the RTS,S vaccine has arrived just in time. The vaccine was created in 1987 and began pilot implementation in endemic countries in 2019. This first-generation malaria vaccine demonstrates modest efficacy against malaria illness and holds promise as a public health tool, especially for children in high-transmission areas where mortality is high.
KEYWORDS: RTS,S; malaria; Plasmodium; adjuvant; AS01; vaccine
Introduction
Malaria
Malaria is a parasitic disease characterized by mosquito transmission, distribution in mostly tropical and subtropical climates, an incubation period of 7–14 days, and systemic symptoms such as fever, chills, abdominal pain, vomiting, muscle and joint pain, fatigue and malaise. Of the five species of malaria that infect humans, P. falciparum exacts the highest toll in terms of human morbidity and mortality, and therefore represents a major public health threat in areas where malaria is transmitted. P. falciparum infection causes asymptomatic infection in as much as 87% of individuals in endemic areas.1 Infected persons may develop clinical signs of illness consistent with uncomplicated malaria, which carries <1% mortality risk. Other infected individuals experiencing more serious disease manifest with symptoms of severe malaria, including impaired consciousness, respiratory distress, multiple convulsions, prostration, shock, abnormal bleeding, and jaundice. A diagnosis of severe malaria carries a 90% risk of death for patients who remain at home and 20% for hospitalized persons.2 For severe malaria, mortality risk increases with age and disease severity,3 and decreases with early initiation of appropriate therapy. Some manifestations of severe malaria carry a more grave prognosis than others.4
According to the World Health Organization (WHO), an estimated 219 million cases of malaria occurred worldwide in 2017, slightly decreased from 239 million in 2010 and increased from 217 million in 2016.5 Approximately 92% of cases in 2017 occurred in sub-Saharan Africa, and the remainder were in Southeast Asia and the Eastern Mediterranean regions. Nearly half of all 2017 malaria cases worldwide occurred in five countries: Nigeria, Democratic Republic of the Congo, Mozambique, India and Uganda. Unfortunately, the ten African countries with highest malaria burden documented increased malaria cases in 2017 compared with the previous year,5 increasing concern that recent gains in malaria control are at high risk of reversal.
Worldwide, malaria caused an estimated 435,000 deaths in 2017, representing a decrease from 451,000 reported in 2016 and 607,000 in 2010.5 Children <5 years of age remain the most vulnerable to malaria death, and represent 61% of all malaria deaths in 2017.5 Despite gains in malaria mortality since 2010, only incremental improvements have been documented since 2015, signaling an urgent need to introduce new strategies to further reduce malaria deaths.
An effective malaria vaccine would be an important tool to combat the enormous socioeconomic burden caused by this disease. Vaccines promote both individual and public health, and are thus considered among the most highly successful public health tools. After provision of clean water and sanitation, vaccination against infectious diseases has contributed the greatest to public health worldwide, compared with other human interventions.
In recognition of malaria’s global importance, the United Nations Sustainable Development Goal 3, to ensure healthy lives and promote well-being for all at all ages, targets a 90% reduction in malaria incidence and mortality by 2030.6 This goal also includes malaria elimination in at least 35 endemic countries. Advances in malaria control include strategies to control the vector using insecticide-treated bed nets, larval source management, and indoor residual spraying. Intermittent preventive therapy provides antimalarials to vulnerable groups to clear parasites and prevent infection. Other efforts that remain in development include mass drug administration and genetically modified mosquitoes. In parallel with these efforts, the first WHO-directed malaria vaccine implementation studies are underway to determine how to maximize public health benefit of the most advanced malaria vaccine to date, the RTS,S vaccine.
Human malaria is caused by five Plasmodia species, of which P. falciparum is the most common and the most deadly. P. vivax is also an important cause of morbidity and mortality worldwide. Malaria parasites are members of the genus Plasmodium, a class of unicellular eukaryotes that are obligate parasites of multiple species of insects and vertebrae, including birds and mammals. Over 100 species of Plasmodium exist in nature. The life cycle includes development in an insect host that feeds on a vertebrate host and injects infectious parasites during a blood meal. Infectious parasites travel from the bite site to the systemic circulation, where they undergo further development and replication before infecting individual red blood cells. Infected red blood cells eventually lyse, causing malaria clinical signs and symptoms. Some infected red blood cells are ingested by mosquitoes during a subsequent blood meal, and perpetuate the parasite life cycle.
For P. falciparum, female anopheline mosquitoes transmit sporozoite stage parasites during a blood meal. These sporozoites travel through the venous circulation to the liver, where they invade hepatocytes and produce 30–40,000 progeny over 6 days, completing the pre-erythrocytic stage of parasite development. During this phase, the parasite does not cause clinical signs and symptoms in the host. Subsequently, infected hepatocytes burst to release progeny merozoites, each of which aims to invade a red blood cell. After erythrocyte invasion, each merozoite then propagates within 48–72 hours to produce ~16-24 merozoites. Infected red blood cells rupture in synchrony, causing increases in cytokine production that lead to clinical symptoms of fever, headache, chills, sweats, vomiting, myalgias and malaise. Malaria symptom severity correlates with parasite load.7 The first stages of clinical illness are characterized by periodic fevers with frequency that is characteristic for different species of human malaria (24 hours for P. knowlesi, 48 hours for P. falciparum, P. ovale and P. vivax, and 72 hours for P. malariae), and correspond to release of a new generation of merozoites in the bloodstream (Figure 1). Infected red blood cells rupture to release merozoites, and each merozoite can invade a new erythrocyte to continue blood stage replication. During this blood stage, parasitized erythrocytes will develop either: 1) progeny asexual merozoites that are released to invade other erythrocytes, or 2) male or female gametocytes that can be ingested by a mosquito during a blood meal. Ingested male and female gametocytes fuse in the mosquito midgut to form the zygote, a diploid phase of development when homologous recombination yields significant genetic variability of progeny. This high degree of genetic polymorphism permits the P. falciparum parasite to evade destruction because human immune responses are generally directed against variable antigenic sites on the parasite. Parasite genetic variability also renders individuals susceptible to repeat and multiple concurrent infections, including individuals vaccinated with the RTS,S/AS01 vaccine. A recent study of vaccinees demonstrated a slightly diminished efficacy against P. falciparum strains that are heterologous to the sequence contained in RTS,S,8 providing evidence for vaccine escape related to genetic variability. No known immunological cross-reactivity is known to exist between P. falciparum and other human malaria species, but this area remains understudied.
Figure 1.
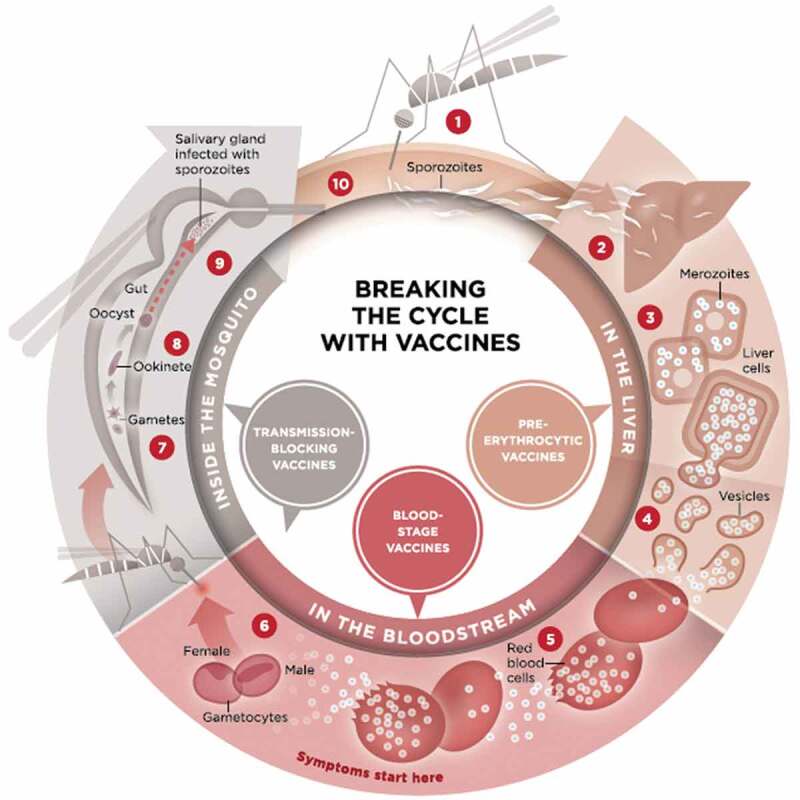
Life cycle of the malaria parasite (courtesy of PATH malaria vaccine initiative, found at http://www.malariavaccine.org/malaria-and-vaccines/vaccine-development/life-cycle-malaria-parasite).
Malaria infection begins when an infected female Anopheles mosquito bites a person, injecting Plasmodium parasites, in the form of sporozoites, into the bloodstream. 2. The sporozoites pass quickly into the human liver. 3. The sporozoites multiply asexually in the liver cells over the next 7–10 days, causing no symptoms. 4. In an animal model, the parasites, in the form of merozoites, are released from the liver cells in vesicles, journey through the heart, and arrive in the lungs, where they settle within lung capillaries. The vesicles eventually disintegrate, freeing the merozoites to enter the blood phase of their development. 5. In the bloodstream, the merozoites invade red blood cells (erythrocytes) and multiply again until the cells burst. Then they invade more erythrocytes. This cycle is repeated, causing fever each time parasites break free and invade blood cells. 6. Some of the infected blood cells leave the cycle of asexual multiplication. Instead of replicating, the merozoites in these cells develop into sexual forms of the parasite, called gametocytes, that circulate in the bloodstream. 7. When a mosquito bites an infected human, it ingests the gametocytes, which develop further into mature sex cells called gametes. 8. The fertilized female gametes develop into actively moving ookinetes that burrow through the mosquito’s midgut wall and form oocysts on the exterior surface. 9. Inside the oocyst, thousands of active sporozoites develop. The oocyst eventually bursts, releasing sporozoites that travel to the mosquito’s salivary glands. 10. The cycle of human infection begins again when the mosquito bites another person.
Malaria vaccines are classified by the parasite developmental stage targeted: pre-erythrocytic vaccines, blood stage vaccines, and transmission blocking vaccines. Some vaccines may target multiple developmental stages. An ideal malaria vaccine would effectively prevent the first stages of parasite development completely, blocking further stages from developing and preventing transmission. As the RTS,S vaccine targets the circumsporozoite protein on the sporozoite surface and targets P. falciparum parasites before they infect liver cells, it is considered a pre-erythrocytic vaccine.
Key issues
P. falciparum malaria causes significant morbidity and mortality worldwide, causing death in 90% of individuals with untreated severe malaria.
In 2017, 92% of malaria cases occurred in sub-Saharan Africa, where 10 countries reported increased cases despite efforts to control the disease.
An efficacious malaria vaccine would be an important tool to combat the enormous socioeconomic burden caused by malaria.
RTS,S/AS01 is the first malaria vaccine to be tested in Phase 3 clinical trials and the first to be assessed in routine immunization programs in malaria-endemic areas.
Results of Phase 3 testing show that among children aged 5–17 months who received 4 doses of RTS,S/AS01, vaccine efficacy against malaria was 36% over 4 years of follow-up.
Phase 3 efficacy was lower among infants who received the vaccine with other childhood vaccines at 6, 10 and 14 weeks of age, and did not justify further use in this age group.
RTS,S/AS01 shows the most benefit in areas with intense malaria transmission, including reductions in malaria cases, overall hospital admissions, and the need for blood transfusions.
WHO has recognized the public health potential of the RTS,S/AS01 vaccine and acknowledged the need for further evaluation before individual countries consider adopting its use in routine vaccination schedules.
RTS,S/AS01 pilot implementation studies are underway in Ghana, Kenya and Malawi to address outstanding questions related to public health use of the vaccine.
Significant hurdles for integration of RTS,S into a country’s vaccination schedule include the need for vaccination during non-routine visits and requirement for at least four doses, including a booster given 18 months after the first dose.
Design
RTS,S vaccine was created in 1987 as part of a collaboration between GlaxoSmithKline (GSK) and the Walter Reed Army Institute of Research (WRAIR) that began in 1984. At the time, both groups were attempting to develop a vaccine based on proof-of-concept studies that radiation-attenuated sporozoites protected against malaria infection.9 The circumsporozoite protein (CSP) antigen was identified as a target of the immune response generated by radiation-attenuated sporozoites, and was subsequently cloned and sequenced by the U.S. National Institutes of Health (NIH) and WRAIR.10,11 As a full-length CSP antigen proved difficult to produce at the time, the research team used GSK’s Escherichia coli elaboration system to produce a subunit antigen based on the central repeat region, work supported by epitope mapping of protective monoclonal antibodies to this region.12 Among four candidate antigens produced, one advanced to clinical testing in humans with controlled human malaria infection (CHMI), and demonstrated protection in a single volunteer.13 Multiple attempts to advance a standalone CSP subunit vaccine continued, but none showed significant clinical efficacy.14
Using expertise gained during development of the Energix-B™vaccine against Hepatitis B,15 researchers at GSK then pioneered the use of hepatitis B surface antigen as a carrier matrix for the CSP central repeat region, and added the CSP C terminal region that contains T- and B-cell epitopes, all based on the P. falciparum NF54 strain. This formed the RTS,S vaccine, in which “R” represents the central repeat region, a single polypeptide chain corresponding to a highly-conserved tandem repeat tetrapeptide NANP amino acid sequence, and “T” represents T-lymphocyte epitopes separated by immunodominant CD4+ and CD8+ epitopes (Th2R and Th3R). This combined RT peptide is genetically fused to the N-terminal of Hepatitis B surface antigen (HBsAg), the “S” (Surface) portion when co-expressed in yeast cells, yielding virus-like particles that display both CSP and S at their surfaces. A second “S” portion is an unfused HBsAg that spontaneously fuses to the RTS component, hence the name RTS,S (Figure 2). The particulate and highly repetitive nature of the RTS,S antigen provides enhanced presentation to the immune system, and likely facilitates the strong anti-CSP antibody and T-cell responses measured in vaccinated individuals.
Figure 2.
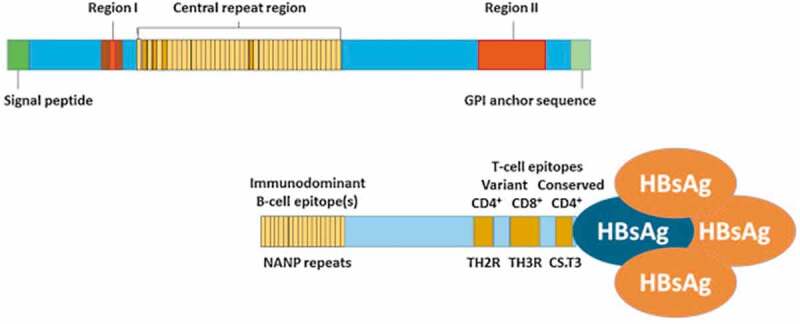
Graphical depiction of circumsporozoite (CSP) and RTS,S structures. CSP comprises an N-terminal region containing a signal peptide sequence and Region I that binds heparin sulfate proteoglycans and has embedded within it a conserved five amino acid (KLKQP) proteolytic cleavage site sequence; a central region containing four-amino acid (NANP/NVDP) repeats; and a C-terminal region containing Region II [a thrombospondin (TSP)-like domain] and a canonical glycosylphosphatidylinositol (GPI) anchor addition sequence. The region of the CSP included in the RTS,S vaccine includes the last 18 NANP repeats and C-terminus exclusive of the GPI anchor addition sequence. Hepatitis B virus surface antigen (HBsAg) monomers self-assemble into virus-like particles and approximately 25% of the HBsAg monomers in RTS,S are genetically fused to the truncated CSP and serve as protein carriers. The CSP fragment in RTS,S contains three known T-cell epitopes: a highly variable CD4 + T-cell epitope before the TSP-like domain (TH2R), a highly variable CD8 + T-cell epitope within the TSP-like domain (TH3R), and a conserved “universal” CD4 + T cell epitope (CS.T3) at the C-terminus. (Figure courtesy of a recent publication16 and open access, http://creativecommons.org/licenses/by/4.0/).
Multiple adjuvants have been tested with RTS,S, almost all from GSK’s adjuvant systems. The very first clinical trial of RTS,S adjuvanted with alum versus alum + 3-O-desacyl-4ʹ-monophosphoryl lipid A (MPL), a combination known as AS04, found AS04 to be more highly immunogenic and protective against CHMI.17 Subsequently, 6 candidate adjuvants were tested with RTS,S in rhesus monkeys and showed that an oil-in-water emulsion + MPL and the saponin QS-21 from Quillaja saponaria extract (AS02A) provided superior antibody and CMI responses. The adjuvants AS02, AS03 (oil-in-water emulsion) and AS04 were each tested in clinical trials of RTS,S with CHMI, and demonstrated equivalent antibody responses in the AS03 and AS04 groups, but the AS02A formulation provided enhanced protection against CHMI.18 Stability studies prompted development of a lyophilized RTS,S formulation for reconstitution with AS02A, and the combination showed equivalent efficacy against CHMI.19 Subsequently, a newer adjuvant system AS01, comprised of liposomes, MPL, and QS-21, provided an opportunity to improve RTS,S immunogenicity. A CHMI study at WRAIR documented greater efficacy and increased anti-CSP antibody and CD4+ T cell responses for AS01 compared with AS02 in malaria-naïve adults,20 findings confirmed in Kenyan adults.21 This RTS,S/AS01 formulation of the vaccine was subsequently tested as part of a Phase 2 program in preparation for the Phase 3 clinical trial.22-26
Phase 3 study
The RTS,S/AS01 vaccine advanced to Phase 3 testing from 2009–2014 in 7 sub-Saharan African countries, and enrolled 15,459 participants, including 8922 children 5–17 months of age and 6537 infants 6–12 weeks of age.27 Participants were randomly assigned at first vaccination in a 1:1:1 ratio to receive three doses of RTS,S/AS01 at months 0, 1 and 2 and a booster dose at month 20; three doses of RTS,S/AS01 and a dose of meningococcal serogroup C conjugate vaccine (Menjugate, Novartis, Basel, Switzerland) at month 20; or a comparator vaccine (Menjugate for infants and rabies vaccine (Verorab, Sanofi Pasteur, Paris, France) for children) at months 0, 1, 2 and 20. The study was conducted in a double-blind manner such that neither participants nor study staff who assessed primary endpoints and adverse events had knowledge of study treatment assignment. Vaccine was given via intramuscular injection. Infant vaccinations were aligned with co-administration of routine oral polio and parenteral DTP-containing vaccines per the Expanded Program of Immunization regimen. The study was conducted in two phases, a double-blind phase from months 0–32 and an extension phase from month 33 until study closure. Infants 6–12 weeks of age were followed over median 38 months, and children 5–17 months of age were followed for median 48 months. The primary aim of the Phase 3 trial was to assess RTS,S/AS01 efficacy over a year of follow-up in 2 age categories. Secondary aims included assessment of a booster dose of RTS,S/AS01 at month 20 with follow-up through a 12 month period. Additional analyses conducted at the end of the extension phase included efficacy against clinical malaria over the entire follow-up period. The primary efficacy outcome was clinical malaria detected by passive surveillance, and was defined as an illness accompanied by an axillary temperature of at least 37.5⁰C and the presence of P. falciparum asexual parasitemia of at least 5000 parasites per mm3, or a case of malaria meeting the primary definition of severe malaria. Severe malaria was defined as the presence of P. falciparum asexual parasitemia of at least 5000 parasites per mm3 with at least one marker of severe disease and without diagnosis of a coexisting illness.27
With regard to the primary aim, efficacy measured by negative binomial regression against first or only episode of clinical malaria in the 12 months after dose 3 was 31.3% (97.5%CI 23.6–38.3%, p < .0001) for infants and 55.8% (97.5%CI 50.6–60.4%, p < .0001) in the 5–17 month age group.27
For the additional efficacy endpoint assessed at the end of study extension of follow-up, the vaccine demonstrated four-dose efficacy measured by negative binomial regression against clinical malaria of 25.9% in infants 6–12 weeks of age, and 36.3% efficacy over median 48 months in children 5–17 months of age.27 Over the same follow-up period, the 3 dose regimen showed reduced efficacy against clinical malaria in both age groups (Table 1). Efficacy against clinical malaria decreased over the follow-up period for all groups and regimens, but waned more slowly in the 4-dose group.27 Among infants 6–12 weeks of age, vaccine efficacy of both the 3 and 4-dose regimens against clinical malaria and severe malaria were lower than efficacy in children 5–17 months of age. Consistent with findings for other longitudinal studies of malaria in children, severe malaria was not a common outcome in this clinical trial, likely because participants received prompt diagnosis and treatment for clinical malaria before progression to severe disease.
Table 1.
RTS,S/AS01 Phase 3 efficacy results.
| Age Group | 6-12 weeks of age (n = 6537) | 5-17 months of age (n = 8922) |
|---|---|---|
| Vaccine Efficacy against clinical malaria, 3-dose group (95% CI) | 18.3% (11.7 to 24.4) | 28.3% (23.3 to 32.9) |
| Vaccine efficacy against clinical malaria, 4-dose group (95% CI) | 25.9% (19.9 to 31.5) | 36.3% (31.8 to 40.5) |
| Vaccine Efficacy against severe malaria, 3-dose group (95% CI) | 10.3% (–17.9 to 31.8) | 1.1% (–23.0 to 20.5) |
| Vaccine efficacy against severe malaria, 4-dose group (95% CI) | 17.3% (–9.4 to 37.5) | 32.2% (13.7 to 46.9) |
With regard to vaccine safety, the RTS,S/AS01 profile is similar to other routine vaccines given to children except for an increased risk of febrile seizures. Children aged 5–17 months at first vaccination were more likely than controls to have a febrile seizure within 7 days after vaccination, especially the third dose. This effect was transient, and all affected children recovered after 7 days. Safety surveillance also suggested a potential increased risk of meningitis and cerebral malaria in this same age group.27 A study in Kenyan children with WHO Stage 1 or 2 HIV disease found that RTS,S/AS01 was well-tolerated in this population, and that they can be safely included in future vaccination programs.28
RTS,S/AS01
The RTS,S vaccine was developed by a public–private partnership in 2001 between GSK and PATH’s Malaria Vaccine Initiative, with support from the Bill and Melinda Gates Foundation to PATH. The goal of the partnership is to develop RTS,S for infants and young children living in malaria-endemic regions in sub-Saharan Africa.
RTS,S antigen is produced by GSK Biologicals. Commercial production of RTS,S purified bulk antigen is a continuous process that begins with fed-batch fermentation of the recombinant yeast strain Saccharomyces cerevisiae RIX4397 from a two-tiered cell bank system, followed by harvesting of the yeast cells, disruption, extraction and purification. The purification process includes different types of chromatography ultracentrifugation, and filtration. RTS,S is stored at −70°C in sterile containers. A single fermentation produces one single fermentation broth from which one single extraction is performed. This leads to a single batch of purified RTS,S antigen without blending or re-processing at any stage of manufacturing. Controls are applied during the manufacturing process to monitor product purity, yield, and integrity. Process validation includes identification and validation of the critical manufacturing parameters and demonstration of process consistency for at least 3 consecutive batches. Release specifications include: visual appearance, pH, identity and antigenic activity, purity, protein content, S to RTS ratio, endotoxin, sterility, size distribution profile, lipid content, and polysaccharide content. RTS,S potency is measured by ELISA with a capture antibody directed against the CS (RT) part of the virus-like particle, and detection antibody raised against the S protein.29
AS01 adjuvant system is formulated at GSK Biologicals. The formulation process includes mixing concentrated liposome bulk intermediate with formulation buffer (PO4/NaCl), then adding QS-21 liquid bulk. After pH check and sterile filtration, the adjuvant is filled into the final containers.29
The reconstituted RTS,S/AS01 product is a preservative-free liquid suspension for injection that appears opalescent, colorless to pale brown. The vaccine consists of two parts. The first is the powder or lyophilized form containing RTS,S, presented in a 3 mL glass vial closed with rubber stoppers and aluminum caps, containing 2 doses of RTS,S antigen. The second is the liquid suspension of the AS01 adjuvant system, presented in a 3 mL glass vial closed with rubber stoppers and aluminum caps, also containing 2 doses of the adjuvant system. The liquid adjuvant is used to reconstitute the RTS,S lyophilized antigen such that 1 vial of each produces 2 doses of vaccine for intramuscular injection.29
The RTS,S component is based on a large segment of the CSP (Amino acids 207 to 395 of the CSP from NF54 strain of P. falciparum).15 The two RTS and S protein components are expressed in genetically engineered Saccharomyces cerevisiae yeast cells, and then fuse spontaneously into virus-like particles displaying CSP and S sequences at their surface.15 The adjuvant system AS01 contains the immunostimulants QS-21 and MPL, and is formulated as a liposome-based adjuvant.
Doses for the ongoing implementation studies continue to be manufactured and donated by GSK, and the company will continue to donate additional vaccine doses for planned Phase 4 studies.
The vaccine was not intended for marketing in malaria-free areas, and the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency was asked to review the Phase 3 data and to provide a scientific opinion based on vaccine quality and the risk/benefit assessment from a regulatory perspective. This process is known as article 58, and is invoked for medicinal products manufactured for human use in a European Union (EU) state that are intended exclusively for markets outside the EU, yet it requires that products meet the same standards as those marketed in the EU. The CHMP performed a scientific evaluation of the vaccine and issued a positive opinion in July 2015, indicating that the risk/benefit assessment is favorable. The CHMP noted that the benefits may be especially important for children in high transmission areas.
Later in October 2015, the WHO Strategic Advisory Group of Experts (SAGE) on immunization and the Malaria Policy Advisory Group (MPAC) reviewed Phase 3 data for RTS,S. The WHO adopted recommendations from these advisory groups to conduct pilot implementation studies of RTS,S/AS01 using a 4-dose schedule in 3 to 5 moderate-to-high transmission settings sub-Saharan Africa. The WHO recommends that these studies assess feasibility of 4-dose administration, the impact on child mortality, and any evidence of increased risk of cerebral malaria in vaccine recipients. The vaccine is currently being piloted in children 5–17 months of age in Ghana, Kenya and Malawi. Results of these implementation studies are expected to guide advisory groups, policy makers and individual countries in their decision for RTS,S/AS01 licensure and deployment.
RTS,S/AS01 vaccination is expected to lead to a later increased risk of malaria in older children. Seven-year follow-up of 5–17 month old children who received 3 doses of vaccine in Kilifi, Kenya and Korogwe, Tanzania demonstrated a waning effect of efficacy over time. After 5 years, vaccinated children suffered increased risk of clinical malaria compared to controls.30 This is not surprising, and is true for other malaria interventions including insecticide-treated nets and seasonal malaria chemoprophylaxis. Whereas this reversal in malaria risk may be viewed as a disadvantage, the goal of early vaccination is to prevent malaria in young children who are at high risk for severe malaria complications, including adverse neurodevelopmental outcomes. Annual booster doses of RTS,S may be considered to alleviate risk for this rebound phenomenon.
Commercial and public health issues
Pricing for RTS,S/AS01 has not yet been determined. Groups including PATH and GSK agree that vaccine should be made available to infants and children at highest risk. Existing mechanisms that provide other vaccines to African children at no cost may help achieve this goal. The RTS,S/AS01 vaccine use fits within Gavi’s mission to save children’s lives and protect people’s health by increasing equitable use of vaccines in lower-income countries. RTS,S/AS01 was part of Gavi’s Vaccine Investment Strategy review in 2018, but was not subject to investment decisions as the malaria vaccine implementation program is ongoing. GSK has stated that pricing will be set at manufacturing cost plus a return of about 5% that will be used to reinvest in vaccines against neglected tropical diseases. After data from pilot implementation studies becomes available, subsequent recommendations from the WHO, including necessary prequalification, may help facilitate financial support for RTS,S/AS01 to reach areas that need it most.
Efficacy of RTS,S/AS01 vaccine is modest, yet still provides significant public health benefit. The Phase 3 results demonstrated that among children who received 4 doses of vaccine, 1744 clinical malaria cases were prevented for every 1000 children vaccinated. This benefit increased in settings of intense malaria transmission. Using Phase 3 data, modeling studies conducted by four different groups under WHO supervision found that 4 doses will avert 116,480 cases of clinical malaria and 484 deaths per 100,000 children vaccinated.31 These modeling studies also determined that RTS,S/AS01 would have marginal impact in areas where malaria prevalence is below 3%, and that the median incremental cost-effectiveness ratio is comparable to other current antimalarial interventions, including $25 USD per case averted and $87 USD per disability-adjusted life years averted, assuming $5 USD per dose of vaccine. Transmission intensity and vaccine cost play a central role in estimates of cost effectiveness. Compared to other interventions targeting infants and children at risk for malaria, including long-lasting insecticide-treated nets and seasonal malaria chemoprophylaxis, RTS,S/AS01 vaccination remains cost-effective as a second or third intervention in areas where parasite prevalence is >10%.31
Ultimately, political will is required for RTS,S uptake and deployment. Fortunately, politicians desire to combat malaria illness and death, but RTS,S vaccination must first be accepted as a viable intervention for malaria, which can take considerable time and effort in the case of a partly protective vaccine. After acceptance, political will can then work to overcome obstacles to vaccine implementation.
Intramuscular vaccine delivery
RTS,S/AS01 is delivered as a lyophilized injection delivered intramuscularly. Four doses are currently indicated for children, with the first dose given at 5 months of age. The first 3 doses are administered monthly, and the third should be completed by 9 months of age. The fourth dose should be administered at 15–18 months.
Immunogenicity
The immunogenicity profile of RTS,S has been extensively investigated, including attempts to correlate vaccine immunogenicity with protection.32-41 In summary, anti-CSP antibody titers against the NANP repeat region, and increased titers and responses have been correlated with a reduced risk of clinical malaria, but no threshold defining protection has been established.42 CD4+ T cell responses are also increased after RTS,S/AS01 immunization, and may be associated with protection.43,44
Immunogenicity has also been explored in vulnerable groups, including children living with HIV. A study of RTS,S/AS01 in Kenyan children with WHO stage 1 or 2 HIV disease reported that anti-CSP antibody geometric mean titer (GMT) was greater in vaccinees than controls 1 month after dose 3 (329.2 EU/mL versus 0.3 EU/mL), but was lower than children in the Phase 3 study (621 EU/mL). Although children in the RTS,S group experienced fewer episodes of clinical malaria, severe malaria, hospital admission due to malaria, and anemia than controls, these differences were not significant, and the study was not powered for these outcomes.28
The Phase 3 clinical trial demonstrated greater vaccine-induced immunogenicity in older children versus infants. Among children aged 5–17 months, anti-CSP antibody increased to 318.2 EU/mL 1 month after the booster dose, compared with 34.2 EU/mL in children who did not receive the boost. One year later, levels dropped to 52.4 EU/mL and 19.3 EU/mL, respectively. In young infants 6–12 weeks of age, anti-CSP antibody increased to 169.9 EU/mL 1 month after the booster dose, compared with 6.2 EU/mL in infants not boosted. One year later, levels dropped to 15.9 EU/mL and 3.7 EU/mL, respectively. Infants in the top tertile of anti-CSP antibody response after the primary series experienced a 36.9% reduction in risk of clinical malaria compared with infants in the lowest anti-CSP antibody tertile. This association was not seen in older children.27 The rapid anti-CSP antibody decline in all participants was characterized by an initial rapid half-life of ~40 days followed by a more gradual loss of vaccine-induced antibody ~600 days.42
Cross-protection
Studies of RTS,S vaccine cross-protection demonstrate mixed evidence of decreased malaria infections with vaccine-type CSP in vaccinees versus controls.45-47 A recent study of variation at the C-terminal sequence, part of RTS,S, demonstrated a slightly diminished efficacy for P. falciparum strains that contain sequences heterologous to RTS,S,8 and suggests that vaccine efficacy may depend somewhat on how closely CSP in the community matches that found in the vaccine. We do not yet know if vaccine deployment will result in increased circulating heterologous parasites resistant to RTS,S, a phenomenon that has occurred with other vaccine products including S. pneumoniae vaccines.
Patient acceptance and preference
Parents of children living in malaria-endemic areas are likely willing to accept a malaria vaccine with a reactogenicity profile similar to scheduled vaccines. Additional potential side effects such as the risk of febrile seizure for a partly effective vaccine will require careful explanation and health information campaigns to inform the public of the risks and benefits of vaccination. Vigilant monitoring for changes in attitudes and practice regarding other antimalarial measures will also be needed, including bednet use, seasonal malaria chemoprophylaxis, and treatment seeking behavior. These measures are known to reduce risk of malaria morbidity and mortality, and should not be replaced by vaccination. Part of the current RTS,S/AS01 implementation study activities include qualitative assessment of behavior change that may occur during vaccine introduction.
Current treatment options
The RTS,S/AS01 vaccine is unique and represents a first for malaria vaccine development. No other malaria vaccine, including vaccines specifically targeting P. falciparum, has advanced to Phase 3 testing, received favorable opinion from the CHMP of the European Medicines Agency, or been considered by the WHO advisory committees for implementation studies in African settings of moderate-to-high malaria transmission.
Other candidate malaria vaccines against P. falciparum in advanced development include PfSPZ Vaccine and R21. Both products continue to be tested for safety and efficacy in malaria-naïve and malaria-experienced individuals. These and other candidate malaria vaccines are listed in the WHO Rainbow Tables48 and have been recently reviewed.49,50
The PfSPZ Vaccine is a live, radiation-attenuated, whole sporozoite vaccine based on the NF54 strain of P. falciparum.51 When injected intravenously, 3–5 doses of PfSPZ Vaccine provide up to 100% protection against homologous CHMI with NF54 strain parasites in malaria-naïve adults,52-54 and durable but incomplete protection against heterologous CHMI with 7G8 strain parasites in malaria-naïve adults.54 In malaria-endemic areas, similar dosing in malaria-experienced adults provides more modest protection against CHMI55 and naturally-occurring malaria.56 Recent testing in malaria-experienced, African adults who received five doses of 2.7 × 105 live, attenuated sporozoites demonstrated 52% efficacy against intense, heterogeneous P. falciparum infection by time-to-event analysis, and 29% efficacy by proportional analysis over a 6-month period.56 Multiple studies of PfSPZ Vaccine are ongoing in both children and adults to optimize dose and regimen, including a large Phase 3 trial planned for Bioko Island in Equatorial Guinea. Like RTS,S/AS01, the PfSPZ Vaccine is designed to target the parasite’s pre-erythrocytic sporozoite stage, and thus prevent infection in the human host. Studies in non-human primates demonstrate that PfSPZ Vaccine generates a local CD8+ T cell response in the liver that inhibits hepatocyte infection.51 Challenges for PfSPZ Vaccine include requirements for vaccine storage in liquid nitrogen and intravenous administration that would necessitate additional infrastructure for vaccine delivery and advanced training for community health workers who administer vaccines.
The R21 vaccine is similar to the RTS,S vaccine, inasmuch as both are virus-like particle-based vaccines based on CSP. R21 particles are formed from a single CSP-hepatitis B surface antigen (HBsAg) fusion protein, which achieves a much higher proportion of CSP displayed on the antigen surface compared with RTS,S. R21 is thus designed to induce a greater anti-CSP antibody response and lower anti-HBsAg antibody response when compared with RTS,S/AS01, and is considered a next-generation RTS,S-like vaccine.57 Like RTS,S/AS01 and PfSPZ Vaccine, R21 targets the parasite’s pre-erythrocytic sporozoite stage. Because it contains a greater amount of CSP per HBsAg particle, R21 may induce higher anti-CSP antibody responses than RTS,S/AS01. As the magnitude of this anti-CSP antibody response to the NANP repeat region seems to correlate with RTS,S/AS01-induced protection,32 the enhanced immune response to CSP induced by R21 may result in superior efficacy and/or duration of protection. When administered with adjuvant Matrix-M, preclinical testing demonstrated enhanced protection against challenge and increased T cell responses when compared with Montanide adjuvant.57 As R21 is early in development, questions remain regarding efficacy against CHMI in malaria-naïve individuals and against naturally-occurring malaria in malaria-experienced persons living in endemic areas.
Future prospects
The ongoing RTS,S/AS01 implementation studies will provide practical information on the feasibility of administering 4 doses of vaccine in children, and on the overall efficacy and safety profile. These critical investigations will inform WHO policy on broad use of RTS,S/AS01. The prospect of vaccine introduction into moderate and highly endemic areas is highly promising for malaria control efforts.
In parallel with implementation studies, alternate dosing schedules for RTS,S/AS01 are being assessed in children in malaria-endemic areas, based on results from a trial in malaria-naïve adults that demonstrated enhanced protection against CHMI when the third dose was delayed and administered as one-fifth of the full dose.58 The immunologic basis for this improved protection includes enhanced CSP-specific antibody avidity and somatic hypermutation in B-cells specific for CSP.58 If results are replicated in ongoing studies in children, this alteration in vaccine schedule would not require changes in vaccine formulation and would dramatically increase vaccine efficacy and overall benefit of RTS,S/AS01 vaccination. Additional considerations include annual boosting of RTS,S vaccination in the most vulnerable groups in efforts to compensate for the rapid decay in vaccine-induced CSP antibody after vaccination.
Alternate adjuvants for RTS,S should also be considered, inasmuch as the antigen depends heavily on a strong adjuvant to enhance height and duration of antibody response. The enhanced immunogenicity provided by adjuvants must also be weighed against potential side effects, and an alternate adjuvant that does not increase risk of febrile seizure in children would offer advantage.
GSK continues to manufacture vaccine for the current implementation studies, but long-term vaccine supply is uncertain. As vaccine manufacturers must plan well in advance of production to meet demand, and countries do not yet know if the WHO will recommend RTS,S for use, additional delays in vaccine production and delivery may occur should implementation studies be favorable for vaccine use. Issues of vaccine transport and storage would also need attention at the national, regional and local levels, as would training of health care personnel. These challenges can surely be overcome through advocacy and partnerships.
Conclusion
After decades of clinical development, the RTS,S/AS01 vaccine has achieved an important milestone as the very first vaccine against malaria to be tested in Phase 3 clinical trials, and now in implementation studies. As the WHO, Gavi and other groups weigh the risk/benefit, cost-effectiveness and practical issues of vaccine implementation capacity in resource-limited settings, consideration should extend to potential impacts on health status, poverty, and social justice for persons living in endemic areas. Future advances, including enhanced protection using a delayed, fractionated dosing schedule and alternate adjuvants are likely, and can continue to be developed to achieve the ultimate goal of malaria eradication.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ayanful-Torgby R, Quashie NB, Boampong JN, Williamson KC, Amoah LE.. Seasonal variations in Plasmodium falciparum parasite prevalence assessed by varying diagnostic tests in asymptomatic children in southern Ghana. PLoS One. 2018;13(6):e0199172. Epub 2018/06/16. PubMed PMID: 29906275; PMCID: PMC6003688. doi: 10.1371/journal.pone.0199172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thwing J, Eisele TP, Steketee RW. Protective efficacy of malaria case management and intermittent preventive treatment for preventing malaria mortality in children: a systematic review for the lives saved tool. BMC Public Health. 2011;11(3):S14. Epub 2011/ 04/29. PubMed PMID: 21501431; PMCID: PMC3231887. doi: 10.1186/1471-2458-11-S3-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, Than M, Htut Y, Mohanty S, Yunus EB, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47(2):151–57. Epub 2008/06/07. PubMed PMID: 18533842. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 4.Severe malaria. Trop Med Int Health: TM & IH. 2014;19(1):7–131. Epub 2014/ 09/13. PubMed PMID: 25214480. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 5.World malaria report 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 6.Towards a global action plan for healthy lives and well-being for all. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 7.Field JW. Blood examination and prognosis in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1949;43(1):33–48. Epub 1949/07/01.PubMed PMID: 18139104. doi: 10.1016/0035-9203(49)90022-x. [DOI] [PubMed] [Google Scholar]
- 8.Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, Lievens M, Abdulla S, Adjei S, Agbenyega T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med. 2015;373(21):2025–37. Epub 2015/ 10/22. PubMed PMID: 26488565; PMCID: PMC4762279. doi: 10.1056/NEJMoa1505819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266(3):169–77. Epub 1973/09/01.PubMed PMID: 4583408. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985;228(4706):1436–40. Epub 1985/ 06/21. PubMed PMID: 2409595. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- 11.Dame JB, Williams JL, McCutchan TF, Weber JL, Wirtz RA, Hockmeyer WT, Maloy WL, Haynes JD, Schneider I, Roberts D, et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984;225(4662):593–99. Epub 1984/ 08/10. PubMed PMID: 6204383. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 12.Young JF, Ballou WR, Hockmeyer WT. Developing a human malaria sporozoite vaccine. Microb Pathog. 1987;2(4):237–40. Epub 1987/04/01.PubMed PMID: 3333800. [DOI] [PubMed] [Google Scholar]
- 13.Ballou WR, Hoffman SL, Sherwood JA, Hollingdale MR, Neva FA, Hockmeyer WT, Gordon DM, Schneider I, Wirtz RA, Young JF, et al. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987;1(8545):1277–81. Epub 1987/ 06/06. PubMed PMID: 2884410. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 14.Ballou WR, Cahill CP. Two decades of commitment to malaria vaccine development: glaxoSmithKline biologicals. Am J Trop Med Hyg. 2007;77(6):289–95. Epub 2008/ 01/31.PubMed PMID: 18165505. [PubMed] [Google Scholar]
- 15.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6(1):90–96. Epub 2009/ 10/07. PubMed PMID: 19806009. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 16.Kaslow DC, Biernaux S. RTS,S: toward a first landmark on the malaria vaccine technology roadmap. Vaccine. 2015;33(52):7425–32. doi: 10.1016/j.vaccine.2015.09.061. [DOI] [PubMed] [Google Scholar]
- 17.Vreden SG, Verhave JP, Oettinger T, Sauerwein RW, Meuwissen JH. Phase I clinical trial of a recombinant malaria vaccine consisting of the circumsporozoite repeat region of Plasmodium falciparum coupled to hepatitis B surface antigen. Am J Trop Med Hyg. 1991;45(5):533–38. Epub 1991/11/01. PubMed PMID: 1835311. doi: 10.4269/ajtmh.1991.45.533. [DOI] [PubMed] [Google Scholar]
- 18.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S malaria vaccine evaluation group. N Engl J Med. 1997;336(2):86–91. Epub 1997/01/09. PubMed PMID: 8988885. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 19.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG Jr., Hall T, Wellde BT, White K, Sun P, Schwenk R, et al. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine. 2007;25(29):5359–66. Epub 2007/06/19. PubMed PMID: 17574311. doi: 10.1016/j.vaccine.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–46. Epub 2009/07/03. PubMed PMID: 19569965. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 21.Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, Cummings JF, Kester KE, Ockenhouse CF, Stewart A, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS One. 2009;4(7):e6465. Epub 2009/08/04. PubMed PMID: 19649245; PMCID: PMC2714466. doi: 10.1371/journal.pone.0006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–32. Epub 2008/12/10. PubMed PMID: 19064627; PMCID: PMC2655100. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, Kai O, Jongert E, Lievens M, Leach A, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS One. 2011;6(10):e25786. Epub 2011/10/15. PubMed PMID: 21998698; PMCID: PMC3188575. doi: 10.1371/journal.pone.0025786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owusu-Agyei S, Ansong D, Asante K, Kwarteng Owusu S, Owusu R, Wireko Brobby NA, Dosoo D, Osei Akoto A, Osei-Kwakye K, Adjei EA, et al. Randomized controlled trial of RTS,S/AS02D and RTS,S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS One. 2009;4(10):e7302. Epub 2009/10/07. PubMed PMID: 19806184; PMCID: PMC2750750. doi: 10.1371/journal.pone.0007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lell B, Agnandji S, von Glasenapp I, Haertle S, Oyakhiromen S, Issifou S, Vekemans J, Leach A, Lievens M, Dubois MC, et al. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabon. PLoS One. 2009;4(10):e7611. Epub 2009/10/28. PubMed PMID: 19859560; PMCID: PMC2763199. doi: 10.1371/journal.pone.0007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnandji ST, Asante KP, Lyimo J, Vekemans J, Soulanoudjingar SS, Owusu R, Shomari M, Leach A, Fernandes J, Dosoo D, et al. Evaluation of the safety and immunogenicity of the RTS,S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J Infect Dis. 2010;202(7):1076–87. Epub 2010/ 08/26. PubMed PMID: 20735271. doi: 10.1086/656190. [DOI] [PubMed] [Google Scholar]
- 27.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. PubMed PMID: 25913272; PMCID: PMC5626001. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otieno L, Oneko M, Otieno W, Abuodha J, Owino E, Odero C, Mendoza YG, Andagalu B, Awino N, Ivinson K, et al. Safety and immunogenicity of RTS,S/AS01 malaria vaccine in infants and children with WHO stage 1 or 2 HIV disease: a randomised, double-blind, controlled trial. Lancet Infect Dis. 2016;16(10):1134–44. Epub 2016/07/11. PubMed PMID: 2739419. doi: 10.1016/S1473-3099(16)30161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agency EM. Assessment report. Mosquirix. European medicines agency, committee for medicinal products for human use, 2015 23 July 2015. Report No.: EMA/CHMP/439337/2015.
- 30.Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, Bejon P. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374(26):2519–29. Epub 2016/06/30. PubMed PMID: 27355532; PMCID: PMC4962898. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S, White MT, Wenger EA, Van de Velde N, Pemberton-Ross P, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387(10016):367–75. Epub 2015/ 11/10. PubMed PMID: 26549466; PMCID: PMC4723722. doi: 10.1016/S0140-6736(15)00725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. Epub 2010/ 01/01. PubMed PMID: 20042088; PMCID: PMC2806383. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campo JJ, Dobano C, Sacarlal J, Guinovart C, Mayor A, Angov E, Dutta S, Chitnis C, Macete E, Aponte JJ, et al. Impact of the RTS,S malaria vaccine candidate on naturally acquired antibody responses to multiple asexual blood stage antigens. PLoS One. 2011;6(10):e25779. Epub 2011/10/25. PubMed PMID: 22022448; PMCID: PMC3192128. doi: 10.1371/journal.pone.0025779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aide P, Dobano C, Sacarlal J, Aponte JJ, Mandomando I, Guinovart C, Bassat Q, Renom M, Puyol L, Macete E, et al. Four year immunogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine. 2011;29(35):6059–67. Epub 2011/03/30. PubMed PMID: 21443960. doi: 10.1016/j.vaccine.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Campo JJ, Sacarlal J, Aponte JJ, Aide P, Nhabomba AJ, Dobano C, Alonso PL. Duration of vaccine efficacy against malaria: 5th year of follow-up in children vaccinated with RTS,S/AS02 in Mozambique. Vaccine. 2014;32(19):2209–16. Epub 2014/ 03/19. PubMed PMID: 24631081. doi: 10.1016/j.vaccine.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Moncunill G, De Rosa SC, Ayestaran A, Nhabomba AJ, Mpina M, Cohen KW, Jairoce C, Rutishauser T, Campo JJ, Harezlak J, et al. RTS,S/AS01E malaria vaccine induces memory and polyfunctional T cell responses in a pediatric African phase III trial. Front Immunol. 2017;8:1008. Epub 2017/09/08. PubMed PMID: 28878775; PMCID: PMC5572329. doi: 10.3389/fimmu.2017.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moncunill G, Mpina M, Nhabomba AJ, Aguilar R, Ayestaran A, Sanz H, Campo JJ, Jairoce C, Barrios D, Dong Y, et al. Distinct helper T cell type 1 and 2 responses associated with malaria protection and risk in RTS,S/AS01E vaccinees. Clin Infect Dis. 2017;65(5):746–55. Epub 2017/05/16. PubMed PMID: 28505356; PMCID: PMC5850568. doi: 10.1093/cid/cix429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ubillos I, Ayestaran A, Nhabomba AJ, Dosoo D, Vidal M, Jimenez A, Jairoce C, Sanz H, Aguilar R, Williams NA, et al. Baseline exposure, antibody subclass, and hepatitis B response differentially affect malaria protective immunity following RTS,S/AS01E vaccination in African children. BMC Med. 2018;16(1):197. Epub 2018/11/01. PubMed PMID: 30376866; PMCID: PMC6208122. doi: 10.1186/s12916-018-1186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobano C, Sanz H, Sorgho H, Dosoo D, Mpina M, Ubillos I, Aguilar R, Ford T, Diez-Padrisa N, Williams NA, et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun. 2019;10(1):2174. Epub 2019/05/17. PubMed PMID: 31092823; PMCID: PMC6520358. doi: 10.1038/s41467-019-10195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobano C, Santano R, Vidal M, Jimenez A, Jairoce C, Ubillos I, Dosoo D, Aguilar R, Williams NA, Diez-Padrisa N, et al. Differential patterns of IgG subclass responses to Plasmodium falciparum antigens in relation to malaria protection and RTS,S vaccination. Front Immunol. 2019;10:439. Epub 2019/04/02. PubMed PMID: 30930896; PMCID: PMC6428712. doi: 10.3389/fimmu.2019.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurtovic L, Agius PA, Feng G, Drew DR, Ubillos I, Sacarlal J, Aponte JJ, Fowkes FJI, Dobano C, Beeson JG. Induction and decay of functional complement-fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med. 2019;17(1):45. Epub 2019/02/26. PubMed PMID: 30798787; PMCID: PMC6388494. doi: 10.1186/s12916-019-1277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, Drakeley C, Gesase S, Lusingu J, Ansong D, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15(12):1450–58. Epub 2015/ 09/08. PubMed PMID: 26342424; PMCID: PMC4655306. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van Den Berg RA, Ballou WR, Jongert E, Wille-Reece U, Ockenhouse C, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017;114(9):2425–30. Epub 2017/02/15. PubMed PMID: 28193898; PMCID: PMC5338562. doi: 10.1073/pnas.1621489114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One. 2013;8(4):e61395. Epub 2013/ 04/25. PubMed PMID: 23613845; PMCID: PMC3628884. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alloueche A, Milligan P, Conway DJ, Pinder M, Bojang K, Doherty T, Tornieporth N, Cohen J, Greenwood BM. Protective efficacy of the RTS,S/AS02 Plasmodium falciparum malaria vaccine is not strain specific. Am J Trop Med Hyg. 2003;68(1):97–101. Epub 2003/01/31.PubMed PMID: 12556156. [PubMed] [Google Scholar]
- 46.Enosse S, Dobano C, Quelhas D, Aponte JJ, Lievens M, Leach A, Sacarlal J, Greenwood B, Milman J, Dubovsky F, et al. RTS,S/AS02A malaria vaccine does not induce parasite CSP T cell epitope selection and reduces multiplicity of infection. PLoS Clin Trials. 2006;1(1):e5. Epub 2006/07/28. PubMed PMID: 16871327; PMCID: PMC1488895. doi: 10.1371/journal.pctr.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waitumbi JN, Anyona SB, Hunja CW, Kifude CM, Polhemus ME, Walsh DS, Ockenhouse CF, Heppner DG, Leach A, Lievens M, et al. Impact of RTS,S/AS02(A) and RTS,S/AS01(B) on genotypes of P. falciparum in adults participating in a malaria vaccine clinical trial. PLoS One. 2009;4(11):e7849. Epub 2009/ 11/20. PubMed PMID: 19924281; PMCID: PMC2773849. doi: 10.1371/journal.pone.0007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tables of malaria vaccine projects globally Geneva: World Health Organization. [accessed 2019. July 14]. https://www.who.int/immunization/research/development/Rainbow_tables/en/.
- 49.Laurens MB. The promise of a malaria vaccine-are we closer? Annu Rev Microbiol. 2018;72:273–92. Epub 2018/09/12. PubMed PMID: 30200856. doi: 10.1146/annurev-micro-090817-062427. [DOI] [PubMed] [Google Scholar]
- 50.Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, Long CA, Seder RA. Malaria vaccines: recent advances and new horizons. Cell Host Microbe. 2018;24(1):43–56. Epub 2018/07/13. PubMed PMID: 30001524; PMCID: PMC6054918. doi: 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334(6055):475–80. Epub 2011/ 09/10. PubMed PMID: 21903775. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 52.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang LJ, Sarwar UN, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016;22(6):614–23. PubMed PMID: 27158907. doi: 10.1038/nm.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–65. PubMed PMID: 23929949. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 54.Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, James ER, Billingsley PF, Gunasekera A, Manoj A, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A. 2017;114(10):2711–16. Epub 2017/02/23. PubMed PMID: 28223498; PMCID: PMC5347610. doi: 10.1073/pnas.1615324114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jongo SA, Shekalaghe SA, Church LWP, Ruben AJ, Schindler T, Zenklusen I, Rutishauser T, Rothen J, Tumbo A, Mkindi C. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99(2):338–49. Epub 2018/06/27. PubMed PMID: 29943719; PMCID: PMC6090339. doi: 10.4269/ajtmh.17-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, Kamate B, Samake Y, Guindo MA, Dolo A, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509. Epub 2017/ 02/22. PubMed PMID: 28216244. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins KA, Snaith R, Cottingham MG, Gilbert SC, Hill AVS. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep. 2017;7:46621. Epub 2017/04/20. PubMed PMID: 28422178; PMCID: PMC5395940. doi: 10.1038/srep46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214(5):762–71. Epub 2016/ 06/15. PubMed PMID: 27296848. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tables of malaria vaccine projects globally Geneva: World Health Organization. [accessed 2019. July 14]. https://www.who.int/immunization/research/development/Rainbow_tables/en/.


