ABSTRACT
Although respiratory syncytial virus (RSV) infection in infants and young children is a global public health issue, development of a safe RSV vaccine has been impeded by formalin-inactivated RSV-enhanced respiratory disease (ERD). In developing a safer yet effective RSV vaccine for children, a strategy to decrease over-reactive T cells and increase neutralizing anti-RSV antibodies should be considered. We previously demonstrated that adult mice immunized with RSV recombinant G protein plus low-dose Cyclosporine A (G+ CsA) could, upon subsequent RSV challenge, produce increased levels of antigen-specific T regulatory cells in lungs that overcame the ERD. Neutralizing anti-RSV antibodies that prevented viral infection were also elicited. In this study, we investigated if such a G+ CsA vaccine could provide infant mice with the same protection from RSV infection without ERD. The results showed that the G+ CsA vaccine could prevent RSV infection with only a mild loss of body weight. Importantly, there was nearly normal morphology and no mucus appearance in lung tissues after RSV challenge. These results demonstrate that the G+ CsA vaccine strategy achieved similar benefits in the neonatal prime and infancy boost model as in the adult mouse model. The G+ CsA immunization strategy is potentially safe and effective in neonates and infants because it suppresses the devastating ERD.
KEYWORDS: RSV, vaccine, vaccine enhanced disease, G protein, CsA, protection, neonate protection
Introduction
Respiratory syncytial virus (RSV) is a ubiquitous negative-sense RNA virus of the Pneumovirus genus and Paramyxoviridae family. It is responsible for acute lower respiratory tract disease among infants and young children around the world.1,2 Almost every infant has experienced at least once RSV infection before reaching two years of age. The RSV-attributed mortality rate for infants aged less than 12 months, as estimated by epidemiological studies over 11 continuous winters, was 2.9 per 100,000, much higher compared to 2.0 for influenza-attributed death.3,4 Furthermore, a growing concern for the potential risk of RSV infection is the development of asthma in later-life following viral-caused bronchiolitis. Though the mechanism is largely unclear, a strong connection between RSV infection-caused bronchiolitis in infancy and later childhood asthma has been reported.5,6
Although RSV is a major public health issue, development of vaccines against RSV has been hampered by the disastrous results obtained with the clinical trial of formalin-inactivated RSV (FI-RSV) vaccine in infants in the 1960s. This vaccine did not protect against RSV infection, rather it enhanced the disease severity and hospitalizations in the vaccinated children who encountered RSV infection post-vaccination.7,8 Causes of enhanced respiratory disease (ERD) remain under investigation. A T-helper-2-type biased (Th2-biased) immune response with the recruitment of eosinophils and neutrophils in lung tissue is thought to be the main cause. But a number of studies also showed that the symptoms can be reduced by depleting total CD4+ or CD8+ T cells, or largely eliminated by depleting both T cells.9–11 CD8+ T cells, particularly the memory CD8+ T cells, are thought to constitute a strong component of protection against RSV infectivity. However, a recent study frustratingly demonstrated that the RSV-specific memory CD8+ T cells are contributing to the exaggerated lung inflammation and injury.12
Additionally, recent studies demonstrated that the key cause of ERD was a lack of regulatory T cells (Treg) in lungs after FI-RSV vaccination and that led to an exaggerated Th2-biased response. The studies have shed light on immunological requirements for the development of novel strategies for a safer and effective RSV vaccine.13,14
Cyclosporine A (CsA) is a widely used immunosuppressant in organ transplantation and treatment of autoimmune diseases.15,16 Moreover, the concept of immunizing with antigen together with an immunosuppressant to induce Treg cells (iTreg) and effectively treat autoimmune diseases has previously been proven in animal models in our lab.17,18 The RSV G protein functions as an attachment protein during RSV infection and has been selected as a vaccine antigen candidate in many approaches.2 These include subunit-, nanoparticle-, peptide-, and bacterial-based RSV vaccines.19–21 We found no report that a combination of CsA with RSV antigen could be useful for RSV vaccine development. But inspired from our iTreg induction strategy, we combined RSV G truncated protein with CsA and formulated the combination as a novel vaccine (G+ CsA). As demonstrated in our previous study, the G+ CsA vaccine induced increased levels of neutralizing anti-RSV antibodies that led to reduced viral titers in lungs. Importantly it also induced iTreg cells in lungs and a robust response to a subsequent RSV challenge with effective suppression of ERD in an adult murine model.22
In this study, we tested the G+ CsA vaccine in a strategy of priming neonates and boosting in infancy using a 5-day-old-mouse model. The newborn (5 to 7-days-old) mouse has been demonstrated to correlate best with the stage of immune maturation of human neonates.23 In the context of RSV vaccine development, the ill-fated FI-RSV was found to elicit a more severe ERD situation in neonatal compared with adult mice.24 Indeed, many RSV vaccines have been tested with a neonatal mouse model.25–27 We demonstrated here that after priming neonatal and boosting infant mice the G+ CsA vaccine could trigger efficient anti-RSV immunity without the ERD in the lungs.
Results
Neonatal priming and infancy boosting with the G+ CsA vaccine can protect pups from RSV challenge by way of an effectively recalled anti-RSV humoral response
Five-day-old mice were immunized on days 0 (first day of immunization) and 14 with 10 μg of RSV A2 recombinant G protein formulated with 10 μg of CsA, and intranasally challenged with 5 × 107 PFU of RSV A2 at day 28 (Figure 1A). At day 33, mice were sacrificed, and lung tissues were homogenized for RSV N gene mRNA expression profiles as detected by qRT-PCR. The results of qRT-PCR assay have been shown to be strongly correlated with tissue culture plaque assay. As depicted in Figure 1B, the G+ CsA vaccine immunization led to significantly reduced viral mRNA loads in lungs 5 days after RSV challenge (G+ CsA versus all control groups, P < .001). Sera were also taken from the sacrificed mice and analyzed by ELISA for total specific anti-G IgG titers and by micro-neutralizing assay for neutralizing antibodies. The G+ CsA vaccinated mice showed significantly increased anti-G IgG antibody titers (Figure 1C, G+ CsA versus G, P < .001; G+ CsA versus FI-RSV, P < .05) and anti-RSV neutralizing antibody titers (Figure 1D, G+ CsA versus G, four-fold increase, P < .001; G+ CsA versus FI-RSV, P < .01) .
Figure 1.
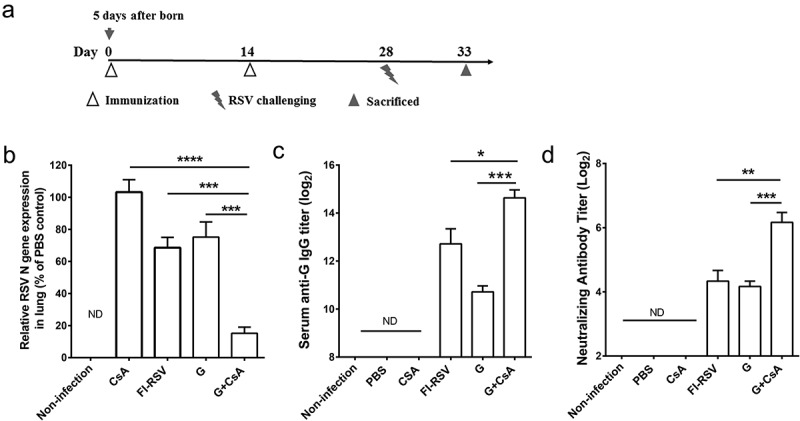
Humoral immune response and protective effect of the G+ CsA vaccine in mice after priming neonates and boosting in infancy. (A) Neonatal mice were immunized at day 0 (5 days after birth) and day 14 then challenged intranasally with RSV at day 28. The mice were sacrificed at day 33. (B) Individual lung tissue RSV load was assessed by qRT-PCR and expressed as % of the load found in the PBS control group (unvaccinated infection control). (C) Serum anti-G IgG antibody titer was tested by ELISA. (D) Serum RSV-neutralizing antibody was tested by plaque assay. Data are mean ± SEM of n = 4–6 mice per group. ND, no detection. *P < .05, **P < .01, ***P < .001, ****P < .0001.
These results demonstrated that, with an immunization strategy of priming neonates and boosting in infancy, the G+ CsA vaccine can protect mice against RSV infection in later life through the effectively recalled anti-RSV humoral responses.
Activation and differentiation of b cells into germinal b cells in neonates primed with the G+ CsA vaccine
Antibody is secreted by differentiated plasma cells. Before that, the naive B cell must be activated by antigen and matured in the germinal center (GC). To further evaluate the effects of the G+ CsA vaccine on the B cell (B220+) activation and differentiation, 5-day-old neonatal BABL/c mice were immunized once and defined as day 0 mice. At day 7 post-immunization, mice were sacrificed for flow cytometry analysis of the percentage of GC B cells (B220+IgD−GL7+) and for expression of B cell activation markers in splenocytes (Figure 2A). We observed that the percentage of IgD−GL7+ cells among B220+ cells was significantly increased in the G+ CsA immunized neonates compared with the control groups (Figure 2C, G+ CsA versus G, P < .01; G+ CsA versus PBS control, P < .01). Immunization with the G+ CsA vaccine also promoted the expression of CD80 and CD86 on B220+ splenocytes, whereas there was no significant difference between neonates vaccinated with G protein alone and the PBS controls (Figure 2D, E). These results further supported the evidence that immunization of the G+ CsA vaccine could promote B cell differentiation and maturation in neonates.
Figure 2.
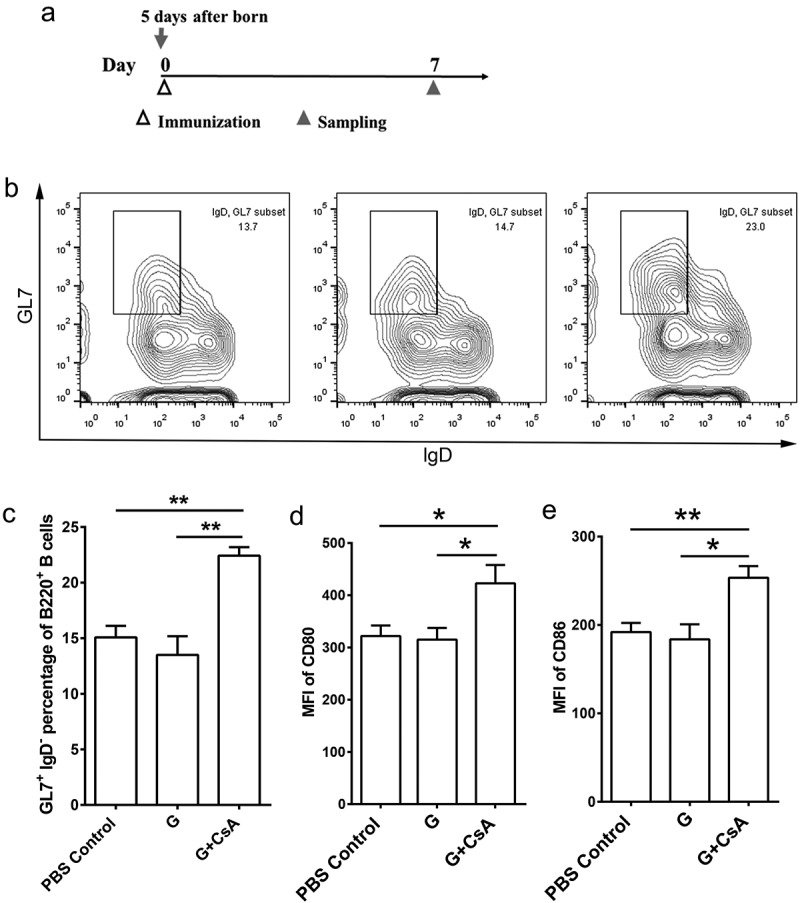
Activation of spleen B cells by the G+ CsA vaccine priming in neonatal mice. (A) Neonatal mice were immunized with vaccines once at day 0 (5 days after birth), and at day 5 the mice were sacrificed for B cell detection in spleens. (B) Representative flow cytometry plots of GC B cells (B220+IgD−GL7+), Left, the PBS control group; Middle, G protein alone group; Right, the G+ CSA group. (C) The frequencies of GC B cells in B220+ splenocytes. (D, E) The expression profile of CD80 (D) and CD86 (E) in B220+ splenocytes are shown as MFI. Data are mean ± SEM of n = 5 mice per group. *P < .05, **P < .01, ***P < .001, ****P < .0001.
G+ CsA vaccinations suppress the enhanced respiratory disease seen upon RSV challenge
Bodyweight loss and histopathology change of lung tissue are indications to assess the disease morbidity caused by ERD. In this study, 5-day-old neonatal mice were immunized on days 0 (first day of immunization) and 14 and intranasally challenged with 5 × 107 PFU of RSV A2 at day 28. Body weights were then measured daily till day 33 when mice were sacrificed, and lung tissues were sectioned for staining with H&E and PAS. Based on the daily monitored weight data after RSV challenge, mice immunized with the G+ CsA vaccine showed changes similar to those of the non-infected age-matched control group. In contrast, the increase in body weight was obviously retarded in other vaccine groups (Figure 3A). We also performed lung pathology analysis by assessing morphological changes in lung tissues stained with H&E and PAS. The H&E staining of lung tissues after RSV infection showed that the G+ CsA-vaccinated mice, though they had some slight inflammation in areas near the bronchia, had significantly less infiltration with inflammatory cells (Figure 3B, G+ CsA versus G, P < .01; G+ CsA versus FI-RSV, P < .001; G+ CsA versus CsA, P = .0558; G+ CsA versus PBS, P < .001). The lung tissues in the FI-RSV-vaccinated or the G protein-vaccinated mice had obvious areas of inflammation with massive cell infiltration and morphological lesions. In addition, positive staining with PAS, indicating the existence of mucus, was seen in FI-RSV and G protein alone immunized groups. In contrast, the lung tissues from the G+ CsA-vaccinated mice had no detectable PAS-positive staining (Figure 3C). These results demonstrated that the G+ CsA vaccine could protect neonatal mice from the RSV infection without inflammation and morphological lesions in their lungs, whereas marked lung injuries and morphological lesions were observed in the control groups after the RSV challenge.
Figure 3.
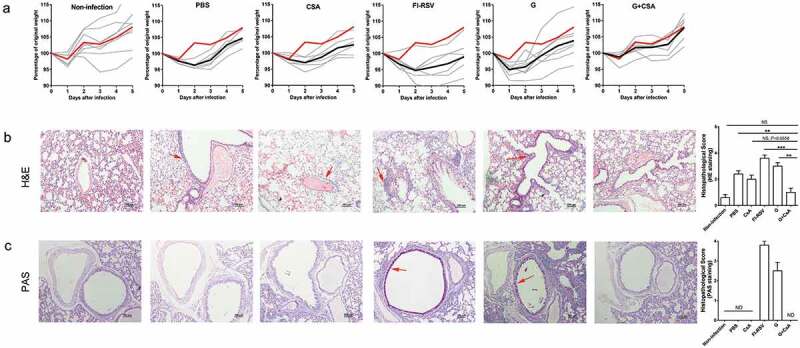
Priming neonates and then boosting infants with the G+ CsA vaccine protects mice from RSV infection without incurring ERD. Neonatal mice (5-day-old) were immunized on days 0 and 14 with vaccines or given control treatments as indicated and then challenged i.n. with RSV on day 28. (A) Body weight changes were recorded daily until day 5 post-infection. Percentage change from the original weight (weight on day 28) is shown (Y axis) plotted against days post-infection (X axis); red solid lines show the means of the non-infected group; black solid lines show the means of the infected groups; gray solid lines show weight changes of individual mice in the groups, n = 6–8). Lung tissues were sectioned and stained with both H&E (B) and PAS (C) at 5 days post-infection. Solid red arrows indicate a typical lymphocyte infiltration seen after H&E staining and typical areas of mucus secretion revealed by PAS staining. Graphs are representative of 5–6 animals per group. The scale bar is 100 μm. Histopathology was scored for severity on a scale of 0 (normal) to 4 (severe). ND, no detection. Data are mean ± SEM, *P < .05, **P < .01, ***P < .001, ****P < .0001.
Priming with the G+ CsA vaccine in neonates induces il-10-expressing iTreg cells in lymph node and spleen
We previously demonstrated that, in adult mice, immunizations with G+ CsA vaccine could induce IL-10-expressing iTreg cells and lead to suppression of ERD. Here we sought to examine if the G+ CsA vaccine immunizations could also induce IL-10-expressing iTreg cells in neonates. Five-day-old newborn BABL/c mice were immunized once with the G+ CsA vaccine, the G protein alone or PBS on day 0. Splenocytes from spleens (SP) and lymphocytes from draining lymph nodes (LN) were sampled at days 3, 7, 10 and 14, (Figure 4A) and analyzed by flow cytometry. The iTreg cells were stained with anti-CD4, CD25 and Foxp3 fluorescent antibodies. The proportion of iTreg cells within the CD4+ T cell population increased during this time in all three groups (Figure 4B,C). The proportions of iTreg in both G+ CsA vaccine and G protein alone groups were consistently higher than the baseline proportions in the PBS control group (normal neonates). Statistical analysis was conducted between G+ CsA vaccine and G protein alone groups. Three days after the vaccination, there was no difference in the proportion of iTreg cells between the two groups. But, on day 7, iTreg cells were significantly induced in both splenocytes and lymphocytes of dLN in the G+ CsA immunized group compared with the G protein alone group (P < .01 in both LN and SP). There were no significant differences between these groups on days 10 and 14.
Figure 4.
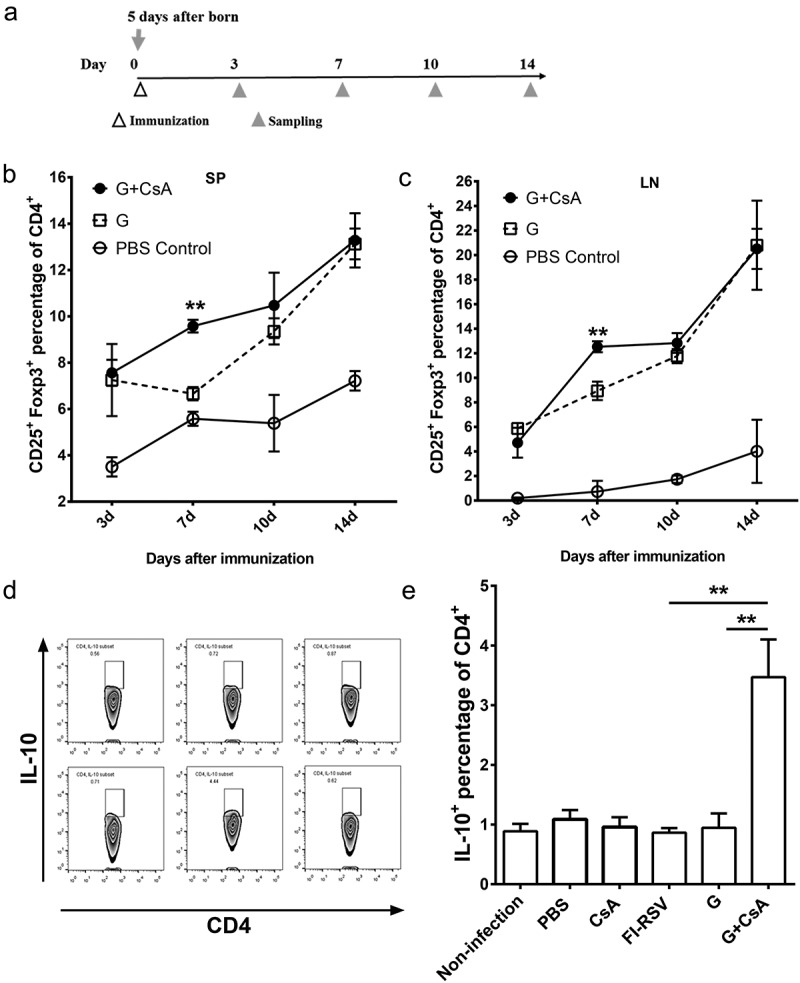
Treg cells were induced in G+ CsA vaccinated neonatal mice and IL-10 was highly expressed in CD4+ T cells after in vitro stimulation. (A) Schematic outline of testing Treg percentage. Neonates were immunized at day 0 (n = 3), spleen (SP) and lymph node (LN) were sampled at days 3, 7, 10 and 14 for Treg cell detection by flow cytometry. (B) Dynamic changes of Treg cells in spleen and (C) in lymph node. (D) Neonatal mice (n = 3–6) were immunized and challenged as in Figure 1 A. After sacrifice, splenocytes were sampled and stimulated in vitro with G protein for 3 days. IL-10 expression were tested in CD4+ cells by flow cytometry. Results of statistical analysis are shown in histograms of mean ± SEM (E) **P < .01.
Since IL-10 serves as an anti-inflammatory cytokine secreted by iTreg cells, we sought to determine the levels of IL-10 expression within the CD4+ T population after two rounds of vaccination followed by RSV challenge. Five-day-old BABL/c mice were immunized at days 0 and 14 and challenged with 5 × 107 PFU of RSV A2 intranasally at day 28. Splenocytes were isolated at day 33 and stimulated in vitro with G protein for 3 days. Then the cells were intracellularly stained with anti-IL-10 antibody and analyzed by flow cytometry (Figure 4D). The G+ CsA-vaccinated neonates had significantly increased levels of IL-10 expressed in CD4+ T cells after the antigen stimulation. In contrast, the levels of IL-10 expression in the other immunized groups were almost as low as in the uninfected control group (Figure 4E, G+ CsA versus G, P < .01; G+ CsA versus FI-RSV, P < .01).
The expression profiles of cytokines and chemokines in the vaccine injection site and draining lymph node
Neonates are sensitive to immunization and show prominent local reactive responses, such as rash, itch and pain. Those reactions may be due to a cascade of activation of proinflammatory or inflammatory cytokines. To examine this, neonatal mice were subcutaneously immunized once in the dorsal skin with the G+ CsA vaccine, the G protein alone or PBS. Three hours after immunization, mice were sacrificed and inguinal lymph nodes near the injection site and pieces of 5 mm × 5 mm skin tissue around the injection pinhole were carefully separated from each animal. Isolated tissues and local lymph nodes were homogenized with the same buffer and volume. Supernatants were applied to a quantitative cytokine/chemokine detection assay with the Legendplex kit (Figure 5A). The results showed that levels of IL-6 (P < .001), CCL17 (P < .01) and CXCL1 (P < .001) were significantly increased in the local tissue around the G+ CsA-injected site compared with the site injected with G protein alone. There was a similar tendency toward increases in these three factors in inguinal LN after G+ CsA injection although there was no significant difference between the two groups. However, the level of IFN-gamma in inguinal LN was significantly increased in the G+ CsA-injected neonates compared with the group receiving G protein alone (P < .05). There were no differences in the other cytokines or chemokines between the two groups (Figure 5B–E).
Figure 5.
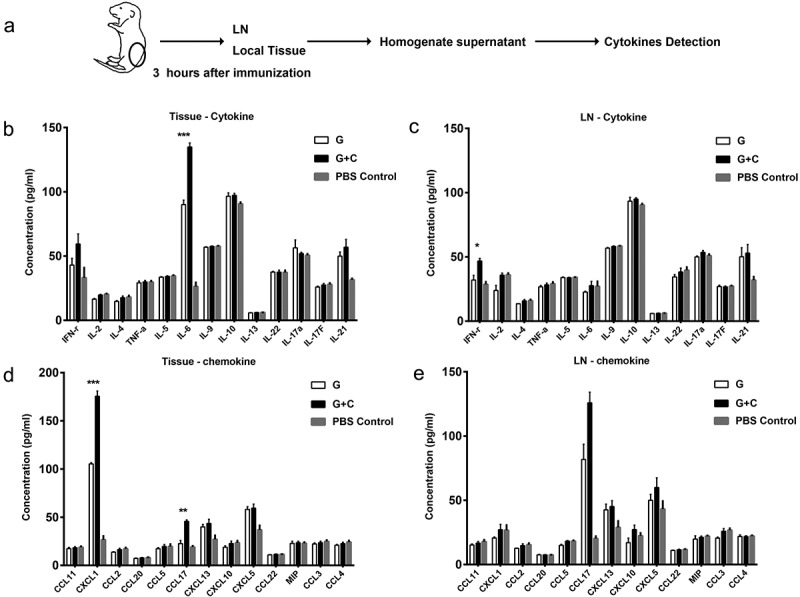
The expression profiles of cytokines and chemokines in local tissue and lymph node of the G+ CsA injection site. (A) Schematic outline . Three hours after neonate immunization (n = 4), local tissue and lymph node near the injection site were sampled for homogenization. The supernatants were screened against the cytokine panel (B and D) and the chemokine panel (D and E) of the Legengplex cytokine panel kit. Statistical analysis compared G+ CsA vaccine group and G protein alone group. Data are mean ± SEM, *P < .05, ***P < .001.
Discussion
The over-reactive host immune response with the risk of elevated immunopathology during RSV infection after FI-RSV vaccination has been found to be tightly associated with the lack of Treg cells.14 To void ERD, vaccine strategies must consider a way to induce or maintain the Treg cells in place in addition to achieving high levels of anti-RSV neutralizing antibodies. Indeed, the ability of this novel vaccine to induce iTreg cells has been documented previously by us in 2016. The combination of the RSV G protein with CsA (G+ CsA), induced a high level of neutralizing anti-RSV antibodies and induced iTregs in the lung, resulting in a near absence of ERD following the RSV challenge in adult animals.22
Here, we investigated the safety and efficacy of this novel G+ CsA vaccine in young mice with a priming and boosting immunization strategy. As neonates are vulnerable to RSV infection, an earlier RSV vaccination should give greater benefit. Accordingly, we developed a strategy of vaccinating by priming neonates (5 days old) and boosting infants (19 days old). Vaccinations with G+ CsA vaccine in neonates effectively prevented the RSV infection after intranasal challenge as seen in reducing viral mRNA loads in lungs. The prevention of RSV infection was consistent with the successfully recalled antibody response and the activated spleen B cells. After challenge with RSV, the least changes in body weight and lung morphology were achieved in the young animals treated with 10 μg of the G+ CsA vaccine (Figure 3). These results demonstrated that the G+ CsA vaccine, when used to prime neonates and boost infants, can provide a potentially safe and effective way to protect vaccinees from subsequent RSV challenge infection and concurrently avoid the problem of ERD.
Similar to the results in adult mice, we found that Treg cells were significantly induced both in spleen and lymph node at day 7 after neonatal vaccination with the G+ CSA vaccine (P < .01). Simultaneously, the expression of IL-10, which is the predominant functional cytokine of Treg cells, was significantly increased in the CD4+ splenocytes of G+ CSA-vaccinated animals after specific antigen simulation in vitro (Figure 4E). Several recent reports have shown that Treg cells and IL-10 play important roles in the maturation of B cells, promoting B cell activation and facilitating antibody production.28–31 The reports suggested that the induction of Treg cells and high secretion of IL-10 could directly contribute to the elevation of the humoral immune response. The higher level of iTreg cells was maintained for 14 days and declined thereafter. Such a leveling off of iTreg was apparently concealable behind a daily increasing trend of nTreg cells. A recalled iTreg response, occurring on encounter with an invasion of RSV, can be anticipated to result in rapid proliferation, chemotaxis into lungs and suppression of ERD as previously described. We and others have previously reported that depletion of Treg or blockage of IL-10 allowed the development of excess pulmonary infiltration and ERD following RSV.
CsA, noted as an immune-modulator at physiological concentration, suppresses T cell function via blocking the phosphatase activity of calcineurin in T cells.32,33 But it is less well known as an immune-modulator at one thousand-fold lower dose and affecting local inflammatory responses as a vaccine adjuvant as described here. We observed that levels of expressed IL-6 and CXCL1 and CCL17 around the local injected tissue were the most increased among the examined cytokines and chemokines, while IFN-gamma also exhibited an increased level in the draining lymph node (Figure 5B–E). IL-6 mainly acts as a proinflammatory cytokine, but numerous studies have reported that IL-6 has a role in promoting activation of myeloid-derived suppressive cells (MDSCs) in both human and mouse models34–36 . CXCL1 is a major neutrophil- and MDSC-activating chemokine and is strongly associated with the mobility of MDSCs37 and CCL17, a ligand of CCR4, plays a crucial role in the accumulation of Treg cells.38,39 This is in agreement with our previous finding that CCL17 plays an important role in the migration of G+ CsA-induced iTreg cells into lung tissue in adult mice.22 Taken together, our findings suggest that G+ CsA immunization in neonatal mice may induce migration of MDSC to the injection site, initially via both IL-6 and CCL17, facilating subsequent iTreg cell induction.40–42 Although we did not find many changes among other cytokines and chemokines, this may be a consequence of examining only one time point. Changes found at one point of time may not be reflected in changes at other times. The dynamics of changes during subsequent times should be further investigated since changes in additional local microenvironmental factors are likely to be implicated in the efficient stimulation of the neonate’s immune responses by the G+ CsA RSV vaccine.
There are two major limitations in this study that should be addressed in future studies. First, the question of how a local cytokine environment influenced the level of iTreg cells in spleen was not examined. Since the infiltration of eosinophils into lung was thought to be the main cause of ERD found in vaccinated young children in the 1960’s clinical trials, such infiltrations after G+ CsA vaccination and RSV challenge must be assessed in future study.
In summary, this study provides further characterization of the G+ CsA vaccine in a neonatal mouse model by using a priming of neonates and boosting of infants immunization strategy. Our data suggest that neonatal immunization with the G+ CsA vaccine can protect mice against RSV infection more safely than other vaccines by suppressing ERD in later life. As no commercial RSV vaccine is available currently, this novel vaccine may have a role in alleviating the staggering burden of morbidity and mortality due to RSV infection around the world.
Materials and methods
RSV, growth conditions and quantification
Plaque-purified human RSV (type A2 strain from the ATCC, Rockville, MD) was propagated in HEp-2 cells, and concentrated by ultracentrifugation (50,000 g for 1 h). RSV loads in the whole lung were measured by quantitative Real-Time PCR (qRT-PCR) with lower limits of detection of 10 copies/reaction, as described previously.26,43 Data were normalized to β-actin, by calculating the ΔCt value. The ΔΔCt values were then calculated (indicated group sample ΔCt – mean ΔCt of PBS control group). The RSV load was expressed as % of the PBS control group (100 × 2-ΔΔCt).
Animals and RSV infection
Parental BALB/c mice were purchased from the Shanghai SLAC Laboratory Animal Co. LTD (Shanghai, China), and neonates were obtained by local inbreeding. Breeding cages were checked daily for new births, and the day of birth was recorded as day 0. Pups were kept with mothers until weaning at the age of 4 weeks. All mice were kept under specific pathogen-free conditions according to the animal welfare guidelines for experimental animals of the Fudan University. Mice were infected intranasally (i.n.) with 5 × 107 PFU of RSV A2 in 50 μl volume under anesthesia on the 14th day after the second immunization.
Vaccines preparation and immunization
The recombinant RSV G protein used in this study was of the same batch used in our previous adult mice study. Basic information about the RSV recombinant G protein was described by Chaofan Li et al.22 In this study, the G protein was dissolved at 400 μg/ml in PBS before use. CsA (Santai, Taishan, Chain) was dissolved in a solution at 400 μg/ml; for the G+ CsA vaccine, G protein solution was mixed with CsA solution at 1:1; for the G protein alone vaccine, G protein solution was mixed with PBS at 1:1; for CsA alone, CsA solution was mixed with PBS at 1:1. The FI-RSV vaccine was formulated as described by Kim et al.8 Briefly, RSV was inactivated by formalin at 4,000:1 (v/v) for 72 h at 37°C, and then centrifuged at 50,000 g for 1 h at 4°C. The pellet was diluted 1:25 in minimum essential medium (MEM) and subsequently mixed with Imject Alum Adjuvant (Thermo Scientific, Rockford, IL) as 10/1 (v/v), suspended in 1/4 of the original volume in serum-free MEM, and stored at 4°C.
For the positive ERD control group, a dose of FI-RSV vaccine which contained 5 × 107 TCID50 inactivated RSV in 50 μl was injected per mouse intramuscularly (i.m.). For the immunization of other groups, 50 μl of the indicated formulation was injected per mouse subcutaneously (s.c.). The subcutaneous route was chosen for the G+ CsA vaccine because we had demonstrated that the s.c. injection significantly increased the antibody response compared with the i.m. route (P< .05).22
Assays of antibodies
To detect serum anti-G-specific IgG antibody, 96-well plates were coated with 100 μl G protein at a concentration of 2 μg/ml (50 mM carbonate-bicarbonate buffer, pH 9.6) at 37°C for 2 h, and blocked with 5% BSA in PBST at 37°C for 1 h. Then, the plates were incubated with serially diluted (2-fold) sera for 1 h at 37°C. Bound antibody was then captured with HRP-conjugated goat anti-mouse IgG (Southern Biotech, Birmingham, USA).
The neutralizing antibody assay was performed as described previously.44 Briefly, sera were heat-inactivated at 56°C for 30 min and serially diluted (5-fold) in a total of 100 μl PBS. Then the diluted sera were incubated with 3 × 103 TCID50 of virus for 2 h at 4°C. Approximately, 5 × 103 HEp-2 cells in 100 μl DMEM-F12 supplemented with 2% FBS were added to each well of a 96-well microtiter plate. The virus-serum mixture was added to the appropriate wells and incubated for 3 days in a 5% CO2 incubator at 37°C. Plates were then washed three times with 0.05% Tween-20 in PBS and fixed with 80% ice-cold acetone in PBS followed by blocking with 3% blocking buffer. Goat anti-RSV antibody (Meridian, ME, USA) was added to the appropriate wells and incubated for 60 min at 37°C. After washing three times, bovine anti-goat IgG-HRP (Santa Cruz, CA, USA) was added, the enzymatic reaction was developed and ODs were read at 450 nm and 620 nm. The neutralization titer was calculated from the average OD of the wells by extrapolating the inverse of the serum dilution that resulted in a 50% reduction of RSV activity. The calculation of neutralization titer is by the method of Reed and Muench.45
Histopathology
For the histopathology analysis, left lung tissue was carefully removed and fixed with 4% paraformaldehyde, and transverse sections (5–7 μm) were stained with hematoxylin and eosin (H&E) or Periodic Acid-Schiff (PAS) for histopathological evaluations. Histopathology was scored for severity in terms of infiltration of inflammatory cells and mucus secretion. The scale of scoring is 0, normal; 1, minimal; 2, slight; 3, moderate; 4, severe.
Flow cytometry and cytokine detection
Samples were stained with Fixable Viability Dye eFluor™ 780 (eBioscience) to remove dead cells before antibody staining. Cells were stained with the following surface antibodies: CD4 FITC or eFluor 450, CD25 PE, B220 APC, IgD FITC, GL-7 PE, MHCII Super Bright 600 (all from eBioscience) and CD80 BV421, CD86 BV510 (both from Biolegend). All intracellular staining was done with the Foxp3/transcription factor staining buffer set (eBioscience) for IL-10 with Percp-Cy5.5, Foxp3 APC (both from eBioscience). The data were collected with BD Fortessa flow cytometer (BD Biosciences, San Diego, CA, USA). Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
For testing the cytokine expression profiles, tissue or lymph node of the injection site were carefully separated. The separated tissues were then chopped and homogenized and centrifuged at 10,000 rpm for 30 min. The supernatants were diluted and tested with Legengplex cytokine panel kit (Biolegend, San Diego, CA, USA) as the instruction book indicated. The kit includes two panels, one of which is for cytokines. The cytokines included were: IFN-r, IL-2, IL-4, TNF-a, IL-5, IL-6, IL-9, IL-10, IL-13, IL-22, IL-17a, IL-17F, IL-21; the chemokine panel included: CCL11, CXCL1, CCL2, CCL20, CCL5, CCL17, CXCL13, CXCL10, CXCL5, CCL22, MIP, CCL3, CCL4. The data was collected with BD Fortessa flow cytometer (BD Biosciences, San Diego, CA, USA). Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Statistics were carried out using GraphPad Prism Software 6.0 (GraphPad, La Jolla, CA, USA) and presented as mean ± SEM. An unpaired Student’s t test analysis was used for all data analysis. * P < .05, ** P < .01, *** P < .001, **** P < .0001.
Funding Statement
This work was partly supported by the Ministry of Science and Technology of China, National Key Technologies R&D Program-Major New Drugs Innovation and Development [2013ZX09102041], Nature Science Foundation of China [31430027], and National High Technology 863 Projects [2012AA02A406] to BW.
Acknowledgments
We would like to thank Dr. Douglas Lowrie (Shanghai Medical College, Fudan University, Shanghai, China) for valuable discussions and proof reading. We would also like to thank Mr. Zhonghuai He and Mr. Hongkai Yang of Beijing Advaccine Biotechnology Co. LTD, for providing samples for this study.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.Piedimonte G, Perez MK.. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2017;35:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev. 2000;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tregoning JS. Respiratory viral infections in infants : causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming DM, Pannell RS, Cross KW. Mortality in children from influenza and respiratory syncytial virus. J Epidemiol Community Heal. 2005;59:586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol. 2013;13:211–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheltema NM, Nibbelke EE, Pouw J, Blanken MO, Rovers MM, Naaktgeboren CA, Mazur NI, Wildenbeest JG, van der Ent CK, Bont LJ. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med. 2018;6:257–64. [DOI] [PubMed] [Google Scholar]
- 7.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–63. [DOI] [PubMed] [Google Scholar]
- 8.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. [DOI] [PubMed] [Google Scholar]
- 9.Bataki EL, Evans GS, Everard ML. Respiratory syncytial virus and neutrophil activation. Clin Exp Immunol. 2005;140:470–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindemans CA, Kimpen JLL, Luijk B, Heidema J, Kanters D, Van Der Ent CK, Koenderman L. Systemic eosinophil response induced by respiratory syncytial virus. Clin Exp Immunol. 2006;144:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors M, Kulkarni AB, Firestone C, Holmes KL, Iii HCM, Sotnikov AV, Murphy BR. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4 + T cells. J Virol. 1992;66:7444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz K, Langlois RA, Harty JT, Varga SM. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 2018;14:e1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta PL, Caballero MT, Fernando P. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol. 2015;23:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loebbermann J, Durant L, Thornton H, Johansson C, Openshaw PJ. Defective immunoregulation in RSV vaccine- augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proc Natl Acad Sci. 2013;110:2987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathis AS, Egloff G, Ghin HL. Calcineurin inhibitor sparing strategies in renal transplantation, part one: late sparing strategies. World J Transplant. 2014;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheffert JL, Raza K. Immunosuppression in lung transplantation. 2014. [DOI] [PMC free article] [PubMed]
- 17.Zheng G, Zhong S, Geng Y, Munirathinam G, Cha I, Reardon C, Getz GS, Van Rooijen N, Kang Y, Wang B, et al. Dexamethasone promotes tolerance in vivo by enriching CD11cloCD40lo tolerogenic macrophages. Eur J Immunol. 2013;43(1):219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Y, Xu L, Wang B, Chen A, Zheng G. Cutting edge: immunosuppressant as adjuvant for tolerogenic immunization. J Immunol. 2014;180:5172–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Sambhara S, Li CX, Ettorre L, Switzer I, Cates G, James O, Parrington M, Oomen R, Du RP, et al. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology. 2000;269(1):54–65. [DOI] [PubMed] [Google Scholar]
- 20.Jorquera PA, Choi Y, Oakley KE, Powell TJ, Boyd JG, Palath N, Haynes LM, Anderson LJ, Tripp RA. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS One. 2013;8:e74905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus-specific Th1 Cells induced by recombinant bacillus calmette-guerin promotes virus clearance and protects from infection. J Immunol. 2010;785:7633–45. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Zhou X, Zhong Y, Li C, Dong A, He Z, Zhang S, Wang B. A recombinant G protein plus cyclosporine A–based respiratory syncytial virus vaccine elicits humoral and regulatory T Cell responses against infection without vaccine-enhanced disease. J Immunol. 2016;196:1721–31. [DOI] [PubMed] [Google Scholar]
- 23.Cormier SA, You D, Honnegowda S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev Anti Infect Ther. 2010;8:1371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotnicky H, Siegrist CA, Aubry JP, Bonnefoy JY, Corvaïa N, Nguyen TN, Power UF. Enhanced pulmonary immunopathology following neonatal priming with formalin-inactivated respiratory syncytial virus but not with the BBG2NA vaccine candidate. Vaccine. 2003;21:2651–60. [DOI] [PubMed] [Google Scholar]
- 25.Martinez X, Li X, Kovarik J, Klein M, Lambert PH, Siegrist CA. Combining DNA and protein vaccines for early life immunization against respiratory syncytial virus in mice. Eur J Immunol. 1999;29:3390–400. [DOI] [PubMed] [Google Scholar]
- 26.Noh Y, Shim B-S, Cheon IS, Rho S, Kim HJ, Choi Y, Kang C-Y, Chang J, Song MK, Kim J-O. neonatal immunization with respiratory syncytial virus glycoprotein fragment induces protective immunity in the presence of maternal antibodies in mice. Viral Immunol. 2013;26:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remot A, Roux X, Dubuquoy C, Fix J, Bouet S, Moudjou M, Eléouët JF, Riffault S, Petit-Camurdan A. Nucleoprotein nanostructures combined with adjuvants adapted to the neonatal immune context: A candidate mucosal RSV vaccine. PLoS One. 2012;7:e37722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao HY, Huang JY, Lin YW, Yu SL, Chitra E, Chang CK, Sung WC, Chong P, Chow YH. Depletion of regulatory T-cells leads to moderate B-cell antigenicity in respiratory syncytial virus infection. Int J Infect Dis. 2015;41:56–64. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs A, Atkinson JP, Fremeaux-Bacchi V, Kemper C. CD46-induced human Treg enhance B-cell responses. Eur J Immunol. 2009;39:3097–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laidlaw BJ, Lu Y, Amezquita RA, Weinstein JS, Vander Heiden JA, Gupta NT, Kleinstein SH, Kaech SM, Craft J. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol. 2017;2:eaan4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckstein LA, Van Quill KR, Bui SK, Uusitalo MS, O’Brien JM. Cyclosporin A inhibits calcineurin/nuclear factor of activated T-cells signaling and induces apoptosis in retinoblastoma cells. Investig Ophthalmol Vis Sci. 2005;46:782–90. [DOI] [PubMed] [Google Scholar]
- 33.Fruman DA, Klee CB, Bierer BE, Burakoff SJ. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992;89:3686–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, Chen WC, Der LK. IL-6-stimulated CD11b + CD14 + HLA-DR - myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, Lee DS. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Te WC, Hsieh CC, Lin CC, Chen WC, Hong JH, Chen MF. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J Mol Med. 2012;90:1343–55. [DOI] [PubMed] [Google Scholar]
- 37.Qin G, Lian J, Huang L, Zhao Q, Liu S, Zhang Z, Chen X, Yue D, Li L, Li F, et al. Metformin blocks myeloid-derived suppressor cell accumulation through AMPK-DACH1-CXCL1 axis. Oncoimmunology. 2018;7(7):e1442167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casares N, Sangro B, Lasarte -J-J, J-I R-B, Echeverria I, Prieto J, Aldabe R, Galeano E, Guembe L, Herrero I, et al. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2010;54(3):422–31. [DOI] [PubMed] [Google Scholar]
- 39.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–93. [DOI] [PubMed] [Google Scholar]
- 40.Park MJ, Lee SH, Kim EK, Lee EJ, Baek JA, Park SH, Kwok SK, La CM. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci Rep. 2018;8:3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Zhao J, Ren JP, Wu XY, Morrison ZD, El Gazzar M, Ning SB, Moorman JP, Yao ZQ. Expansion of myeloid-derived suppressor cells promotes differentiation of regulatory T cells in HIV-1+ individuals. AIDS. 2016;30:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoso A, Mazza EMC, Bicciato S, Mandruzzato S, Bronte V, Serafini P, Inverardi L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur J Immunol. 2014;44:3307–19. [DOI] [PubMed] [Google Scholar]
- 43.Galloux M, Le Goffic R, Gault E, J-F E, M-A R-W, Rémot A, Riffault S, P-L H, Sourimant J, Yu Q. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nat Commun. 2014;5:5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh SR, Dennis VA, Carter CL, Pillai SR, Jefferson A, Sahi SV, Moore EG. Immunogenicity and efficacy of recombinant RSV-F vaccine in a mouse model. Vaccine. 2007;25:6211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed LJ, Müench H. A simple method of estimating 50 percent end-points. Am J Hyg. 1938;27:493–97. [Google Scholar]


