ABSTRACT
Vaccination against influenza during pregnancy provides direct protection to pregnant women and indirect protection to their infants. Trivalent inactivated influenza vaccines (IIV3s) are safe and effective during pregnancy, but quadrivalent inactivated influenza vaccines (IIV4s) have not been evaluated in pregnant women and their infants. Here, we report the results of a randomized phase IV study to evaluate the immunogenicity and safety of IIV4 vs. IIV3 in pregnant women. Participants aged ≥18 years at weeks 20 to 32 of gestation were randomly assigned in a 2:1 ratio to receive a single dose of IIV4 (n = 230) or IIV3 (n = 116). Between baseline and 21 days after vaccination, hemagglutination inhibition (HAI) antibody titers increased in both groups by similar magnitudes for the two influenza A strains and single B strain common to IIV4 and IIV3. For the additional B strain in IIV4, HAI titers were higher in IIV4 recipients than IIV3 recipients (post-/pre-vaccination geometric mean titer ratio, 6.3 [95% CI: 5.1 − 7.7] vs. 3.4 [95% CI: 2.7 − 4.3]). At delivery, in both groups, HAI antibody titers for all strains were 1.5 − 1.9-fold higher in umbilical cord blood than in maternal blood, confirming active transplacental antibody transfer. Rates of solicited and unsolicited vaccine-related adverse events in mothers were similar between the two groups. Live births were reported for all participants and there were no vaccine-related adverse events in newborns. These results suggest IIV4 is as safe and immunogenic as IIV3 in pregnant women, and that maternal immunization with IIV4 should protect newborns against influenza via passively acquired antibodies.
KEYWORDS: Immunization, influenza vaccines, newborn infant, pregnancy, safety, seasonal influenza, transplacental antibody transfer, vaccination
Introduction
Pregnant women and young infants are among the population subgroups at greatest risk of severe illness, complications, and death from influenza.1,2 Hospital admissions for influenza illness are more frequent among pregnant women than non-pregnant women,3,4 and influenza in young infants frequently leads to hospitalization,5 bacterial co-infections,6,7 and a higher mortality rate than in older children.8 Severe influenza illness during pregnancy has also been associated with preterm birth and fetal death in the 2009 influenza A/H1N1 pandemic.9
Maternal vaccination during pregnancy is considered the most effective strategy to protect pregnant women and newborn infants against influenza.1 The World Health Organization (WHO)1 and other advisory bodies10–12 recommend that pregnant women are prioritized for influenza vaccination, since this provides both direct protection to pregnant women and indirect protection to their infants via transplacental maternal antibody transfer.2,13–16 This strategy is especially important for preventing influenza illness in infants aged less than 6 months because influenza vaccines are not licensed for use in this age group.
Inactivated influenza vaccines have been shown to be safe, immunogenic, and effective during pregnancy in several randomized controlled studies.13–15,17 Importantly, vaccination was shown to reduce the incidence of laboratory-confirmed influenza cases in pregnant women and their newborns by around 50%.13–16,18,19 Studies have also identified reduced rates of influenza-related hospitalization among infants born to women vaccinated against influenza during pregnancy.19–21 However, in many countries, few pregnant women receive influenza vaccines, often due to low awareness of the risks posed by the disease and concerns about the safety and efficacy of influenza vaccination during pregnancy.22
Until recently, most influenza vaccines have been trivalent, containing antigens from two influenza A subtypes (H1N1 and H3N2) and one influenza B lineage virus.1,23 However, since the 1980s, two antigenically distinct influenza B lineages have co-circulated globally, which has complicated the selection of the correct B lineage for each new influenza season.24 Global data indicate that differences frequently occur between the trivalent vaccine and the predominant circulating B lineage strains, which has resulted in suboptimal protection in several previous influenza seasons.25–27 To help ensure protection against influenza B, quadrivalent inactivated influenza vaccines (IIV4s), which contain the two A strains and a strain from each B lineage, have been developed and are generally replacing trivalent inactivated influenza vaccines (IIV3s) worldwide.24 Although there are no specific data on influenza B infection in pregnant women, influenza B is known to cause similar morbidity to influenza A across different populations and age groups.28 Vaccination during pregnancy with IIV4 instead of IIV3 would therefore extend protection to cover both circulating B lineages in pregnant women and newborns.
Whereas vaccination during pregnancy with IIV3s has been shown safe, effective, and to result in transplacental maternal antibody transfer,13–15 IIV4s have not been evaluated in pregnant women and their infants. Here, we report the immunogenicity and safety of an IIV4 in pregnant women, as well as birth outcomes and transplacental transfer of vaccine-induced antibodies.
Patients and methods
Study design
This was a Phase IV, randomized, blind observer, controlled, multi-center study conducted in pregnant women in Finland between September, 2017 and June, 2018 (EudraCT number: 2016-004763-40).
During the study enrollment period, the National Immunization Program of Finland offered vaccination with IIV3 free of charge for pregnant women at any stage of pregnancy.29 This led to a lower than expected rate of enrollment and, consequently, the required study power was not achieved for the original primary (non-inferiority of IIV4 immune responses vs. IIV3) and secondary (superiority of IIV4 immune response against the additional B strain) immunogenicity objectives. The study protocol was amended by replacing the immunogenicity objectives by a description of the immune response.
The revised primary objectives of the study were (a) to describe the immune response and (b) to describe the safety of one dose of IIV4 (VaxigripTetra, Sanofi Pasteur) or IIV3 (Vaxigrip, Sanofi Pasteur) 21 days after vaccination in pregnant women. Secondary objectives were to evaluate the transplacental transfer of antibody from mother to newborn from the cord blood after one dose of IIV4 or IIV3, and to evaluate the safety profile of IIV4 and IIV3 in pregnant women and in terms of birth outcome. The study was approved by the national ethics committee and conducted in accordance with the Declaration of Helsinki, Good Epidemiological Practice, and national regulations. All participants provided their written informed consent before inclusion.
Participants
The study included pregnant women aged ≥18 years at 20 to 32 weeks of pregnancy. This gestational age was chosen to assess transplacental antibody transfer at birth. Potential subjects were excluded if they had received any vaccine within 4 weeks before study vaccination; already been vaccinated against influenza in the 2017 − 2018 season; experienced pregnancy complications during the current pregnancy; or had a chronic illness that, in the opinion of the investigator, might interfere with the study assessments. Further exclusion criteria are provided in the Supplementary Materials.
Vaccines
The study used the WHO-recommended 2017–2018 Northern Hemisphere formulations of IIV4 and IIV3. Both vaccines were inactivated, split-virion, thimerosal-free, and were provided in pre-filled syringes of 0.5 ml. IIV3 contained 15 µg hemagglutinin per strain of A/Michigan/45/2015 (H1N1)pdm09-like virus, A/Hong Kong/4801/2014 (H3N2)-like virus, and B/Brisbane/60/2008-like virus (B/Victoria lineage). IIV4 contained 15 µg hemagglutinin of each of the above strains plus 15 µg hemagglutinin of B/Phuket/3073/2013-like virus (B/Yamagata lineage).
Study conduct
Participants were randomly assigned in a 2:1 ratio to receive a single dose (0.5 ml) of IIV4 or IIV3 by intramuscular injection. Randomization was performed using the permuted block method and scratchable randomization lists were used at each site to communicate which vaccine was to be injected. The study was conducted in a blind observer manner, where neither the participants nor the investigators responsible for safety assessment knew which vaccine was administered. Blood samples were taken from the study participants at baseline (day 0) and 21 days after vaccination to determine hemagglutination inhibition (HAI) antibody titers. To study the transfer of maternal antibodies, blood samples were also taken from the participants within 4 days of delivery and from the umbilical cord on the day of delivery.
Immunogenicity assessments
Anti-hemagglutinin antibody levels were measured in the participants before vaccination (day 0) and 21 days after the last vaccination, and in the participants and umbilical cord blood after delivery, by HAI assay as described previously.30 Geometric mean titers (GMTs), the geometric mean of the individual ratios of post-vaccination vs. pre-vaccination (day 0) titers, percentages of individuals with titers ≥1:40, and seroconversion or significant increase in HAI titer were calculated. Seroconversion was defined as a pre-vaccination HAI titer <1:10 and a post-vaccination titer ≥1:40; and a significant increase as a pre-vaccination HAI titer ≥1:10 and ≥4-fold increase in post- vs. pre-vaccination HAI titer.
Safety assessments
Safety was assessed according to International Conference on Harmonization guidelines.31 Immediate unsolicited adverse events (AEs) were defined as those occurring within 30 min following vaccination. Subjects recorded information about solicited reactions in a diary card for up to 7 days after vaccination, and about unsolicited AEs up to 21 days after vaccination. Any serious AEs were reported to investigators throughout the study, from inclusion until delivery. The newborn baby underwent a physical examination after delivery. Any complications during pregnancy or delivery were considered as AEs, and in some cases were considered serious AEs (e.g., spontaneous abortions, fetal death, stillbirth, and congenital anomalies reported in the baby). Investigators assessed unsolicited AEs and serious AEs as unrelated or possibly related to the vaccination. AEs were coded with Medical Dictionary for Regulatory Activities (MedDRA) terminology (version 19.0).
Sample size
Under the original trial protocol, with 486 subjects planned for enrollment in the IIV4 group and 243 in the IIV3 group, the study was planned to have an overall power of >80% for the original non-inferiority and superiority immunogenicity endpoint analyses. Following the low rate of enrollment, the study protocol was amended to make the immunogenicity objectives descriptive only, and so recalculating the sample size was not required.
Statistical analysis
All analyses were descriptive. 95% confidence intervals (CIs) were calculated using SAS® version 9.4 (SAS Institute, Cary, NC, USA). Missing or incomplete data were not replaced, with the exception that all HAI titers under the lower limit of quantitation (10) were assigned a value of 5 and all HAI titers above the upper limit of quantitation (10,240) were assigned a value of 10,240. Immunogenicity was analyzed in all eligible subjects who were vaccinated according to protocol and who had valid serology results. Safety was analyzed in all subjects who received a study vaccine.
Results
Study population
The study enrolled 346 subjects in Finland between September 15, 2017 and January 26, 2018. Enrollment was stopped on January 26, 2018, before reaching the number of subjects to achieve the study power for the original primary (non-inferiority of IIV4 immune responses vs. IIV3) and secondary (superiority of IIV4 immune response against the additional B strain) immunogenicity objectives. This was due to an unforeseen increase in free-of-charge IIV3 vaccination among pregnant women under the Finnish influenza vaccination program.
By the end of the enrollment period, 230 subjects had been randomized to receive IIV4 and 116 had been randomized to receive IIV3 (Figure 1). The study ended on June 14, 2018 and was completed by all subjects except for two vaccinated with IIV4 (one voluntarily withdrawal and one due to noncompliance with the study protocol [missing visit] because of pre-term delivery, which was considered unrelated to the vaccine) and one vaccinated with IIV3 (lost to follow-up).
Figure 1.

Study design and disposition of participants. Pregnant women aged ≥18 years at 20 to 32 weeks of pregnancy were randomly assigned 2:1 to receive IIV4 or IIV3 by intramuscular injection.
Subject demographics were similar between the two groups: the median age was 32.1 years (range: 20.3 − 44.3 years) for the women vaccinated with IIV4 and 30.7 years (range: 20.5 − 42.5 years) for those vaccinated with IIV3, and the median gestational age at enrollment was 25 weeks (interquartile range [IQR]: 22 − 29 weeks) for the IIV4 recipients and 24 weeks (IQR: 21 − 27 weeks) for the IIV3 recipients.
Immunogenicity
Baseline HAI antibody titers for all vaccine strains were similar between the pregnant women vaccinated with IIV4 and those vaccinated with IIV3 (Table 1). At 21 days after vaccination, HAI antibody titers had increased in IIV4 recipients for all vaccine strains by 3.8-fold to 8.6-fold from baseline titers. The increase in HAI antibody titers was similar in IIV3 recipients for the A/H1N1, A/H3N2, and B/Victoria strains common to both vaccines (post-/pre-vaccination GMT ratios: 5.3 − 9.6). However, IIV4 HAI titers were higher than IIV3 titers for the B/Yamagata strain (GMT ratios: 6.3 [95% CI: 5.1 − 7.7] vs. 3.4 [95% CI: 2.7 − 4.3]). At least 95.8% of IIV4 recipients and 94.5% of IIV3 recipients had titers ≥1:40 against individual vaccine strains at 21 days after vaccination. Rates of seroconversion/significant increase in titers were 38% to 61% in IIV4 recipients and 41% to 62% in IIV3 recipients for the three common vaccine strains. For B/Yamagata, more participants vaccinated with IIV4 seroconverted or had significant increases in titers than those vaccinated with IIV3 (59.7% [95% CI: 52.9 − 66.3%] vs. 38.5% [95% CI: 29.4 − 48.3%]).
Table 1.
HAI antibody responses.
| A/H1N1 |
A/H3N2 |
B/Victoria |
B/Yamagata |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Day | IIV4 | IIV3 | IIV4 | IIV3 | IIV4 | IIV3 | IIV4 | IIV3a |
| N | - | 216 | 109 | 216 | 109 | 216 | 109 | 216 | 109 |
| HAI GMT (95% CI) | 0 | 138 (114 − 166) | 121 (88.4 − 166) | 39.6 (32.2 − 48.6) | 40.0 (29.4 − 54.5) | 67.1 (55.2 − 81.4) | 72.5 (54.7 − 96.1) | 159 (131 − 193) | 155 (120 − 202) |
| 21 | 525 (466 − 592) | 638 (529 − 769) | 341 (286 − 407) | 369 (283 − 483) | 568 (496 − 651) | 697 (569 − 855) | 993 (870 − 1134) | 529 (415 − 674) | |
| HAI titer ≥1:40, % (95% CI) | 0 | 86.1 (80.8 − 90.4) | 78.0 (69.0 − 85.4) | 55.1 (48.2 − 61.8) | 53.2 (43.4 − 62.8) | 69.4 (62.8 − 75.5) | 65.1 (55.4 − 74.0) | 85.2 (79.7 − 89.6) | 83.5 (75.2 − 89.9) |
| 21 | 99.5 (97.4 − 100) | 100 (96.7 − 100) | 95.8 (92.2 − 98.1) | 94.5 (88.4 − 98.0) | 100 (98.3 − 100) | 99.1 (95.0 − 100) | 100 (98.3 − 100) | 97.2 (92.2 − 99.4) | |
| GMT ratiob (95% CI) | 21/0 | 3.8 (3.1 − 4.7) | 5.3 (3.7 − 7.6) | 8.6 (6.9 − 10.9) | 9.2 (6.6 − 13.0) | 8.5 (6.8 − 10.6) | 9.6 (6.9 − 13.4) | 6.3 (5.1 − 7.7) | 3.4 (2.7 − 4.3) |
| Seroconversion or significant increasec, % (95% CI) | 21/0 | 38.0 (31.5 − 44.8) | 41.3 (31.9 − 51.1) | 59.3 (52.4 − 65.9) | 62.4 (52.6 − 71.5) | 61.1 (54.3 − 67.7) | 60.6 (50.7 − 69.8) | 59.7 (52.9 − 66.3) | 38.5 (29.4 − 48.3) |
Values are for all subjects completing the study according to protocol. IIV3 contained 15 µg hemagglutinin per strain of A/Michigan/45/2015 (H1N1)pdm09-like virus, A/Hong Kong/4801/2014 (H3N2)-like virus, and B/Brisbane/60/2008-like virus (B/Victoria lineage). IIV4 contained 15 µg hemagglutinin of each of the above strains plus 15 µg hemagglutinin of B/Phuket/3073/2013-like virus (B/Yamagata lineage). Abbreviations: CI, confidence interval; GMT, geometric mean titer; HAI, hemagglutination inhibition; IIV4, quadrivalent inactivated influenza vaccine; IIV3, trivalent inactivated influenza vaccine
a The IIV3 formulation did not include the B/Phuket/3073/2013-like virus (B/Yamagata strain)
b Geometric mean of the individual ratios of the post-vaccination (day 21) HAI titer divided by the pre-vaccination (day 0) HAI titer
c Seroconversion was defined as a pre-vaccination (day 0) HAI titer <1:10 and a post-vaccination (day 21) HAI titer ≥1:40, and a significant increase was defined as a pre-vaccination HAI titer ≥1:10 and a ≥ 4-fold increase in HAI titer
At delivery, HAI antibody titers for the A/H1N1 strain were almost twice as high in the umbilical cord blood as in the maternal blood in both groups (1.9-fold higher in the IIV4 group, 1.8-fold higher in the IIV3 group) and were between 1.5 and 1.7 times as high for the A/H3N2 strain and two B-lineage strains (Figure 2). The cord blood to maternal blood HAI GMT ratios were similar between the IIV4 and IIV3 groups for all four vaccine strains.
Figure 2.
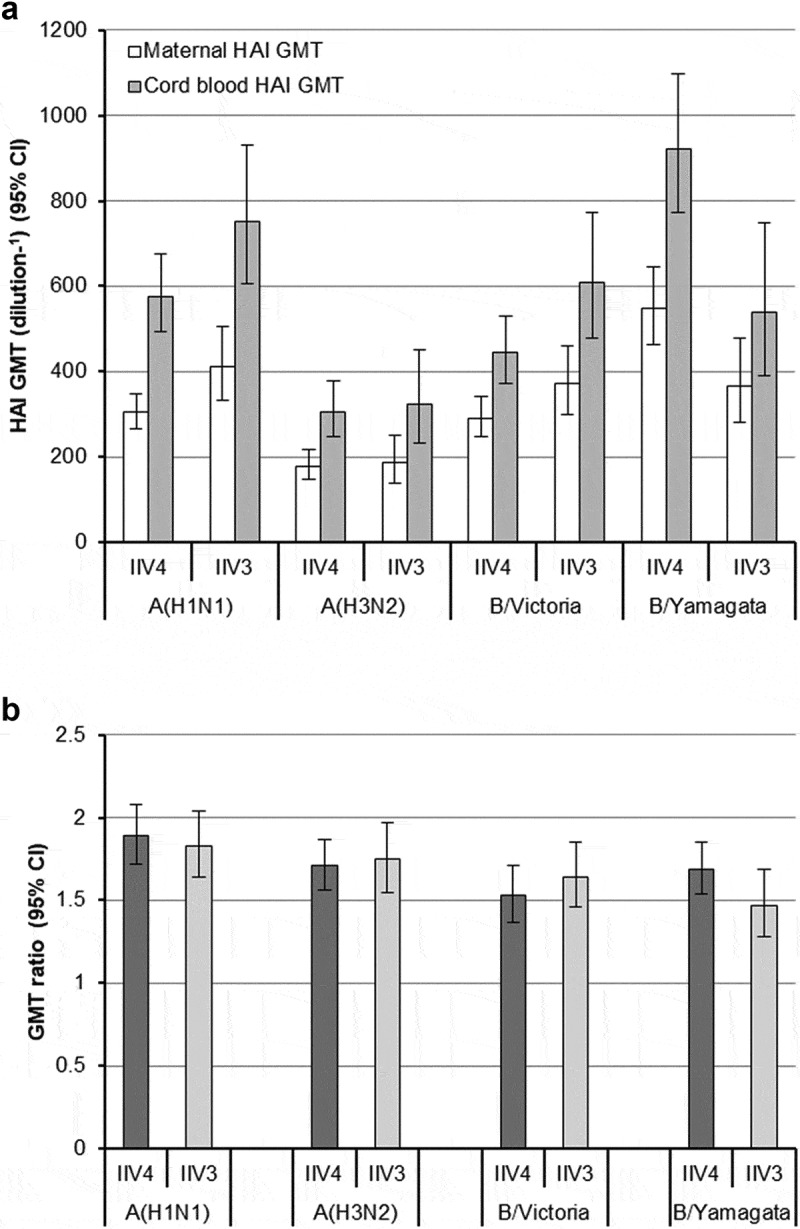
Maternal and cord blood hemagglutination inhibition (HAI) antibody titers at delivery. (a) Maternal and cord blood HAI geometric mean titers (GMTs). (b) GMT ratio: the geometric mean of the individual ratios of the cord blood HAI titer divided by the maternal HAI titer at delivery. The trivalent inactivated influenza vaccine (IIV3) contained A/H1N1, A/H3N2, and a strain from the B/Victoria lineage. The quadrivalent inactivated influenza vaccine (IIV4) contained each of the above strains plus a strain from the B/Yamagata lineage. Values are for all subjects completing the study according to protocol who provided blood and cord blood samples at delivery. For GMT ratios (b), mothers that had twins were counted twice. Abbreviation: CI, confidence interval.
Safety
The proportions of subjects reporting solicited reactions and unsolicited vaccine-related AEs were similar between the two vaccine groups (Table 2). Injection site reactions were reported by most subjects vaccinated with IIV4 (90.0%) and IIV3 (80.9%). These were typically mild (grade 1) in intensity and resolved within 3 days. Pain at the injection site was more frequently reported by subjects vaccinated with IIV4 than those vaccinated with IIV3 (88.7% [95% CI: 83.9 − 92.5%] vs. 76.5% [95% CI: 67.7 − 83.9%]) (Supplemental Table 1). The most frequently reported solicited systemic reaction in the two vaccine groups was headache. The most common vaccine-related unsolicited AEs were injection site pruritus, fatigue, and oropharyngeal pain (Supplemental Table 1). None of the subjects reported grade 3 (severe) solicited reactions or unsolicited vaccine-related AEs.
Table 2.
Proportions of subjects experiencing adverse events within 21 days after vaccination.
| IIV4 |
IIV3 |
|||
|---|---|---|---|---|
| (N = 230) |
(N = 116) |
|||
| Event | n | % (95% CI) | n | % (95% CI) |
| Immediate unsolicited AE | 0 | 0.0 (0.0 − 1.6) | 0 | 0.0 (0.0 − 3.1) |
| Solicited reaction | 214 | 93.0 (88.9 − 96.0) | 106 | 92.2 (85.7 − 96.4) |
| Solicited injection site reaction | 207 | 90.0 (85.4 − 93.6) | 93 | 80.9 (72.5 − 87.6) |
| Solicited systemic reaction | 155 | 67.4 (60.9 − 73.4) | 83 | 72.2 (63.0 − 80.1) |
| Unsolicited AE | 127 | 55.2 (48.5 − 61.8) | 61 | 52.6 (43.1 − 61.9) |
| Vaccine-related | 29 | 12.6 (8.6 − 17.6) | 17 | 14.7 (8.8 − 22.4) |
| Non-serious | 126 | 54.8 (48.1 − 61.3) | 61 | 52.6 (43.1 − 61.9) |
| Non-serious vaccine-related | 29 | 12.6 (8.6 − 17.6) | 17 | 14.7 (8.8 − 22.4) |
| Injection site non-serious vaccine-related | 9 | 3.9 (1.8 − 7.3) | 4 | 3.4 (0.9 − 8.6) |
| Systemic non-serious vaccine‑related | 23 | 10.0 (6.4 − 14.6) | 13 | 11.2 (6.1−18.4) |
| AE leading to study discontinuation | 0 | 0.0 (0.0 − 1.6) | 0 | 0.0 (0.0 − 3.1) |
| Serious AE | 2 | 0.9 (0.1 − 3.1) | 0 | 0.0 (0.0 − 3.1) |
| Vaccine-related | 0 | 0.0 (0.0 − 1.6) | 0 | 0.0 (0.0 − 3.1) |
| Death | 0 | 0.0 (0.0 − 1.6) | 0 | 0.0 (0.0 − 3.1) |
Abbreviations: AE, adverse event; CI, confidence interval; IIV4, quadrivalent inactivated influenza vaccine; IIV3, trivalent inactivated influenza vaccine
Two serious AEs were experienced within 21 days of vaccination (one hospitalization for pneumonia and one hospitalization for pre-eclampsia), although neither was considered vaccine-related. No immediate unsolicited AEs were reported and none of the participants had AEs leading to discontinuation from the study.
Birth outcomes
All birth outcomes reported were live births. One subject vaccinated with IIV4 and three subjects vaccinated with IIV3 gave birth to twins. Baby birth weights and heights, and the proportion of participants having vaginal and C-section deliveries were also similar between the two groups.
Six congenital abnormalities were reported in babies of IIV4-vaccinated mothers (2.6%) and five in babies of IIV3-vaccinated mothers (4.2%), most of which were hip dysplasias. There were no vaccine-related adverse events in newborns.
Discussion
By including strains from both circulating B lineages, IIV4s extend the protection provided by IIV3s against influenza. However, the safety and efficacy of IIV4s in pregnant women – a group prioritized for vaccination1 – has not been previously evaluated. The results from this randomized study showed that IIV4 had similar immunogenicity to IIV3 and was well tolerated in pregnant women vaccinated during the second or third trimester of pregnancy. In addition, high HAI antibody titers were found in the cord blood after delivery, suggesting maternal immunization conferred protection to newborns via transplacental antibody transfer.
Consistent with studies in non-pregnant adults,30,32,33 IIV4 induced similar HAI antibody titers to IIV3 in pregnant women for the three common vaccine strains, and higher HAI titers for the additional B lineage strain. Baseline HAI antibody titers for A/H1N1 and B/Yamagata were high in the study population. The high baseline A/H1N1 titers might be explained by previous vaccination with the 2009 adjuvanted pandemic A/H1N1 vaccine, which was received by more than one-half of Finland’s population.34 The high baseline B/Yamagata titers could be related to the high circulation of this lineage in 2017 − 2018,27 and could have limited the difference in titer increase for this strain between IIV4 and IIV3. Nonetheless, more than 95% of IIV4 recipients had titers of 1:40 or higher against each individual vaccine strain on day 21.
As reported previously,35 cord blood HAI antibody titers were higher than those from maternal blood, which indicates the newborns should have been passively immunized against the influenza strains of each vaccine. In previous clinical studies, newborns of IIV3 recipients had higher HAI titers for all vaccine strains, and were more likely to have titers of 1:40 or higher, than did newborns of placebo or control vaccine recipients.13,15 Compared with IIV3 recipients, IIV4 recipients did not have clearly increased cord blood to maternal blood HAI GMT ratios for the additional B/Yamagata strain, perhaps again due to high baseline B/Yamagata HAI titers in the population.
The pregnant women vaccinated with IIV4 experienced no differences in solicited local and systemic reactions, or unsolicited vaccine-related AEs to those vaccinated with IIV3. The frequently observed injection site reactions, headache, and myalgia after vaccination are expected reactions from influenza vaccines, and have been reported in similar proportions of participants in other studies.13–15,30 Solicited injection site reactions were more frequently reported in IIV4 recipients than in IIV3 recipients (90% vs. 81%), though this slight difference was not considered medically significant. Moreover, nearly all of the solicited reactions were mild in intensity and short-lived.
None of the reported pregnancy complications or congenital abnormalities were considered vaccine-related. This is consistent with the large body of evidence affirming no increased risk of adverse pregnancy outcomes following influenza vaccination during pregnancy.9,36,37 The observed incidence of hip dysplasias (17.1 per 1000) was within the expected incidence rate for Scandinavian countries (0.9 − 28 per 1000).38
Our study is the first to evaluate the immunogenicity and safety of IIV4 specifically in pregnant women. The study was strengthened by its randomized design and by comparing to the IIV3 formulation, which has been shown safe and effective during pregnancy.13–15 However, due to low enrollment, our study could only report descriptive analyses. The originally planned, larger sample size should not have considerably increased the rates of observed rare serious vaccine-related AEs, given that much larger studies of IIV4,30 and of IIV3 in pregnant women,13–15 show very low rates of these events. Our study was also limited by only including pregnant women in late second or third trimester, whereas the WHO recommends influenza vaccination at any stage of pregnancy.1 Nonetheless, other studies have shown IIV3 has a similar safety profile at other gestational ages.13–15,17 Finally, the study was conducted only in Finland. Including pregnant women from other countries might have increased the size of the study and helped confirm consistency across different regions.
Consistent with the recognized safety and effectiveness of IIV3 during pregnancy,1,11,13–15,39 our results support the use of IIV4 to protect pregnant women against influenza. The high cord blood HAI antibody titers also suggest maternal immunization with IIV4 would protect newborns against influenza, at least as well as IIV3,13,15 via passively acquired antibodies. The results from this study might encourage healthcare professionals to recommend IIV4 to pregnant women, for whom vaccination coverage is often poor due to concerns about influenza vaccine safety and efficacy during pregnancy.22
Funding Statement
This study was funded by Sanofi Pasteur.
Acknowledgments
We thank Drs. Tiina Karppa, Anitta Ahonen, Aino Forstén, Pauliina Paavola, Satu Kokko, Marita Paassilta, and Ilkka Seppä for recruiting participants for this study. Medical writing was provided by Dr. Jonathan Pitt (4Clinics, Paris, France) and funded by Sanofi Pasteur.
Disclosure of potential conflicts of interest
C.E., N.L., A.L.C., and V.G.B. are full-time employees of Sanofi Pasteur. T.V., M.V. and S.H. declare no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.World Health Organization . Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87(47):461–76. [PubMed] [Google Scholar]
- 2.Fell DB, Azziz-Baumgartner E, Baker MG, Batra M, Beaute J, Beutels P, Bhat N, Bhutta ZA, Cohen C, De Mucio B, et al. Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine. 2017;35(43):5738–50. doi: 10.1016/j.vaccine.2017.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertz D, Geraci J, Winkup J, Gessner BD, Ortiz JR, Loeb M.. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta-analysis of observational studies. Vaccine. 2017;35(4):521–28. doi: 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, MacDonald N. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176(4):463–68. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz FM. Influenza virus infection in infancy and early childhood. Paediatr Respir Rev. 2003;4:99–104. [PubMed] [Google Scholar]
- 6.O’Brien KL, Walters MI, Sellman J, Quinlisk P, Regnery H, Schwartz B, Dowell SF. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30(5):784–89. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 7.Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991;145(4):445–48. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- 8.Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr. 2014;173(3):265–76. doi: 10.1007/s00431-013-2023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fell DB, Savitz DA, Kramer MS, Gessner BD, Katz MA, Knight M, Luteijn JM, Marshall H, Bhat N, Gravett MG, et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG. 2017;124(1):48–59. doi: 10.1111/1471-0528.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . Pregnant women and influenza (flu); 2019. [accessed 2019 February28]. https://www.cdc.gov/flu/protect/vaccine/pregnant.htm.
- 11.The American College of Obstetricians and Gynecologists . ACOG committee opinion: influenza vaccination during pregnancy; 2018. [accessed 2019 February28]. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co732.pdf?dmc=1&ts=20190228T0855428827.
- 12.European Centre for Disease Prevention and Control . Seasonal influenza vaccination and antiviral use in EU/EEA Member States: An overview of vaccine recommendations for 2017–2018 and vaccination coverage rates for 2015–2016 and 2016–2017 influenza seasons. Stokholm (Sweden): ECDC; 2018. [Google Scholar]
- 13.Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, Adrian PV, van Niekerk N, Treurnicht F, Ortiz JR, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–31. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 14.Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, Stewart L, Mullany LC, Chu HY, LeClerq SC, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17(9):981–89. doi: 10.1016/S1473-3099(17)30252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapia MD, Sow SO, Tamboura B, Teguete I, Pasetti MF, Kodio M, Onwuchekwa U, Tennant SM, Blackwelder WC, Coulibaly F, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16(9):1026–35. doi: 10.1016/S1473-3099(16)30054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 17.Munoz FM, Jackson LA, Swamy GK, Edwards KM, Frey SE, Stephens I, Ault K, Winokur P, Petrie CR, Wolff M, et al. Safety and immunogenicity of seasonal trivalent inactivated influenza vaccines in pregnant women. Vaccine. 2018;36(52):8054–61. doi: 10.1016/j.vaccine.2018.10.088. [DOI] [PubMed] [Google Scholar]
- 18.Sakala IG, Honda-Okubo Y, Fung J, Petrovsky N. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12(12):3065–71. doi: 10.1080/21645515.2016.1215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omer SB, Clark DR, Aqil AR, Tapia MD, Nunes MC, Kozuki N, Steinhoff MC, Madhi SA, Wairagkar N. Maternal influenza immunization and prevention of severe clinical pneumonia in young infants: analysis of randomized controlled trials conducted in Nepal, Mali and South Africa. Pediatr Infect Dis J. 2018;37(5):436–40. doi: 10.1097/INF.0000000000001914. [DOI] [PubMed] [Google Scholar]
- 20.Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004;21(6):333–39. doi: 10.1055/s-2004-831888. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355–61. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014;32(36):4602–13. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 23.Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013;12(9):1085–94. doi: 10.1586/14760584.2013.824709. [DOI] [PubMed] [Google Scholar]
- 24.Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8(1):81–88. doi: 10.4161/hv.8.1.17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings L, Huang QS, Barr I, Lee PI, Kim WJ, Buchy P, Sanicas M, Mungall BA, Chen J. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia-Pacific region. Influenza Other Respir Viruses. 2018;12(3):383–411. doi: 10.1111/irv.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin Infect Dis. 2014;59(11):1519–24. doi: 10.1093/cid/ciu664. [DOI] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control . Seasonal influenza, 2017–2018. ECDC Annual epidemiological report for 2017 Stokholm (Sweden); 2018.
- 28.Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103(3):e43–51. doi: 10.2105/AJPH.2012.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Insitute for Health and Welfare. Finland . Influenza vaccinations in pregnancy [in Finnish]; 2019. [accessed 2019 April2]. https://thl.fi/fi/web/rokottaminen/rokotteet/kausi-influenssarokote/raskaana-olevat.
- 30.Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013;31(47):5572–78. doi: 10.1016/j.vaccine.2013.08.069. [DOI] [PubMed] [Google Scholar]
- 31.International Conference on Harmonisation . Clinical safety data management: definitions and standards for expedited reporting E2A; 1994. doi: 10.3168/jds.S0022-0302(94)77044-2 [DOI]
- 32.Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, Santos J, Montalban MC, de Looze FJ, Eizenberg P, Hall S, Dupuy M, Hutagalung Y, et al. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: a randomized, controlled, phase III trial. Vaccine. 2015;33(21):2485–92. doi: 10.1016/j.vaccine.2015.03.065. [DOI] [PubMed] [Google Scholar]
- 33.Sesay S, Brzostek J, Meyer I, Donazzolo Y, Leroux-Roels G, Rouzier R, Astruc B, Szymanski H, Toursarkissian N, Vandermeulen C, et al. Safety, immunogenicity, and lot-to-lot consistency of a split-virion quadrivalent influenza vaccine in younger and older adults: A phase III randomized, double-blind clinical trial. Hum Vaccin Immunother. 2018;14(3):596–608. doi: 10.1080/21645515.2017.1384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syrjanen RK, Jokinen J, Ziegler T, Sundman J, Lahdenkari M, Julkunen I, Kilpi TM. Effectiveness of pandemic and seasonal influenza vaccines in preventing laboratory-confirmed influenza in adults: a clinical cohort study during epidemic seasons 2009–2010 and 2010–2011 in Finland. PLoS One. 2014;9(9):e108538. doi: 10.1371/journal.pone.0108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis. 1993;168(3):647–56. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 36.McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine. 2015;33(18):2108–17. doi: 10.1016/j.vaccine.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 37.Giles ML, Krishnaswamy S, Macartney K, Cheng A. The safety of inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccin Immunother. 2019;15(3):687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loder RT, Skopelja EN. The epidemiology and demographics of hip dysplasia. ISRN Orthop. 2011;2011:238607. doi: 10.5402/2011/238607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Advisory Committee on Immunization Practices . Committee on immunization practices. Prevention and control of seasonal influenza with vaccines. recommendations of the advisory committee on immunization practices–United States, 2013-2014. MMWR Recomm Rep. 2013;62:1–43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention . Pregnant women and influenza (flu); 2019. [accessed 2019 February28]. https://www.cdc.gov/flu/protect/vaccine/pregnant.htm.
- The American College of Obstetricians and Gynecologists . ACOG committee opinion: influenza vaccination during pregnancy; 2018. [accessed 2019 February28]. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co732.pdf?dmc=1&ts=20190228T0855428827.


