ABSTRACT
Significant concerns have arisen over the past 3 y from the increased global spread of the mosquito-borne flavivirus, Zika. Accompanying this spread has been an increase in cases of the devastating birth defect microcephaly as well as of Guillain–Barré syndrome in adults in many affected countries. Currently there is no vaccine or therapy for this infection; however, we sought to develop a combination approach that provides more rapid and durable protection than traditional vaccination alone. A novel immune-based prophylaxis/therapy strategy entailing the facilitated delivery of a synthetic DNA consensus prME vaccine along with DNA-encoded anti-ZIKV envelope monoclonal antibodies (dMAb) were developed and evaluated for antiviral efficacy. This immediate and persistent protection strategy confers the ability to overcome shortcomings inherent with conventional active vaccination or passive immunotherapy. A collection of novel dMAbs were developed which were potent against ZIKV and could be expressed in serum within 24–48 h of in vivo administration. The DNA vaccine, from a previous development, was potent after adaptive immunity was developed, protecting against infection, brain and testes pathology in relevant mouse challenge models and in an NHP challenge. Delivery of potent dMAbs protected mice from the same murine viral challenge within days of delivery. Combined injection of dMAb and the DNA vaccine afforded rapid and long-lived protection in this challenge model, providing an important demonstration of the advantage of this synergistic approach to pandemic outbreaks.
KEYWORDS: Zika virus, antibodies, dMAb-DNA encoded monoclonal antibodies, vaccination, immunotherapy, DNA vaccine
Introduction
Zika virus (ZIKV), a member of the genus Flavivirus of the Flaviviridae family, was first isolated from a sentinel monkey in the Zika forest of Uganda in 1947. Like other flaviviruses including dengue (DENV), yellow fever (YFV), West Nile (WNV), Japanese encephalitis (JEV) and tick-borne encephalitis (TBEV), ZIKV is an arbovirus, with mosquitoes of the Aedes genus, particularly Aedes aegypti, transmitting ZIKV between human and non-human primate (NHP) hosts.1–3 Most ZIKV infections are asymptomatic, but some individuals develop mild symptoms after infection that include fever, headaches, lethargy, rash, arthralgia, and/or myalgia. Outbreaks of ZIKV infections across islands in the Pacific Ocean and in the Americas (primarily Brazil) between 2007 and 2016, however, have indicated both previously unreported modes of transmission including sexual, vertical, and through blood transfusions/organ transplant as well as new, severe signs of ZIKV infection.3–5 The most significant of these new manifestations is a dramatic increase in the incidence of microcephaly, congenital blindness, and other severe birth defects in babies born to mothers infected with ZIKV during pregnancy. Subsequent studies in mice and humans have since confirmed that ZIKV is a teratogen.3,6–9 Additionally, ZIKV infection was also found to increase the risk for development of Guillain–Barré syndrome post recovery.8 This alarming increase in cases of microcephaly and other birth defects following ZIKV infections prompted the World Health Organization (WHO) to declare ZIKV infection a global health emergency in 2016.4,10
There are currently no licensed vaccines or treatments to prevent or ameliorate ZIKV infection and disease, but new findings about ZIKV infection have accelerated efforts to develop such therapeutics and vaccines. Vaccines targeting ZIKV have been made in several formulations including purified inactivated virus, protein subunits, adenovirus vectors, DNA plasmids, and RNA.11–16 Each of these has been shown to induce ZIKV-specific immune responses in mice and NHPs that can protect these animal models against morbidity and mortality following ZIKV challenge. Some of these vaccines are now being evaluated in phase I clinical trials.11 A second line of investigation for ZIKV therapeutics is the isolation and production of anti-ZIKV monoclonal antibodies (mAbs) for passive transfer into infected patients. Several antibodies have been identified and isolated from ZIKV-infected patients that are capable of neutralizing in vitro infection by a broad panel of ZIKV isolates from Africa, Asia, and the Americas.
Therapeutic mAbs can be an effective means of combating infectious diseases, but factors including a laborious production process and the need for repeated dosing to maintain protective serum levels make them cost-prohibitive, which limit their clinical application. To overcome some of these barriers, our group has pioneered a method that uses DNA plasmid technology as a delivery vehicle for these antibodies. Delivery of DNA plasmids encoding genes of therapeutic monoclonal antibodies (dMAbs) into muscle followed by electroporation stimulates long-term, in vivo production of the mAbs which significantly reduces costs by eliminating both the need for ex vivo production and purification of protein mAbs and the need for repeated dosing. Furthermore, using DNA plasmids as vectors for mAb gene delivery has additional advantages including; (i) a strong safety profile in numerous clinical trials; (ii) the ability for re-dosing since DNA vectors are non-immunogenic, and (iii) the possibility for long-term gene expression even though DNA vectors do not integrate into cellular DNA. Synthetic DNA technology allows for manipulation of mAb sequences to improve in vivo expression levels and/or modify effector function(s) of the antibodies. We have demonstrated that delivery of dMAbs targeting Pseudomonas, Chikungunya, Dengue, and Influenza into mice produces biologically relevant mAb serum levels that can protect animals from challenge by each pathogen.17–20 Additionally, dMAbs can be co-delivered with DNA vaccines to provide immediate protection during the eclipse period when conventional vaccine-induced immunity is developing.19
Here we describe the identification and cloning of a panel of humanized IgG monoclonal antibodies isolated from ZIKV DNA vaccine-immunized mice as well as derived from ZIKV-infected rhesus macaque monkeys (RhMac). The genetic sequences of the anti-ZIKV mAbs were modified to synthetically produce human IgGs while retaining each mAb’s complementarity-determining region (CDR). The genetic sequences were optimized to improve protein translation and then cloned into DNA vectors. The dMAb plasmids were shown to direct production of ZIKV-specific antibodies in vitro and in vivo. Mice administered ZIKV-dMAb plasmids were protected from morbidity and mortality after challenge with a contemporary clinical ZIKV strain. Most importantly, the dMAb plasmids provide immediate protection from ZIKV which could be co-administered with a ZIKV DNA vaccine to provide comprehensive protection from ZIKV during the eclipse period of the vaccine-induced immune responses. The anti-ZIKV mAbs described here may be useful in providing protection against this rapidly emerging virus.
Materials and methods
Cells
Human Embryonic Kidney 293T cells and Vero cells were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco–Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 IU of penicillin per ml, 100 μg of streptomycin per ml and 2mM L-glutamine as described previously.19
Generation and evaluation of DNA-encoded monoclonal antibody (dMab) plasmids which express monoclonal antibodies (mAb) from generated and developed anti-ZIKV hybridomas
For screening of antibody-producing mouse hybridoma clones, viral preparation and recombinant soluble ZIKV-Env protein was used as ZIKV antigens in an indirect ELISA. Approximately 50–60 antibody-producing mouse hybridoma clones were identified as having antibody binding at least four-fold higher than the background level reactivity. For the RhMac clones, we used phage display methods as outlined in Cold Spring Harbor Laboratory (CSHL) manual to generate mAb clones with high affinity for the ZIKV envelope protein. Positive hybridoma clones reacting with sucrose-purified ZIKV virions, as well as recombinant soluble ZIKV-Env protein, were characterized by an indirect ELISA. Positive clones were identified by nucleotide sequencing of the variable H chain (VH) and variable L chain (VL) sequences as described.18–20 The coding sequences for the RhMac clone antibodies against ZIKV-Ig were obtained from the phage display assay.21–23 We created synthetic antibody constructs (Ig) that encode VH and VL regions from anti-ZIKV-Ig and were utilized for the cloning organization of IgG constructs in a novel synthetic dual promoter plasmid strategy for developing anti-ZIKV-IgG.
Measurement of expression of anti-ZIKV-Env antibody from ZIKV-dMAb by Western blot analysis
Samples of anti-ZIKV-dMAb construct transfected cells (293T) were utilized for expression analysis using the GeneJammer transfection reagent (Agilent Technologies). Samples were collected and compared to the control lysate from the empty vector control. For each analysis, 20μg of sample were added to SDS loading buffer and loaded into each lane of a 4–12% Bis-Tris PAGE gel (Life Technologies). The gel was run and transferred onto a nitrocellulose membrane using iBlot2 (Life Technologies). Samples were separated on a polyacrylamide gel (12% NuPAGE Novex, Invitrogen) and transferred to a PDF membrane (Invitrogen) that was blocked using a commercial buffer (Odyssey Blocking Buffer, LiCor BioSciences) and incubated overnight at 4°C with specific primary antibodies raised in mice as well as beta-actin (Santa Cruz). IRDye800 and IRD700 goat anti-human or anti-mouse secondary antibodies were used for detection (LiCor Biosciences).
Immunofluorescence analysis
For immunofluorescence analysis, chamber slides (Nalgene Nunc) were seeded with Vero cells (1x104) from stock cultures. Cells were grown until they reached approximately 80% confluency after which cells were infected for 2 h with ZIKV at a multiplicity of infection (m.o.i.) of 0.01 so that cells expressed the antigens. Fixed cells on the slides were incubated for 1 h at 37°C with twofold dilutions of sera beginning at 1:100 dilution from the ZIKV-IgG administered mice for 90 min at 37°C in a humidified chamber. After washing thrice with PBS, the cells were incubated for 60 min at 37°C with a FITC-conjugated goat anti-human IgG (Santa Cruz Biotechnology Inc). The additional nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) was performed at room temperature for 20 min. After each incubation step, the samples were washed once with PBS. The samples were subsequently mounted onto glass slides using DABCO and were viewed under a confocal microscope (LSM710; Carl Zeiss). The resulting images were analyzed using the Zen software (Carl Zeiss).
Quantitative and binding ELISA
ELISA assays were performed with sera from mice administered ZIKV-dMAb or pVax1 in order to measure the expression kinetics of the antibody construct and target antigen binding. Quantification of IgG in murine immunization studies was performed using 96-well black MaxiSorp plates (Nalgene Nunc) coated overnight at 4°C with 10 μg/mL goat anti-Human IgG (H + L) (Pierce). Plates were blocked with Casein Blocker (Thermo), and serum samples and a standard curve (10 μg/mL of ChromPure Human IgG, whole molecule) (Jackson Labs) were serially diluted. Purified human IgG kappa or lambda (Bethyl Laboratories, Montgomery, TX) was used as a standard. Following incubation, samples were probed with an anti-human IgG antibody conjugated to horseradish peroxidase (Bethyl Laboratories) at a 1:20,000 dilution. Plates were developed using o-phenylenediamine dihydrochloride substrate (OPD, Sigma Aldrich, St. Louis, MO) and stopped with 2 N H2SO4. Plates were then read at 450 nm using a Biotek EL312e quantification. A standard curve was generated using purified human IgG/kappa (Bethyl Laboratories). All sera samples were tested in duplicate. For avidity ELISA, serum samples were applied to wells in duplicate. After 1-h incubation, one set of samples was incubated with wash buffer and another with 4 M urea (Sigma Aldrich) for 5 min and washed twice with wash buffer, and the ELISA protocol was completed as described.
ZIKV viral challenge study
Five-to-seven-week-old A129 mice deficient in interferon (IFN)-α/β receptors were used for this study and were bred under specific pathogen-free conditions in an animal facility at The Wistar Institute. Mice were injected with a total volume of 50μl of either pVax1 DNA (100 μg) or ZIKV-IgG (100 μg) diluted in sterile water in the quadriceps muscle using optimized electroporation (EP) for DNA delivery (CELLECTRA®; Inovio Pharmaceuticals, Inc., PA, USA). The pulsing parameters for EP delivery were as previously described.19,24 Mice were challenged with a total of 1 × 106 PFU (25μl) of ZIKV-PR209 virus in 50 μL of PBS by subcutaneous (s.c.) injection in the left hind footpad. Following virus challenge, mouse weight, morbidity, and mortality were monitored daily. A 1-to-5 morbidity scale was adapted as described before: (1) Healthy (no disease); (2) Displaying mild signs – decreased mobility; (3) early signs of hunched posture and decreased mobility; (4) Fur ruffling, increased lethargy and limited mobility, and signs of paralysis in one hind limbs; and (5) Moribund, minimal mobility consistent with inability, paralysis or both hind limbs. Mice were euthanized if weight loss was equal to or greater than 20% of their original weight and/or if they scored “5” on the morbidity scale. Mice were monitored daily for survival and signs of infection (i.e., body weight and lethargy) over the 2-week post-challenge observation period. Blood samples were collected 7–14 d post-challenge. Two independent experiments were performed. All animal experiments complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and were approved by the Animal Care and Use Committee of the Wistar Institute.
Statistical analysis
Statistical analyses, using either a Student t-test or the nonparametric Spearman’s correlation test, were performed using Graph Pad Prism software (Prism Inc.). Correlations between the variables in the control and experimental groups were statistically evaluated using the Spearman rank correlation test. For all the tests, p values less than 0.05 were considered significant.
Results
Generation and characterization of antibodies targeting ZIKV envelope
The major target of the host humoral immune response and of neutralizing Abs against flaviviruses is the envelope glycoprotein, which is a 56-kDa protein and the major antigen represented on the surface of virions.5,25,26 Our group has recently developed and tested in an animal model a DNA vaccine and passive antibody immunotherapy against ZIKV infection and disease.12 This strategy has demonstrated protective efficacy in mice, non-human primates (NHP) and shows induction of protective immunity in passive transfer studies from vaccinated humans.27 These studies and others12–14,28 support the role of antibodies directed against the pre-membrane: envelope protein complex (prM+Env) in mediating protection against infection and disease.7,12 Furthermore, within the prME complex antibodies targeting the E antigen are associated with the ability to transfer protection in animal models. Passive antibodies may have value in therapy of infected persons to lower viral load as well as in rapid protection strategies to protect at risk women of childbearing years or the immune compromised. Neutralizing monoclonal antibodies (mAbs) have been demonstrated to be effective in the treatment of several infectious diseases as well as in preliminary in vitro and in vivo models of flavivirus-related infections.25,26,28 Given their specific antiviral activity as being well-tolerated molecules with limited side effects, mAbs could represent a new therapeutic approach for the development of an effective treatment, as well as useful tools in the study of the host-virus interplay and in the development of more effective immunogens.
Two approaches were used to create antibodies in mice and RhMacs capable of binding to the ZIKV Envelope (Env) protein for protective studies. In the first approach, C57/B6 mice were immunized three times by EP-enhanced vaccination using 50μg of a DNA vaccine encoding a synthetic, consensus sequence of ZIKV pre-membrane/membrane and envelope (prME) antigens. A similar DNA vaccine can induce robust antibody responses in mice and NHPs, that can protect mice from ZIKV challenge.12 Following the third immunization, sera from immunized mice were screened by ELISA to confirm the presence of antibodies targeting ZIKV Env. Subsequently, B lymphocytes from immunized mice splenocytes were isolated and used to create hybridomas by conventional means.19 The hybridomas were screened to identify clones producing antibodies with the highest affinity against ZIKV-Env, resulting in 10 mouse mAbs: 1C2A6, 1D4G7, 3F12E9, 8D10F4, 8A9F9, 2B7D7, 4D6E8, 5E6D9, 6F9D1, and 9F7E1 being selected. Supernatants from each of these hybridoma clones bound purified recombinant soluble ZIKV-Env protein by indirect ELISA and western blot analysis, respectively (Figure 1a,b). Anti-ZIKV ENV mAbs also bound to ZIKV-PR 209 virus-infected Vero cells as demonstrated via immunofluorescence assay (Figure 1c).
Figure 1.
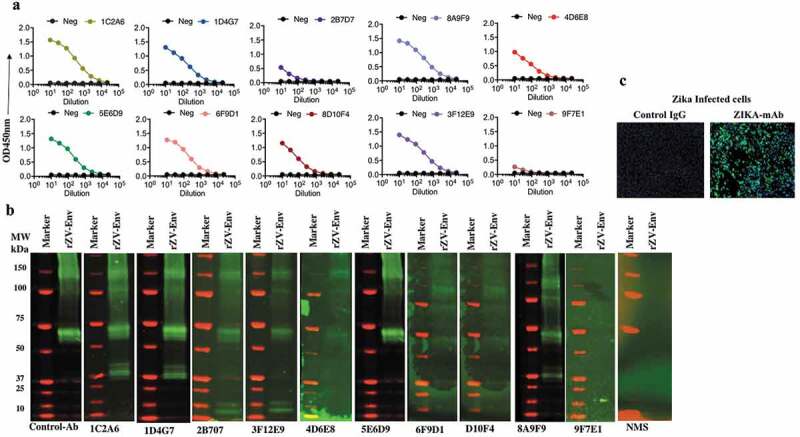
Characterization of ZIKA-mAbs.
A binding ELISA of recombinant ZIKV ENV protein (rZIKV-E) and monoclonal antibodies from hybridoma supernatants. The clones are derived from DNA vaccine, prME1, immunized murine spleen. A total of nine clones were selected and narrowed down, for further analysis, to ID4G7, 8D10F4, 8A9F9, and 3F12E9 based on the binding affinity. (b) Western blot analysis of each of the nine clones’ binding capability against the ZIKV ENV protein (rZIKV-E). The monoclonal antibodies were probed with anti-mouse IgG-IRDye CW-800 as a secondary antibody. (c) IFA (immunofluorescence) analysis demonstrated specific binding of antibodies to ZIKV-infected Vero cells. ZIKV-mAbs bound to ZIKV Env antigens were probed with anti-mouse IgG labeled with Alexa Fluor 488 (fluorescent green).
The second approach used to generate anti-ZIKV Env mAbs utilized blood and splenic tissue collected from five RhMacs that were challenged twice with a contemporary strain of ZIKV, PR209. All macaques developed viremia after the first challenge, but none had detectable viremia after re-challenge. Spleen and whole blood mononuclear cells were harvested from the macaques 15 d-post re-challenge. Subsequently, scFv/phage-display technology combined with solid-phase selection against immobilized Zika antigen was used. RNA from peripheral blood B cells was prepared from each of the five macaques and pooled. Similarly, splenic RNA was prepared from each macaque and pooled. RT-PCR to amplify variable heavy (VH), variable kappa (Vκ), and variable lambda (Vλ) gene segments was performed separately on peripheral blood RNA and splenic RNA, and then VHs, Vκs, and Vλs from each source of RNA were pooled. RT-PCR was performed as described21–23 using a combination of human immunoglobulin primers previously described and supplemented with sets of macaque-specific VH, Vκ, and Vλ as summarized in supplementary data Table 1. Separate VH/Vκ and VH/Vλ scFv phage display libraries were constructed using the pComb3X vector as described21,22 using TG1 electrocompetent E. coli and comprised 1.6 × 109 and 1.5 × 109 independent transformants, respectively. VH/Vκ and VH/Vλ libraries were panned separately on immobilized Zika ENV protein using solid-phase methods previously described.21 Positive clones were identified using phage ELISA, and heavy and light chain scFv nucleotide sequences were identified as described21,23 and analyzed for homology to macaque immunoglobulin germline gene segments using IMGT/V-Quest.29 Of 44 randomly selected clones, 11 unique RhMac anti-ZIKV Env mAbs were isolated after screening: KP401, KP412, LP401, LP305, LP314, LP306, LP320, LP311, LP312, LP402 and LP408 where “K” and “L” designations indicate whether the scFv comprises a kappa or lambda light chain, respectively. Clones LP305 and LP314 were clonally related (identical heavy chain and light chain CDR3 regions) as were clones LP311 and LP320 so only clones LP305 and LP311 were selected for dMAb conversion.
The nucleotide sequences of all mouse and RhMac anti-ZIKV mAbs were used for phylogenetic analysis and structural modeling of the antibody variable regions. An unrooted phylogenetic tree based on the CDR sequences of 16 mAbs (5 of the 10 murine antibodies and all 11 of the macaque antibodies) shows a high level of similarity between them suggesting they may all target the same or related epitopes. Predicted CDR structures of each mAb made using Discovery Studio software show similar CDR conformations between the most closely related mAbs as expected (Figure 2a). Docking analysis between the models of mAbs 8A9F9, LP314 and LP408 and the structure of ZIKV Env was performed using Discovery Studio software’s ZDOCK function. This analysis suggests that each of these may bind to ZIKV Env Domain 3 (EDIII), a known target of neutralizing antibodies in related flaviviruses.26,30–32
Figure 2.
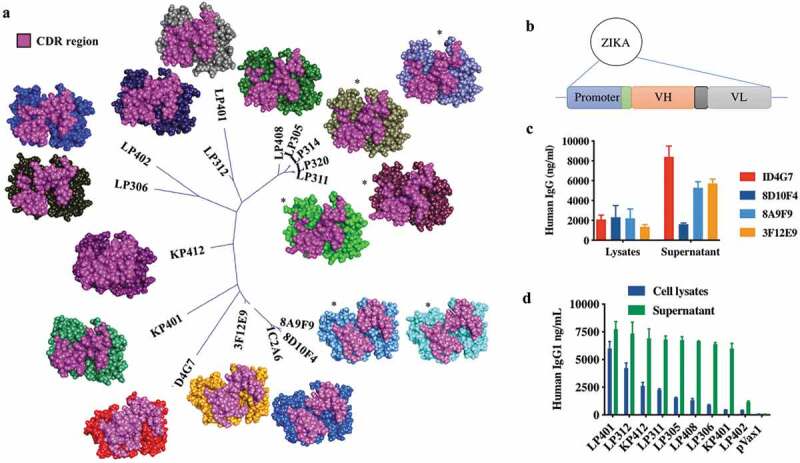
Comparison of Zika envelope antibodies by CDR phylogeny and molecular modeling.
(a) An unrooted phylogenetic tree based on CDR comparisons (only) of 16 anti-Zika envelope antibodies was generated to demonstrate relatedness and by extension the potential for epitope targeting similarity. Shown nearby to each antibody location on the tree is a molecular model generated with standard antibody modeling protocols as implemented in Discovery Studio. Models are shown in CPK format and CDRs are highlighted. As sequence relatedness translates to structural similarities, it can be noted that clusters of nearly or completely identical CDRs adopt similar conformations. Of the Fv models shown, LP408, LP314, and 8A9F9 have been subjected to docking analysis using ZDOCK as implemented in Discovery Studio and scored with ZRANK. In all cases, the EDIII region (a known target for effective neutralizing antibodies) contains multiple top poses for the antibodies in question. In particular, LP408 and LP314 demonstrate the top 15 of 20 poses total in this region. * indicates similar CDR conformations between closely related mAbs and a potential ZIKV ENV Domain III (EDIII) binding for clones 8A9F9, LP314, and LP408. (b) Schematic diagram of ZIKV-specific antibody heavy and light chain encoded as a codon-optimized single cassette into the pVax1 DNA plasmid. Construction and expression of ZIKV-dMAb plasmid constructs from (c) ZIKV DNA plasmid-vaccinated murine spleen-derived and (d) ZIKV-infected RhMac spleen-derived. Each dMAb construct was transfected into 293T cells in order to determine in vitro expression via enzyme-linked immunosorbent assays (ELISAs). The bar represents the level of IgG present at 48-h post transfection for both cell lysates and supernatants per construct.
Construction and pharmacokinetic evaluation of anti-ZIKV- dMAbs
The heavy and light chain sequences of each mouse and RhMac mAb were cloned as a single cassette into the pVax1 DNA plasmid to create dMAb plasmids (Figure 2b). Each antibody was cloned as a full-length human IgG1 constant region sequence to take advantage of IgG1 properties including longer circulating half-lives, better tissue penetration, lower retention times, and better recruitment of effector functions by the antibody constant (Fc) component.18,19 Antibodies produced after transfection or injection of these plasmids will henceforth be referred to as dMAbs. Each synthetically designed dMAb plasmid cassette consisted of an antibody sequence fused downstream of an enhanced leader sequence, and each cassette was codon and RNA structure optimized to drive increased in vivo protein production.12,18,33 In vitro expression of antibody from each plasmid was evaluated by performing a quantitative ELISA for IgG concentration on cell lysates and supernatants collected from plasmid-transfected HEK293T cells. Supernatants from cells transfected with either mouse or RhMac dMAb plasmids contained IgG at levels of 2000–9000 ng/ml at 48-h-post-transfections while lysates generally had lower levels at approximately 2000 ng/ml (Figure 2c,d).
Pharmacokinetic, binding and avidity activity of ZIKV-dMAbs in vivo
The in vivo expression of dMAb was evaluated by injecting B6.Cg-Foxn1nu/J mice with 100 μg of dMAb plasmids intramuscularly followed by adaptive in vivo EP. Sera collected from mice at various times post-injection and EP were evaluated by quantitative ELISA for chimeric human IgG to assess DMAb production and longevity. As noted in Figure 3, mouse and RhMac dMAb plasmids both were evaluated for production of IgG in vivo. Peak serum antibody levels varied between the different mouse- and RhMac dMAbs evaluated. Mouse-derived ZIKV dMAb clones were observed at the highest serum concentration (ng/mL) between 15 and 25 dpost-injection (Figure 3a). Importantly, these in vivo dMAbs retained the ability to bind to ZIKV Env protein by Western blot analyses (Figure 3b). The expression of RhMac dMAbs was detectable out to 60 dpost injection, and these in vivo dMAbs in circulation were also able to bind ZIKV Env protein (Figure 3c,d). These results indicate that EP-enhanced intramuscular delivery of dMAb plasmids mediates in vivo production of functional ZIKV Env-specific monoclonal antibodies.
Figure 3.
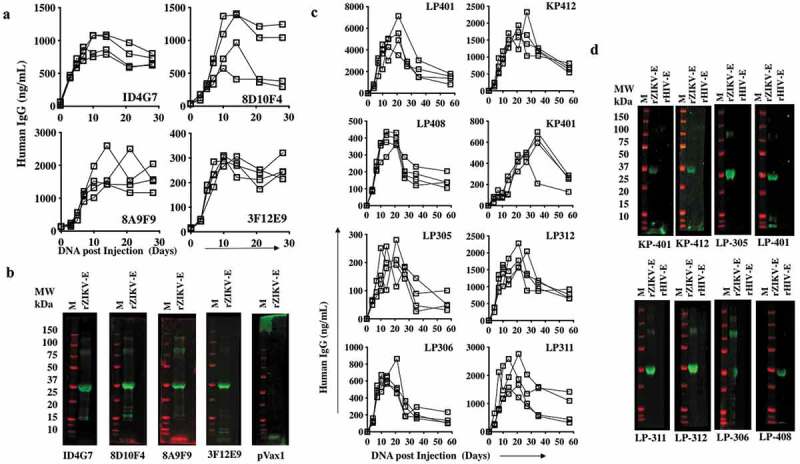
Construction and expression of the ZIKV DNA plasmid-vaccinated murine spleen-derived ZIKV dMAb plasmid constructs.
In vivo expression of ZIKV dMAb. CAnN.Cg-Foxn1nu/Crl nude mice aged 6–8 weeks received 100 μg intramuscular injection in the right tibialis anterior muscle followed by CELLECTRA® enhanced electroporation delivery. Serum IgG level was measured at various time points and quantified via ELISA. (a and c) Binding of recombinant ZIKV Env protein (rZIKV-E) with sera from anti-ZIKV dMAb-administered mice. rZIKV-E coated ELISA plates were probed with D 14 sera from ZIKV dMAbs or pVax1-injected mice. The mean OD450mn values are shown ±SD. (b and d) Expression of the ZIKV dMAb clones ID4G7, 8D10F4, 8A9F9, and 3F12E9 by SDS-polyacrylamide gel electrophoresis and Western Blot. The Western blot analysis shows rZIKV-E probed with mouse sera containing dMAbs 14 d post the dMAb plasmid injection, and the murine sera bound to anti-human IgG-IRDye 800-CW conjugated secondary antibodies.
The binding affinity of an antibody to its target is defined by natural affinity and avidity. Natural affinity is the strength of monovalent binding between an antibody and an antigenic epitope, often measured by binding to IgG fragment to a target antigen. Avidity is the supportive force of engaging multiple antibody paratope/antigen epitope pairs between one antibody and one antigen. In other words, avidity is a functional consequence of antibody-binding multivalency. We therefore investigated IgG binding and avidity of these mAbs and analyzed in an indirect IgG and avidity ELISA. Using the traditional antigen-capture sandwich ELISA, all of these mAbs were tested. Shown are average ELISA signals plotted against input antibody concentration, a dose-dependent binding curve could be readily obtained, and the signals reached saturation at higher antibody concentrations (Figure 4a,b). Furthermore, we also assessed the monoclonal antibody activity of recombinant ZV-envelope III proteins by ELISA. EP-enhanced intramuscular delivery of dMAb plasmids produced immune sera was tested by ELISA for detection and showed the presence-binding activity of the antibodies against DIII protein (Figure 4c).
Figure 4.
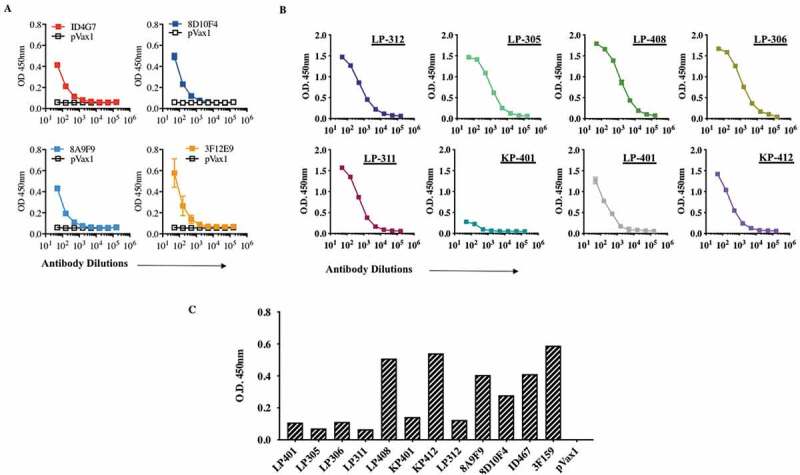
Binding characteristics of Zika-mAbs IgG s and avidity to Zika-Env.
ZIKV-mAbs samples were tested for anti-ZIKV-Env-specific binding as well as avidity using a modified ELISA with 4 M urea washes against rZIKV-E protein. (A and B) Binding of in vivo-produced human IgG DMAbs against rZIKV-E protein. (a) A half-log dilution ELISA of mouse-derived human IgG DMAb clones. The serum level was normalized at 10ng/ml for each clone (b) A half-log dilution ELISA of RhMac-derived human IgG dMAb clones. The serum level was normalized at 10ng/ml for each clone (c) Specificity of antibodies against ZIKV envelope DIII domain. ZIKV-DIII protein was incubated with the indicated anti-ZIKV mAbs. Binding was determined by ELISA and the results are representative of two independent experiments performed in triplicate. (d) Avidity ELISAs were performed with the addition of 5‐min incubation with either PBST or 4 M urea. Differences between PBS and urea treated samples are indicated. Symbols indicate mean OD; error bars indicate standard deviation.
Figure 4.
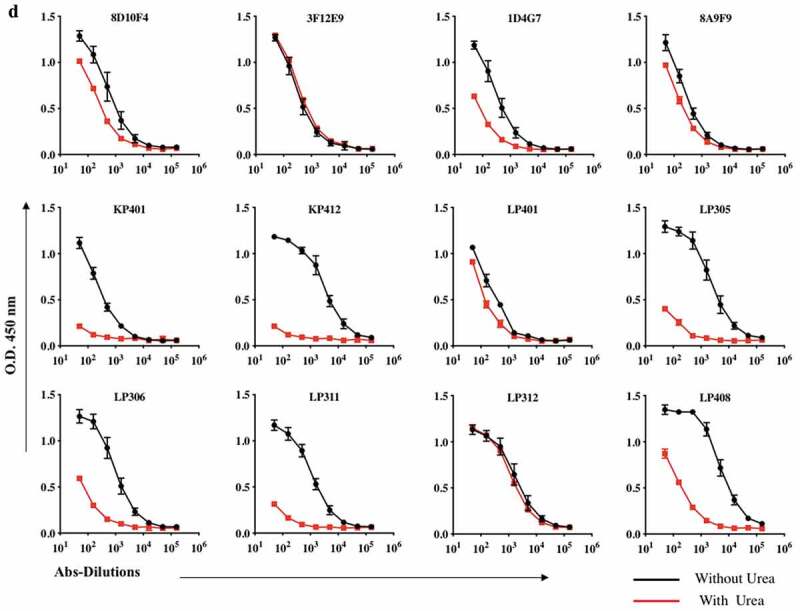
(Continued.)
Avidity characteristics of anti-ZIKV mAbs
To investigate whether the affinities of these interactions were engaging multiple antibodies, we studied each mAb’s binding toward its Zika-Envelop recombinant antigen in using urea 4 M. ELISA titers for these ZIKV dMAbs exhibit high affinity to ZIKV antigen were also comparable, although the titer for 8D10F4, 3F12E9, 8A9F9, LP401, and LP312, were significantly stronger and higher whereas we found that low-avidity IgG antibodies namely ID4G7, KP401, KP412, LP305, LP306, LP311 and LP408 were detected (Figure 4d).
ZIKV-dMAbs protect mice against ZIKV challenge
The ZIKV-dMAbs were next evaluated for their ability to offer protection from ZIKV infection. For these studies, B6.129S2-Ifnar1tm1Agt/Mmjax (A129) mice were used, as it has been demonstrated that the lack of functional type I interferon signaling in these mice allows for ZIKV replication and subsequent disease.12,16,34 Groups of A129 mice were injected intramuscularly with 100 μg of either a ZIKV-dMAb plasmid or an empty pVax1 vector, both formulated in sterile water, followed by EP. All mice were challenged 2 d later with 106 plaque-forming units (PFU) of ZIKV PR209. Physical health indicators, including body weight and weakness, were monitored daily for 2 weeks post-infection. All mice injected with pVax1 experienced severe weight loss (data not shown) and eventually succumbed to ZIKV infection by d 9 post-challenge. Most mice that received either mouse clones (Figure 5a) or RhMac clones (Figure 5b) of anti-ZIKV dMAb prior to challenge survived the infection. Furthermore, there was notably less ZIKV-associated morbidity following viral challenge in mice pre-injected with either mouse clones (Figure 5c) or RhMac clones (Figure 5d) compared to the pVax1-injected cohort exhibiting the most severe disease-state on average.
Figure 5.
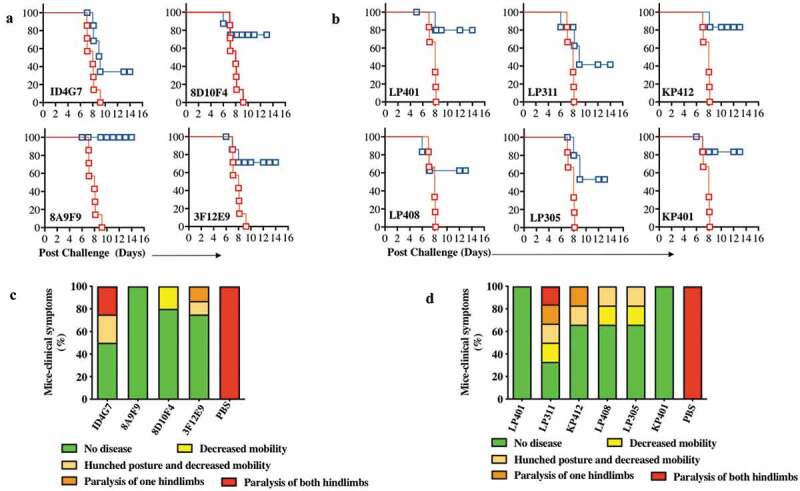
Characterization of ZIKV dMAb protection in vivo in mice from ZIKV.
B6.129S2-Ifnar1tm1Agt/Mmjax (A129) mice (n= 7 per group) were injected with 100 μg of ZIKV dMAb plasmid or an empty pVax1 vector i.m. followed by CELLECTRA® enhanced electroporation delivery 2 d prior to being challenged with 106 plaque-forming units (PFU) of ZIKV PR209. (a and c) ZIKV dMAb clones tested for this challenge, ID4G7, 8D10F4, 8A9F9, and 3F12E9, were derived from ZIKA prME1 DNA-vaccinated murine spleen. (b and d) ZIKV dMAb clones tested for this challenge, LP401, LP408, LP305, LP311, KP401, and KP412 were derived from ZIKV-infected RhMac peripheral blood B cells and spleen. (a) A Kaplan-Meier survival curve of murine ZIKV challenge 2 d prior to mouse-derived ZIKV dMAb administration. Red lines show pVax1-injected cohort, and blue lines indicate dMAb-injected cohort. (b) A Kaplan-Meier survival curve of murine ZIKV challenge 2 d prior to RhMac-derived ZIKV dMAb administration. Red lines show pVax1-injected cohort, and blue lines indicate dMAb-injected cohort. (c-d) Bar graphs of ZIKV-associated clinical scores post challenge at D 9. Each mouse was scored from 1 (green, no disease) to 5 (red, paralysis of both hindlimbs) and recorded as a percentage. The body weight and clinical signs of symptoms were examined and documented daily post challenge. The disease burden was the most severe between D 7 and D 9, where all mice in the control group exhibited a paralysis of both hind limbs or in a moribund state. Non-surviving or moribund mice by D 9 were recorded as 5 (red, paralysis of both hindlimbs) (c) Percent of clinical symptoms in ZIKV-PR209 challenged A129 mice at D 9 receiving mouse-derived anti-ZIKV dMAb clones or pVax1 empty vector control. (d) Percent of clinical symptoms in ZIKV-PR209 challenged A129 mice at D 9 receiving RhMac-derived anti-ZIKV dMAb clones or pVax1 empty vector control.
In vivo evaluation of protective responses generated by co-delivery of anti-ZIKV-dMAb and ZIKV expressing DNA vaccine plasmids
Combinations of the synthetic ZIKV-DNA vaccines, ZIKV-dMAbs, and combination of both were evaluated systematically. We have previously reported on the effectiveness of a DNA vaccine containing a novel consensus prME ZIKV antigen in inducing protective humoral and cellular immune responses in mice, NHPs12 and humans.27 As with most vaccines, optimal levels of protective immune responses are not achieved until at least a week post administration, and often, one or two boosting immunizations are needed to achieve durable, protective responses. In an outbreak situation, it is imperative to get health-care professionals and other personnel to hot zones rapidly, and often before they can receive a boost of any vaccine available to protect them from the threat.33 For these situations, we hypothesized that formulating a dMAb plasmid with a DNA vaccine could create a novel field strategy capable of providing both immediate and persistent protective responses against a pathogen. DNA is a useful vector for this strategy as it is non-immunogenic and allows for multiple administrations without loss of potency.17,19 For this study, we injected groups of A129 mice intramuscularly + EP with ZIKV DNA vaccine plasmid co-formulated with mouse-derived- or RhMac-derived- anti-ZIKV dMAb plasmids as described in Figure 6a. Half of the mice in each group were challenged with 106 PFU of ZIKV PR209 2 d later (D 2) while the other half in each group were challenged 3 weeks after the first immunization (D 21). As expected, ZIKV challenge of vaccine+dMAb groups conducted 2 d post the first vaccine+dMAb injection was able to protect the mice from morbidity and mortality (90% protection). The other half of each group that were challenged 3 weeks post the initial injection conferred 100% protection from ZIKV morbidity and mortality (Figure 6b,c). These results indicate that neither the mouse nor RhMac ZIKV dMAbs interfered with the induction of immune responses by the ZIKV DNA vaccine-making co-administration of these plasmids a viable strategy for providing immediate and persistent protection from ZIKV.
Figure 6.
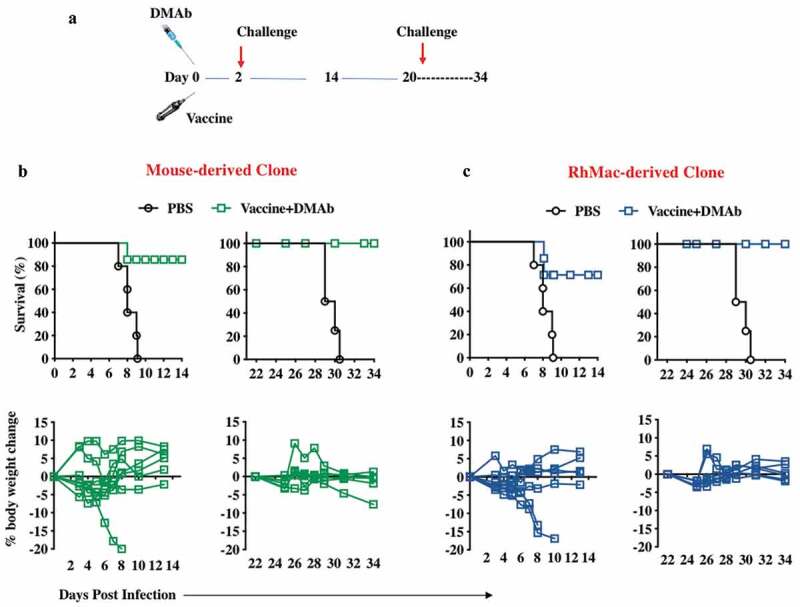
A combination immunotherapy with ZIKV dMAb and the ZIKV DNA vaccine prME1, given concurrently, confers immediate and persistent protection against ZIKV challenge.
(a) A schematic depiction of the combination immunotherapy schedule and challenge. A129 mice of 3–6 weeks of age and mixed gender were immunized with 25μg of pVax1 vector or ZIKV prME1 DNA vaccine i.m. in the left tibialis anterior muscle, and 100μg of pVax1 vector or the ZIKV dMAb plasmids i.m. in the right tibialis anterior muscle, as both injections were followed by CELLECTRA® enhanced electroporation delivery (n= 24, n= 12 for pVax1+ pVax1 group, n= 12 for prME1+ ZIKV dMAb group). Six mice from each group were challenged with 105 PFU of ZIKV PR209 2 d post DNA vaccine + ZIKV-dMAb combination. These remaining 12 mice were challenged with 106 PFU of ZIKV PR209 at 3 weeks after the first immunization. A combination immunotherapy of ZIKV DNA vaccine plus a ZIKV dMAb selected from mouse-derived clones (b) and rhesus macaque (RhMac)-derived clones (c). ZIKV dMAb clones of both origins confer protection in mice from an acute and prolonged challenge. The top and bottom left graphs refer to the ZIKV challenge 2 d post the first combination immunotherapy (vaccine+dMAb), where the top indicates a Kaplan-Meier survival curve and the bottom indicates percent body weight change over time. The top and bottom right graphs refer to the ZIKV challenge 3 weeks post the initial vaccine+dMAb administration, where the top graph indicates a Kaplan-Meier survival curve and the bottom graph charting percent body weight change over time.
Discussion
ZIKV infection is an emerging disease threat worldwide for which there is currently no vaccine or drug therapy approved for human use.27,35 As of January 2019, there are over 20 ZIKV vaccines in the pipeline in preclinical development and two candidates having entered phase II clinical trials (#NCT03110770, #NCT03014089). Current ZIKV vaccine platforms utilize various modalities including nucleic acids (both DNA and mRNA); purified, inactivated whole virus vaccines (PIV); viral-vectored vaccines; subunit proteins-virus-like particles (VLPs); and live-attenuated vaccines. Developing ZIKV vaccines based on a single strain of virus is feasible because the two genetic lineages of ZIKV, African and Asian/American, have limited variability.36 As for ZIKV therapeutics, anti-ZIKV monoclonal antibodies for both prophylaxis and treatment have been developed including the EDIII-specific ZKA190 clone which is broadly neutralizing and the ZIKV-117 clone that reduces infection in pregnant and non-pregnant mice. Small molecule antiviral agents have been tested that limit ZIKV infection in various ways including nucleoside analogs, peptidomimetic agents, adenosine analogs, antimalarials, anthelmintics, and cyclin-dependent kinase inhibitors (CDK).37 With both ZIKV vaccines and therapeutics for prophylactic and therapeutic usages, one of the biggest hurdles in advancing agents to the clinic has been the selection of the most suitable clinical endpoint.37 Given the endemic potential of ZIKV encompassing several continents and the broad spectrum of clinical manifestations, it is difficult to discern a naïve population without extensive laboratory testing. Ethical considerations for the inclusion of pregnant women and their safety related to ZIKV-related neurological complications, notably microcephaly and Guillain–Barré Syndrome, have also hindered the clinical evaluation of ZIKV vaccines and therapeutics.
In this study, we used two distinct strategies to generate a panel of mouse and RhMac antibodies capable of binding to the Envelope protein of ZIKV. The antibody gene sequences were encoded into DNA plasmid vectors to create dMAb plasmids that were shown to drive production of significant serum levels of functional anti-ZIKV antibodies in mice after a single EP-enhanced intramuscular injection. Delivery of these anti-ZIKV dMAb plasmids protected mice from morbidity and mortality following ZIKV challenge. Importantly, the ZIKV dMAb plasmids described here could be co-formulated with an anti-ZIKV DNA vaccine to provide immediate and persistent anti-ZIKV immune responses, respectively. As such, this can be a promising strategy for protecting individuals traveling to a ZIKV endemic region.8,38
While the dMAb plasmids were constructed in a similar fashion, in vivo serum levels of ZIKV dMAbs varied greatly. The clones producing peak serum antibody levels of 1√g/ml or more include human IgGs 8A9F9 and 8D10F4 originated from mice, and the human IgGs LP301, LP311, LP312, and KP412 originating from RhMac. There is no discernible pattern to explain the variable expression levels of each dMAb in vivo with factors including antibody folding, antibody charge, and antibody glycosylation all likely contributing factors. To improve expression levels in vivo, changes in dMAb plasmid formulation are being explored along with two-plasmid strategies where the heavy and light chains of each antibody are delivered on separate plasmids. DNA plasmids co-formulated with hyaluronidase have been shown to increase in vivo expression.20,39,40 Despite variable expression levels, the ability of the different antibodies to bind to ZIKV envelope is similar at normalized serum concentrations. An area of concern for antibody therapies for flaviviruses, particularly dengue virus, is the potential for antibody-dependent enhancement (ADE) of infection where sub-neutralizing levels of antibodies can mediate greater levels of infection by allowing uptake of virus into cells expressing the Fc receptor. There are findings that cross-reactive dengue virus mAbs can exacerbate ZIKV infection of placental macrophages using Hoffbauer cells.41 However, such effects had not been strongly demonstrated so far with ZIKV mAbs and increased ZIKV infections. Given the terminal disease state of severe ZIKV infections in humans, sub-neutralizing ZIKV mAbs with therapeutic efficacy warrants further investigation into higher animal models.
These ZIKV dMAb studies have several limitations to address. Our efforts were concentrated on identifying myriad of potent anti-ZIKV clones that bind to the prM and envelope regions of ZIKV to mainly affect receptor binding targeting Domain III of envelope as well as the stem and transmembrane regions that alter virion entry, assembly, and membrane fusion.42,43 Yet other structural and non-structural proteins may also be targeted to prevent or treat ZIKV infections. Monoclonal antibodies binding to ZIKV-NS1 region may confer advantages during an active viral replication phase of ZIKV infection, whereas clones that bind to ZIKV capsid anchor (Ca) region may block proper virion assembly as the cis-binding of Ca with PrM is strictly required to produce infectious particles.42 Broadly neutralizing capability of these anti-ZIKV clones has not been evaluated in this study since the dMAb-administered animals were challenged with a single strain of ZIKV-PR209. Despite the limitations in study designs, ZIKV has limited strain variability in which the African and Asian/American lineages are classified as a single serotype.36 ZIKV-PR209 is of the Asian/American lineage and one of the most virulent strains of wild-type ZIKV in recent years, which has been used in numerous published studies to test neutralization and in animal challenges to validate therapeutic or prophylactic efficacy of ZIKV agents.12,27,44 Moreover, sequence alignments of 58 complete ZIKV genome and 5 ZIKV envelope sequences compiled as of April 2016 show highly conserved amino acid variations and only eight variation sites in the envelope region.45
To our knowledge, these results are the first report of the in vivo expression of anti-ZIKV antibodies from a vector system. As ZIKV has spread throughout the islands of the Pacific Ocean and into South and Central America over the last 10 y, more serious signs and symptoms of ZIKV infection including birth defects and Guillain–Barré syndrome have become evident and prompted a concerted effort to develop therapeutics that could protect from and/or treat ZIKV infection.8 The mAbs described here have the potential to provide rapid prophylaxis against ZIKV infection and can be further combined with a ZIKV DNA vaccine to provide a durable protection to the virus. Furthermore, the mAb isolation process and the novel dMAb delivery strategy described here would be highly useful during an emerging infectious disease outbreak as the development and characterization stages are rapid and effective. The potential to broaden the clinical use of this novel mAb delivery strategy as well as dMAb therapies to resource-poor regions of the world warrants further study.
Funding Statement
This work was supported by the Wistar Science Discovery fund awarded to K.M.
Acknowledgments
The studies described in this manuscript were funded by a grant from the Wistar Science Discovery Award to KM. We would like to thank the Wistar Flow Cytometry Facility and Animal Facility for their technical assistance. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
DBW and KM contributed to conceptualization. HC, SBK, ELR, KA, PB, SA, LVD, CCR, GG contributed to methodology, and investigation; SR, KEB, LMH, JJK, KEU, and DLS contributed to resources; HC, SBK, DBW, and KM contributed to writing-review and editing; KM contributed to supervision; SBK and HC contributed equally to this work.
Conflict of Interest
CCR, SR, KEB, LMH, and JJK are employees of Inovio Pharmaceuticals and as such receive salary and benefits, including ownership of stock and stock options. KM discloses grant funding, industry collaborations, speaking honoraria, and fees for consulting. He has received consulting fees from Inovio Pharmaceuticals related to DNA and dMAb vaccine development. He has a patent application for DNA vaccine development and delivery of DNA-encoded monoclonal antibodies pending to Inovio Pharmaceuticals. Remuneration includes direct payments. DBW discloses grant funding, SAB and Board service, industry collaborations, speaking honoraria, and fees for consulting. His service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments and/or stock or stock options. He notes potential conflicts associated with this work with Pfizer, Bristol Myers Squibb, Inovio Pharmaceuticals, Merck, VGXI, Geneos, AstraZeneca, and potentially others. Licensing of technology from this laboratory has created over 150 jobs in the biotech/pharma industry. The other authors declare no competing financial interests.
References
- 1.Butler D. Zika virus: Brazil’s surge in small-headed babies questioned by report. Nature. 2016;530:13–14. doi: 10.1038/nature.2016.19259. [DOI] [PubMed] [Google Scholar]
- 2.Musso D, Baud D. Zika virus: time to move from case reports to case control. Lancet Infect Dis. 2016. doi: 10.1016/S1473-3099(16)00096-7. [DOI] [PubMed] [Google Scholar]
- 3.Samarasekera U, Triunfol M. Concern over Zika virus grips the world. Lancet. 2016;387:521–24. doi: 10.1016/S0140-6736(16)00257-9. [DOI] [PubMed] [Google Scholar]
- 4.Bogoch II, Brady OJ, Kraemer MU, German M, Creatore MI, Kulkarni MA, Brownstein JS, Mekaru SR, Hay SI, Groot E, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–36. doi: 10.1016/S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D. Zika virus genome from the Americas. Lancet. 2016;387:227–28. doi: 10.1016/S0140-6736(16)00003-9. [DOI] [PubMed] [Google Scholar]
- 6.Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2017;168:542. doi: 10.1016/j.cell.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Griffin BD, Muthumani K, Warner BM, Majer A, Hagan M, Audet J, Stein DR, Ranadheera C, Racine T, De La Vega MA, et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat Commun. 2017;8:15743. doi: 10.1038/ncomms15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med. 2016. doi: 10.1056/NEJMe1601862. [DOI] [PubMed] [Google Scholar]
- 9.Roa M. Zika virus outbreak: reproductive health and rights in Latin America. Lancet. 2016. doi: 10.1016/S0140-6736(16)00331-7. [DOI] [PubMed] [Google Scholar]
- 10.Lucey DR, Gostin LO. The emerging Zika pandemic: enhancing preparedness. JAMA. 2016. doi: 10.1001/jama.2016.0904. [DOI] [PubMed] [Google Scholar]
- 11.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, et al. Safety and Immunogenicity of an anti–Zika virus DNA vaccine — preliminary report. N Engl J Med. 2017. doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, Kraynyak KA, Duperret EK, Keaton AA, Chung C, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines. 2016;1:16021. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–51. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–78. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boigard H, Alimova A, Martin GR, Katz A, Gottlieb P, Galarza JM. Zika virus-like particle (VLP) based vaccine. PLoS Negl Trop Dis. 2017;11:e0005608. doi: 10.1371/journal.pntd.0005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Nganga D, Nanayakkara O, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–32. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, DiGiandomenico A, Keller AE, Smith TRF, Park DH, Ramos S, Schultheis K, Elliott STC, Mendoza J, Broderick KE, et al. An engineered bispecific DNA-encoded IgG antibody protects against Pseudomonas aeruginosa in a pneumonia challenge model. Nat Commun. 2017;8:637. doi: 10.1038/s41467-017-00576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flingai S, Plummer EM, Patel A, Shresta S, Mendoza JM, Broderick KE, Sardesai NY, Muthumani K, Weiner DB. Protection against dengue disease by synthetic nucleic acid antibody prophylaxis/immunotherapy. Sci Rep. 2015;5:12616. doi: 10.1038/srep12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthumani K, Block P, Flingai S, Muruganantham N, Chaaithanya IK, Tingey C, Wise M, Reuschel EL, Chung C, Muthumani A, et al. Rapid and long-term immunity elicited by DNA encoded antibody prophylaxis and DNA vaccination against Chikungunya virus. J Infect Dis. 2016. doi: 10.1093/infdis/jiw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott STC, Kallewaard NL, Benjamin E, Wachter-Rosati L, McAuliffe JM, Patel A, Smith TRF, Schultheis K, Park DH, Flingai S, et al. DMAb inoculation of synthetic cross reactive antibodies protects against lethal influenza A and B infections. NPJ Vaccines. 2017;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostertag EM, Kacir S, Thiboutot M, Gulendran G, Zheng XL, Cines DB, Siegel DL. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 1. Structural and functional characterization in vitro. Transfusion. 2016;56:1763–74. doi: 10.1111/trf.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andris-Widhopf J, Steinberger P, Fuller R, Rader C, Barbas CF 3rd.. Generation of human Fab antibody libraries: PCR amplification and assembly of light- and heavy-chain coding sequences. Cold Spring Harb Protoc. 2011. Sep 1;2011(9). [DOI] [PubMed] [Google Scholar]
- 23.Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, Tsunoda K, Amagai M, Stanley JR, Siegel DL, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–99. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise MC, Hutnick NA, Pollara J, Myles DJ, Williams C, Yan J, LaBranche CC, Khan AS, Sardesai NY, Montefiori D, et al. An enhanced synthetic multiclade DNA prime induces improved cross-clade-reactive functional antibodies when combined with an adjuvanted protein boost in nonhuman primates. J Virol. 2015;89:9154–66. doi: 10.1128/JVI.00652-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barouch DH, Thomas SJ, Michael NL. Prospects for a Zika virus vaccine. Immunity. 2017;46:176–82. doi: 10.1016/j.immuni.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016. Aug 4;536(7614):48–53. [DOI] [PubMed] [Google Scholar]
- 27.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, et al. Safety and Immunogenicity of an anti-Zika virus DNA vaccine - preliminary report. N Engl J Med. 2017. doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, Wakamiya M, Tesh RB, Barrett AD, Wang T, et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med. 2017;23:763–67. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–8. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–26. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 31.Dai L, Song J, Lu X, Deng Y-Q, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, et al. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, et al. Structural basis of Zika virus-specific antibody protection. Cell. 2016;166:1016–27. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muthumani K, Falzarano D, Reuschel EL, Tingey C, Flingai S, Villarreal DO, Wise M, Patel A, Izmirly A, Aljuaid A, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7:301ra132. doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond M. a mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–30. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Bardelli M, Espinosa DA, Pedotti M, Ng T-S, Bianchi S, Simonelli L, Lim EXY, Foglierini M, Zatta F, et al. A human bi-specific antibody against Zika virus with high therapeutic potential. Cell. 2017;171:229–41 e15. doi: 10.1016/j.cell.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, et al. Broadly neutralizing activity of Zika virus-immune sera identifies a single viral serotype. Cell Rep. 2016. Aug 9;16(6):1485–91. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilder-Smith A, Vannice K, Durbin A, Hombach J, Thomas SJ, Thevarjan I, Simmons CP. Zika vaccines and therapeutics: landscape analysis and challenges ahead. BMC Med. 2018;16:84. doi: 10.1186/s12916-018-1067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. Guillain–Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–39. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perales-Puchalt A, Wojtak K, Duperret EK, Yang X, Slager AM, Yan J, Muthumani K, Montaner LJ, Weiner DB. Engineered DNA vaccination against follicle-stimulating hormone receptor delays ovarian cancer progression in animal models. Mol Ther. 2019. Feb 6;27(2):314–25. doi: 10.1016/j.ymthe.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perales-Puchalt A, Duperret EK, Yang X, Hernandez P, Wojtak K, Zhu X, Jung SH, Tello-Ruiz E, Wise MC, Montaner LJ. DNA-encoded bispecific T cell engagers and antibodies present long-term antitumor activity. JCI Insight. 2019. Apr 18;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe. 2018;24:731–42 e6. doi: 10.1016/j.chom.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rana J, Slon Campos JL, Leccese G, Francolini M, Bestagno M, Poggianella M, Burrone OR. Role of capsid anchor in the morphogenesis of Zika virus. J Virol. 2018. Oct 29;92(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin SR, Zou G, Hsieh SC, Qing M, Tsai WY, Shi PY, Wang W-K. The helical domains of the stem region of dengue virus envelope protein are involved in both virus assembly and entry. J Virol. 2011;85:5159–71. doi: 10.1128/JVI.02099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esquivel RN, Patel A, Kudchodkar SB, Park DH, Stettler K, Beltramello M, Allen JW, Mendoza J, Ramos S, Choi H, et al. In Vivo delivery of a DNA-encoded monoclonal antibody protects non-human primates against Zika virus. Mol Ther. 2019;27:974–85. doi: 10.1016/j.ymthe.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaver JT, Lelutiu N, Habib R, Skountzou I. Evolution of two major Zika virus lineages: implications for pathology, immune response, and vaccine development. Front Immunol. 2018;9:1640. doi: 10.3389/fimmu.2018.01640. [DOI] [PMC free article] [PubMed] [Google Scholar]


