ABSTRACT
The leishmaniases are a collection of vector-borne parasitic diseases caused by a number of different Leishmania species that are distributed worldwide. Clinical and laboratory research have together revealed several important immune components that control Leishmania infection and indicate the potential of immunization to prevent leishmaniasis. In this review we introduce previous and ongoing experimental research efforts to develop vaccines against Leishmania species. First, second and third generation vaccine strategies that have been proposed to counter cutaneous and visceral leishmaniasis (CL and VL, respectively) are summarized. One of the major bottlenecks in development is the transition from results in animal model studies to humans, and we highlight that although American tegumentary leishmaniasis (ATL; New World CL) can progress to destructive and disfiguring mucosal lesions, most research has been conducted using mouse models and Old World Leishmania species. We conclude that assessment of vaccine candidates in ATL settings therefore appears merited.
KEYWORDS: Leishmaniasis, American Tegumentary Leishmaniasis, Leishmania spp, immune response, vaccines
Introduction
Through their residence in endemic regions, approximately 350 million people are currently at risk of infection with protozoan parasites of the genus Leishmania and subsequent development of leishmanaisis.1,2 Estimates suggest that the disease is present in 98 countries and about 2 million new cases occur each year. Three different clinical manifestations can be observed: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL) and visceral leishmaniasis (VL). The presenting form is determined by which of the more than 20 Leishmania species that can infect humans is actually manifesting disease,3,4 with many different Leishmania species associated with CL. CL is associated with a lower mortality than VL, but it is more widespread and afflicts a higher number of individuals. In the Old World L. major and L. tropica are the major causes of CL, while in the New World the disease is more commonly referred to as American tegumentary leishamaniasis (ATL) and is caused by infection with L. amazonensis, L. braziliensis, L. guyanensis, L. panamensis, L. mexicana, among others. Although ATL is usually relatively benign and can cure spontaneously if given enough time, in some cases symptoms exacerbate to cause MCL that presents with disfigurement and causes loss of productivity.4 Currently, the strategies to reduce leishmaniasis are limited to vector control and treatment of patients with outdated and toxic antimonial drugs for which there are increasing reports of resistance.5 Thus, there is both an opening and an urgent need for a safe and effective vaccine against leishmaniasis.
Although a considerable amount has been revealed with regard to the development of immunity during Leishmania infection, most of the knowledge, especially of the parasite-host relationship, has been derived from experimental studies in animal models.6,7 Leishmania infection is established when promastigotes, the flagellated form of the parasite, are transmitted during the bite of an infected sand fly on mammalian hosts. Once in the skin, the parasites are phagocytosed by different cell types, including inflammatory monocytes and dendritic cells (DCs) that are recruited to the site of infection. Over the first few days following infection, these cells become the predominant foci of Leishmania.8 Among the most important innate mechanisms that control the infection are the production of reactive oxygen species (ROS), generated by the respiratory burst during phagocytosis, and production of nitric oxide (NO), generated by inducible NO synthase (iNOS), followed by the activation of interferon gamma (IFN-γ) mediated cells.9 The interaction of parasites and DCs is one of the main factors that determines the outcome of Leishmania infection with DC-mediated development of Leishmania-specific CD4 and CD8 T lymphocytes well established as being important for protection.10,11 In murine models, the activation of Th1 and CD8 T cells depends upon antigen presentation through major histocompatibility complexes/human leukocyte antigen (MHC/HLA) complexes on the surface of infected DC in the presence of interleukin (IL)-12, driving the T cells to produce IFN-γ that in turn activates macrophages to produce NO. This cascade of events contributes to the elimination of the parasites, whereas T cell production of IL-4 and TGF-β in the local microenvironment inhibits DC secretion of IL-12 and favors parasite survival.9
The observation that recovery from primary infection with L. major, and to some extent with L. braziliensis is typically associated with long term protection against reinfection, indicates that vaccines can be developed against CL.12,13 Spontaneous cure has been documented in endemic areas for L. braziliensis infection (usually occurring with a clinical evolution of less than 3 months) and this suggests that an immune response that controls the infection has developed.14 Carvalho et al.15 discovered that patients with rapidly self-healing L. braziliensis ATL exhibited positive intradermal skin test results, lymphocyte proliferation and IFN-γ production, indicating a strong T-cell response to Leishmania antigens. Infection with L. braziliensis is often difficult to heal, however, and sometimes produces mutilating lesions in the nasopharyngeal mucosa with responses significantly higher in healed patients than in those with active disease.
It is noteworthy that majority of our understanding of Leishmania infections has been generated in mice using tightly controlled L. major infection models, and the better understanding of experimental immunity against L. major might be one of the reasons why most current vaccine candidates are focused on Old World species instead of those found throughout the New World. Until recently it was believed that long-lived memory cells generated during chronic L. major infection were required for durable protection, and vaccination strategies based on generating such cells were therefore desirable. Peters et al demonstrated that for T helper 1 (Th1) concomitant immunity, in which protection against reinfection coincides with a persisting primary infection, preexisting CD44(+)CD62L(-)T-bet(+)Ly6C+ effector T cells that are short-lived in the absence of infection and are not derived from memory cells reactivated by secondary challenge, mediate immunity.16 Such gaps in our knowledge regarding immunity against Leishmania infections in humans may be confounding vaccine development, and it is noteworthy that the New World Leishmania species induce a response pattern distinct form that induced by Old World Leishmania species.17 IL-17 has been linked to a massive influx of inflammatory cells that lead to disease exacerbation,18 while IL-10 has been linked with strong immunosuppression and exacerbated pathology.19 The immune response against Leishmania in humans is not fully understood, and a far more complex response appears to occur that likely involves important interactions of a wide variety of cytokines and cells that dictate clinical outcome. It remains unclear how to best generate the immune memory that prevents reinfection and the immune correlates that would be beneficial in determining this are also not fully defined.9,20,21 Nevertheless, we believe that identifying antigens that appropriately target of the anti-Leishmania response is fundamental for the development of an effective vaccine against CL/ATL. Indeed, many different peptides and antigens from New World Leishmania species have been screened using modern bioinformatics tools and appear to have potential within vaccines for ATL.22,23 Most, however, remain in research stages and have not been fully developed as vaccines ready for clinical trials.
Currently, five vaccines have been licensed and approved against Leishmania species. Two of these are approved for administration to humans, with one in Brazil using killed L. amazonensis parasites for immunotherapy of CL and the other in Uzbekistan using live L. major parasites. The others use recombinant proteins for prophylactic immunization of dogs in Brazil.5,24–27 Although these vaccines were approved by the respective national regulatory agencies, their effectiveness remain controversial. For example, the vaccines used for immunotherapy cannot be used alone but rather in combination with conventional chemotherapies.28 Whole parasites need to be used with caution since they can induce chronic lesions; and although the vaccine for dogs was proven to be effective in the short term, its long term impact remains unknown and further studies are needed to determine if its use can reduce the spread of the disease.25,29 To date, there are no licensed vaccines for human CL but those that have advanced to clinical trials include two killed L. amazonensis vaccines,30,31 each of which generated inconsistent results between vaccine and placebo groups, and the defined polyprotein-containing subunit vaccine LEISH-F1+ MPL-SE, which was demonstrated to be safe while generating an antigen-specific Th1 response.32
Vaccine development
A considerable challenge in developing a vaccine is identifying the best type or set of antigens against which to direct an appropriate immune response. Additional variables such as the specific gene or protein targeted, the amount of antigen/vector used, the number of immunizations, the Leishmania species prioritized for evaluation and the type of experimental challenge, can confound results between studies and somewhat confuse the vaccine landscape. As an example, it became understood only recently that components within sand fly saliva can alternatively inhibit or assist in promoting the anti-Leishmania Th1 response.33 It is clear, however, that a variety of different targets and strategies can be used to develop a vaccine for leishmaniasis, and in this regard the likelihood that an effective vaccine will emerge should be considered as high.
In a simplified form, the composition of a vaccine consists of two key elements: antigen(s) to generate a pathogen-specific response and the adjuvant to initiate and direct the immune response.34 In this sense, to direct and adequately stimulate immunity against Leishmania, the vaccines require specific parasite antigens and immunostimulatory molecules. In first generation vaccines the antigen component is derived from use of the whole parasite, either in a live attenuated manner or inactivated and killed by chemical or physical processes. In first generation vaccines the adjuvant component is typically also inherent to the parasite, with multiple pathogen-associated molecular patterns (PAMP) established and defined for Leishmania species.35,36 Second generation vaccines are composed of molecularly defined components, using recombinant antigens, which can be one or more proteins, and specified adjuvants. The panel of defined adjuvants that can be used with recombinant proteins to tailor the vaccine-induced response continues to expand,36–39 and a variety of defined adjuvants have now been used in studies with vaccines against Leishmania.32,40,41 Regarding third generation vaccines, these include vaccines that use the pathogen-specific DNA or RNA, or a platform/carrier that contains genetic component(s) of the pathogen to target the immune response (Figure 1).
Figure 1.
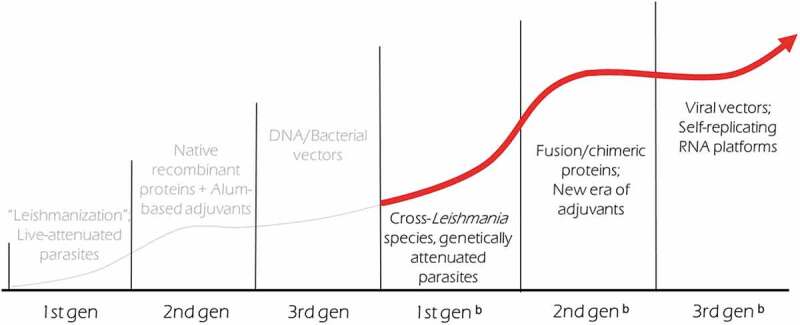
Maturation and expansion of Leishmania vaccine development.
Antigen target selection
Concurrent with the development of new adjuvants, advances in molecular and in silico tools in recent years has led to a dramatic increase in the number of Leishmania-specific targets that have been evaluated in vaccine studies.22,42,43 Genome sequencing revolutionized vaccine development because the availability of pathogen genomes has informed discovery of novel antigens while the exclusion of targets that have homology with human genes. The number of Leishmania species genomes that are available in public data banks has grown over time, making it now possible to infer the complete proteome of different species (predicted proteome). The use of these genome sequences to make in silico predictions of suitable targets (reverse vaccinology)44 provides an efficient means with which to identify important epitopes of both CD4+ and/or CD8 + T cells.45,46 Numerous research groups are now applying reverse vaccinology to identify antigens that are common, or highly homologous, across the published genomes of various Leishmania spp. One approach has used linear methods to search for epitopes in different Leishmania species proteomes,47 while another has focused on identifying epitopes within already known antigens.48 The latter approach led to the development of a refined vaccine candidate containing multi-epitope peptides of Leishmania Cysteine Protease A (CPA160–189) that provided partial protection against L. infantum infection in BALB/c mice.49 Freitas-Silva et al., used a combination of in silico methods to identify epitopes of CD4 and CD8 T cells within the predicted proteome of Leishmania (Viannia) braziliensis.22 These peptides had a high binding affinity to both MHC/HLA complexes and the immunogenicity of some was demonstrated by stimulation of peripheral blood mononuclear cells (PBMC) from healthy controls and post-treatment ATL patients. To validate the use of bioinformatics tools for epitope prediction, protein-protein interaction networks and metabolic pathways, Brito et al., integrated data from experimental and in silico studies and demonstrated that some of these methods correlated with protection observed in murine models.23 This indicates that reverse vaccinology may not only be important for screening of potential candidates, but suggest that it may also help us better understand parasite virulence mechanisms and how the host immune response can curtail them.
Phage display, a high-throughput method where mimotopes are presented on the surface of phages and are recognized by specific target ligands, provides another strategy to rapidly select potential candidates.50 A recent study used phage display and PBMC from ATL patients to identify T cell-specific mimotopes, then to evaluate the immunogenicity of two of the identified clones in mice after challenge with L. amazonensis.51 The results were promising as polarized Th1 responses were generated and immunized mice had significant lower numbers of parasites when compared with controls, but further testing is needed to determine if these can advance. It is also noteworthy that despite being selected by screening among ATL patients, animal testing was conducted against L. amazonensis, a strain also associated with VL.
First generation vaccines
First generation vaccines consist of live-attenuated or killed parasites that are used to generate broad immunity. Leishmanization (LZ; the inoculation of a low dose of live Leishmania parasites to generate a minor lesion but also a natural anti-Leishmania response) was the first vaccine strategy proven to be effective against leishmaniasis in humans and it was widely used among soldiers in the Soviet Union.52 Clinical trials in humans based on LZ were performed due to its high efficacy, and motivated the return of this practice in high-incidence regions.53 In Venezuela a relatively large number of ATL patients received immunotherapy with monthly intradermal injections of a combined vaccine containing autoclaved promastigotes of L. mexicana amazonensis MHOM/VE/84/MEL and viable Bacillus Calmette- Guerin (BCG) during the 1980s and 90s.54 Clinical healing varied from 91.2 to 98.7%, with an average of 95.7%. The high percentage of clinical cures achieved with this immunotherapy (> 90%) supported further use in the routine treatment of localized ATL, and the immunotherapy was modified to use promastigotes of L. braziliensis strain MHOM/BR/84/LTB 300 killed by pasteurization.54,55
Although first generation vaccines are attractive for developing countries because they can be produced at a relative low cost,56 sustaining a consistent product can present a barrier. There can be difficulties in standardizing culture conditions to obtain the immunogen and progressive declines in infectivity can occur when subculturing the parasites.40,57 This situation is analogous to the Bacille-Calmette Guerin (BCG) vaccines used to prevent tuberculosis, where numerous substrains, each of which provide varying degrees of efficacy, are now used worldwide.58
L. major LZ can provide protection against infection with different Leishmania species in mice and suggests the possibility of using L. major LZ to prevent VL.59 Among the important considerations when using live Leishmania parasites in human populations is not only safety in the vaccinee but also safety of introducing parasites into the local population. Ideally, live parasites should induce a protective immune response in the vaccinated individual but should be cleared and not persist in the long term.60 LZ has the capacity of inducing non-healing lesions in immunocompromised patients, and persistent parasites could support recrudescence in the event of immune suppression while also potentially serving as a reservoir for transmission to susceptible individuals and the introduction of this species into a non-endemic region. Murine studies have shown that although heat-killed Leishmania can stimulate an early Th1 response and protection, protective immunity is not sustained and wanes with time.61–63 One hypothesis is that a long-term anti-Leishmania response is not generated because mice immunized in this way are not able to induce effector memory cells following a secondary challenge. When repeated boosts of killed parasites were provided, however, both effector and central memory T cells (TEM and TCM, respectively) were produced and a prolonged protection against virulent challenge could be observed.64 A number of strategies are being developed to enhance LZ, including the concomitant inoculation of adjuvant molecules (such as CpG-ODNs) to prompt the innate immune response to rapidly kill the parasites or generate long term immunity.64 Attempts to improve the efficacy of attenuated/killed Leishmania vaccines against CL using different types of immunization, different immunization schemes and different ways of attenuation (i.e. photosensitization;65 impairing the mannose activation pathway66) and challenge have indicated promise but have not yet reached advanced phases of clinical development.67–69
Targeted genetic modification to attenuate or delete specific virulence genes within Leishmania is considered a powerful strategy with the potential to provide cross-protective parasites with improved safety profiles, examples are described on Table 1. While the first attempts using attenuated Leishmania parasites rendered protection in murine models, lack of knowledge regarding potential reversion to a wild type genotype left the parasites unsuitable for use in human populations.73–77 Advances in genetic manipulation have provided new methods with which to address these issues, however, and various reverse genetic modifications of Leishmania parasites have now been characterized.78 Genetically engineered parasites lacking essential genes such as dhfr-ts (L. major), lpg2 (L. mexicana and L. major), SIR2 (L. infantum), P27 (L. major), Centrin1 (L. donovani) and ΔCPB (L. mexicana) have been developed and evaluated in animal models.70–72,79–82 In addition to targeted genetic attenuation, use of naturally attenuated Leishmania species (i.e., those species that are nonpathogenic in humans) also appears possible as indicated by the use of, L. tarentolae, a species with the genus Sauroleishmania83 that typically infects lizards. L. tarentolae is advancing on the basis that it could also potentially be used as a prophylactic or immunotherapeutic vaccine in immunocompromised patients.84–87
Table 1.
Examples of first generation vaccine candidates using genetically modified parasites against CL associated with protection.
| SPECIES | MODIFICATION | OLD WORLD OUTCOME | NEW WORLD OUTCOME | REFERENCE |
|---|---|---|---|---|
| L. mexicana | Mannose pathway impairment | Not tested | Protection (live parasites challenge) – No protection (killed parasites challenge) | 66 |
| L. mexicana | Lack of the dhfr-ts gene | Not tested | No protection | 70 |
| L. major | Lack of the dhfr-ts gene | Protection | Not tested | 71 |
| L. major | Lack of the P27 gene | Partial protection | Not tested | 72 |
Second generation vaccines
In contrast to first generation vaccines, second generation vaccines use defined products to generate the desired immune responses. Native proteins from Leishmania have been used either crudely or in a purified manner to generate protective responses and the majority of Leishmania vaccines currently being explored include antigenic proteins from the parasite or different individual antigens produced as recombinant products.88 The search for antigenic proteins of Leishmania is becoming more common and different strategies are being used to characterize these antigens, including genomic or proteomic approaches using serum samples or T cells from Leishmania-infected individuals28,88 and bioinformatics tools, which have facilitated the recombinant production of a variety of targets.62,89 The most common production methods are prokaryotic expression systems using Escherichia coli or those using yeast.90,91 Proteins alone are insufficient as vaccines, however, and they need to be co-injected with adjuvants to induce a satisfactory T cell response.63 Experimental evidence also suggests that multiple injections of most sub-unit vaccines are required to generate lasting immunity. Despite this, in addition to a high degree of control and reproducibility in production, one of the major advantages of the second-generation vaccine approach is overall cost. Mathematical modeling indicates that a vaccine dose costing 2 USD or less would be much more economically beneficial then the currently available leishmaniasis treatments.92
Many Leishmania proteins have now been purified or recombinantly expressed for evaluation as defined subunit vaccine candidates (Table 2). Due to the fact that it is expressed in both the insect and vertebrate host phases of the parasite lifecycle, Leishmania homolog of receptors for activated C kinase (LACK) has been widely evaluated and mice immunized with LACK resist L. major infection.105,106 L. major H2B histone protein, and its divergent N-terminal region, have also been used to confer protection against experimental L. major infection.107 When histone H1 was tested in conjunction with the Montanide ISA 720 adjuvant in monkeys, it reduced the lesions formed after L. major infection and these self-healed over time.108 Among the purified proteins, the parasite cell surface metalloprotease GP63 mediated a robust protection against challenge with both L. mexicana and L. major in mice but conferred only partial protection in monkeys.109–111 With regard to using proteins from one Leishmania species to protect against an alternate species, sterol methyl transferase (SMT) of L. infantum, protected mice against L. major challenge when formulated with MPL-SE.112 Another example of a protein that could potentially yield cross-protection due to high sequence homology between Leishmania species is L. donovani nucleoside hydrolase (NH36), which protected mice against both L. (L.) amazonensis and L. (V.) braziliensis infection.113,114 Alves-Silva et al. recently extended this initial finding by assessing the efficacies of NH36, its F1 and F3 domains, and the recombinant chimera F1F3, each formulated with Riedel de Haen saponin, against L. braziliensis mouse infection, observing some variance in the onset and magnitude of multifunctional IL-2+TNF-α+IFN-γ+ antigen-specific CD4 and CD8 T cells but finding that the F1F3 chimera resulted in the greatest reduction of the ear lesions sizes.112 Similarly, L. major recombinant ribosomal proteins L3 and L5 combined with CpG-ODNs induced a Th1 response in BALB/c mice that conferred protection against L. major and L. braziliensis challenge.115
Table 2.
Examples of second generation candidate antigens tested in mice.18
| SPECIES | CANDIDATE ANTIGEN | FUNCTION | OLD WORLD OUTCOME | NEW WORLD OUTCOME | REFERENCE |
|---|---|---|---|---|---|
| L. braziliensis | Thiol-specific antioxidant (TSA) LeiF LACK |
Tryparedoxin peroxidase | Protection against L. major | No protection | 93 |
| Leishmania putative eukaryotic initiation factor | Protection against L. major | No protection | 93 | ||
| Leishmania homolog of receptors for activated C-kinase | Protection against L. major | Partial protection | 93 | ||
| L. amazonensis | P4 nuclease Cysteine proteinase HSP20 GP46 |
Endonuclease activity Cysteine-type peptidase Activity Heat-shock protein Membrane glycoprotein |
Not tested Not tested Not tested Not tested |
Protection Partial protection No protection Protection |
94 95 96 97 |
| L. mexicana | GP63 | Metalloendopeptidase activity | Not tested | Protection | 98 |
| L. major | LmTSI GP63 PSA2 TSA Histone H1 LACK |
Stress-Induced protein sti1 Metalloendopeptidase activity Promastigote surface antigen protein 2 Typaredoxin peroxidase DNA binding Leishmania homolog of receptors for activated C-kinase |
Protection Protection No protection No protection Protection Partial protection |
No protection (L. braziliensis) Protection (L. mexicana) Protection (L. mexicana) Not tested Not tested Not tested |
99 100 101 102 103 104 |
Interestingly, the impact of sand fly saliva has been systematically investigated over the last few years and it is now understood that sand fly components inoculated during feeding can modify the bite site environment.116 Several proteins found in the saliva of Lutzomyia longipalpis (Lu. longipalpis), one of the main vectors of the disease in the New World, appear to enhance the parasite’s pathogenesis117 and based on these observations, vaccine candidates containing sand fly salivary proteins have been proposed. Indeed, evaluation of these salivary proteins either alone or in conjunction with Leishmania proteins has generated promising results in animal models.117–120 Mice immunized with the LJM11 protein from Lu. longipalpis have significantly reduced parasite numbers in the ear and lymph node following challenge with L. braziliensis plus Lu. longipalpis salivary gland sonicate, but not when the parasites were inoculated with salivary gland sonicate from an alternate sand fly.121
Recombinant methods also make it possible to manipulate and combine proteins and/or complementary epitopes into a single polyepitope/polyprotein product. Leish-111f, an antigen made of 3 fused proteins (L. major thiol-specific antioxidant [TSA], L. major stress-inducible protein-1 [STI1], and L. braziliensis elongation and initiation factor [LeIF]) provided protection against experimental L. major infection.41,122 The combination of Leish-111f with the adjuvant monophosphoryl lipid A plus squalene (MPL-SE) became the first defined vaccine candidate to advance to phase 1 and phase 2 clinical trials, where it was found to be safe and immunogenic.40 When injected with the Toll-like receptor (TLR)-4 agonist glucopyranosyl lipid A (GLA), KSAC, a recombinant protein made of KMP-11, SMT, A2 and CPB, conferred protection in susceptible BALB/c mice against sand-fly transmission of L. major.123 A fusion protein made of L. major cysteine proteinases (CP) A and B and L. pifanoi cysteine protease has also been shown to provide partial protection against L. amazonensis in mice.124,125 It is not clear, however, if these vaccines can provide protection against ATL-causing Leishmania species.
Third generation vaccines
In the last few years, genetic immunization consisting of either nucleic acids alone or as genes added into delivery vectors, has emerged as another alternative, namely third generation vaccine strategy. These nucleic acid-based vaccines also have a significant logistical advantage because their typically high stability appears to make them practical for use in tropical areas. Additional advantages provided by this method include the selective expression of proteins that are assumed to be folded close to their native/natural conformation; production or persistence of the antigen over several days; and induction of antigen-specific memory cells.126 Both DNA and RNA platforms have been developed, but those using DNA need to access the nucleus and currently appear to work better in small animal models. To date, higher doses of DNA have been required in larger animals and humans and this has raised some safety concerns.127,128 Furthermore, the low immunogenicity of DNA vaccines observed in dogs and humans has limited their advance and has necessitated the use of approaches such as in vivo electroporation, microneedle-based delivery and DNA encapsulation to increase their immunogenicity.52 Despite this, a wide repertoire of antigens has been investigated in the context of DNA vaccines.129 The gene coding for GP63 was the first DNA vaccine developed against leishmaniasis, mediating solid protection against L. major infection in mice.130-132 LACK is perhaps the most extensively studied DNA vaccine against Leishmania and in clinical trials pairing of LACK with IL-12 increased the protection achieved relative to that observed with LACK alone.106 DNA-encoding the A2 protein has also mediated protection in mice infected with L. amazonensis.133 In a recent report, a TSA-based DNA vaccine was successful in promoting a Th1 immune response and protection against L. major challenge.134 Experiments using the iron superoxide dismutase of L. donovani demonstrated protection against L. amazonensis in BALB/c mice by inducing IFN-γ that reduced the parasite burden.135 Studies involving the pcDNA3H3H4 plasmid expressing L. major histone proteins H3 and H4 resulted in a partial resistance to L. major associated with the development of Th1/Th2-type responses and a reduced number of parasite-specific regulatory T cells at the infection site.136 L. infantum histone genes H2A, H2B, H3, and H4 have also been able to control both L. major and L. braziliensis infections in BALB/c mice.137,138 Similar to the ability to produce chimeric fusion proteins, multiple genes can be fused together for use within third generation vaccines. A recent study compared the responses generated by genes encoding either LACK alone, TSA alone or a fusion of these two genes named LACK-TSA, and found that LACK-TSA triggered a stronger protective response against challenge with L. major than the individual genes.139
In contrast to DNA, RNA platforms need access only to the cytoplasm and have been demonstrated to be effective in both small and large animal models.140–143 RNA can be generated in non-replicating platforms optimized and/or modified to avoid their detection by the immune system, or in self-replicating platforms that use viral replication machinery. Self-replicating RNA vaccines can engage the innate immune system in a manner similar to the parent virus and essentially provide a self-adjuvanting function.144 Unlike the responses generated with second generation vaccines that typically consist of antigen-specific CD4 T cells exclusive of CD8 T cells, an important feature of third generation vaccines is the induction of both antigen-specific CD4 and CD8 T cells.145 A fusion of the kinetoplastid membrane protein 11 (KMP11) and hydrophilic acylated surface protein B (HASPB) genes was cloned into a lentiviral vector (pCDH-cGFP) that generated a protective response with significant increases in both pro- and anti-inflammatory markers (IFN-γ and IgG2a versus IL-10 and IgG1).146 The higher levels of IFN-γ and IgG2a indicated skewing toward a desirable Th1 response.
The influenza virus has also been used as a vector, and a recombinant influenza virus expressing the single MHCII-restricted peptide LACK158–173 achieved protection against L. major while generating considerable levels of peptide-specific IFN-γ.147 Recently, the first human trial using a third-generation vaccine designed for VL and post-kala azar dermal leishmaniasis (PKDL) was safely conducted and confirmed the ability of a simian adenovirus (ChAd63) that encodes two Leishmania proteins (KMP11 and HASPB) to generate antigen-specific responses.148 Similar studies in primates and/or humans have not yet been conducted against CL-causing Leishmania species.
In addition to the use of viral vectors, a number of bacteria including Listeria monocytogenes, Mycobacterium bovis BCG and Salmonella enterica serovar Typhimurium, have been used as delivery systems for Leishmania antigens.62,131 An example of this approach was shown in a study where novel Leishmania antigens were selected through a proteomic/in silico approach then expressed in Salmonella Typhimurium SL3261. Immunization of mice with individual serovars of Salmonella expressing the antigens LinJ08.1190 and LinJ23.0410, or a pool of these constructs, significantly delayed progression of L. major infection and increased resistance against L. donovani.149 Some examples of these candidates are listed on Table 3.
Table 3.
Examples of third generation (DNA) vaccine candidates against CL associated with protection.
| SPECIES | GENE | OLD WORLD OUTCOME | NEW WORLD OUTCOME | REFERENCE |
|---|---|---|---|---|
| L. major | GP63 | Protection | Not tested | 131 |
| L. major | LACK | Protection | Not tested | 106 |
| L. amazonensis | A2 | Protection (L. chagasi) | Protection (L. amazonensis) | 133 |
| L. major | TSA | Protection | Not tested | 134 |
| L. major | H3, H4 | Partial protection | Not tested | 136 |
| L. infantum | H2A, H2B, H3, H4 | Protection against L. major | Protection against L. braziliensis | 134 |
| L. major | KMP11+ HASPB | Protection | Not tested | 146 |
Conclusions
Cutaneous leishmaniasis (CL) and American tegumentary leishmaniasis (ATL) remain important neglected tropical diseases that directly or indirectly impact millions each year. In addition to sand fly vector control efforts, the current control strategies for leishmaniasis rely upon early and accurate diagnosis (when attainable) coupled with chemotherapeutic approach to limit disease symptoms and ongoing parasite transmission. These efforts are having more success on the Indian subcontinent (against VL) than in South America, where VL- and CL-causing Leishmania species co-exist.
It is our belief that integrating a vaccine within leishmaniasis control strategies would have the greatest and most sustainable impact. It is quite conceivable that a combination of approaches may be used to achieve effective immunization. Indeed, we modeled such an approach against VL-causing Leishmania species, providing mice with a heterologous immunization regimen that involved second and third-generation vaccines with the same target proteins/inserts. This heterologous immunization scheme stimulated robust antigen-specific T cell responses and was capable of protecting against experimental L. donovani infection.150 This approach could potentially provide the same benefit against CL-causing Leishmania species. Until now, most attempts to develop a vaccine against CL have been based on Old World Leishmania species, however, and unfortunately these species appear to have a response pattern that differs from the species that predominate in the New World and especially so from L. braziliensis. Even though most of the preclinical evaluations have used experimental L. major infection to determine vaccine efficacy, immunization to prevent ATL should be attainable given the plethora of targets and variety of platforms under preclinical investigation. For this to happen, however, it will be necessary to ensure that both ATL and its complications remain a priority among public health policy decision makers and that vaccine development efforts evaluate promising candidates against ATL-causing species that are endemic throughout South America.
Funding Statement
This work was supported by the CRDF Global [62966];Conselho Nacional de Desenvolvimento Científico e Tecnológico [14/2014];Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [001];Fundação Oswaldo Cruz [Tesouro];Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco;National Institute of Allergy and Infectious Diseases [R01AI025038];Global Health Innovative Technology Fund [G2018-111].
Acknowledgments
BCO is the recipient of a PhD scholarship from the Foundation of Science and Technology Support of Pernambuco (FACEPE) and a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES – Doutorado Sanduíche) – Finance code 001.
Leishmania research at IDRI has been funded by grants from CRDF Global (#62966), National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI025038, the Bill and Melinda Gates Foundation (#631 and #39129) and The Global Health Innovative Technology Fund (G2018-111).
Leishmania research at FIOCRUZ Pernambuco is funded by Tesouro Fiocruz and the National Council for Science and Technology Development (CNPq), Brazil. Universal, Process number 14/2014.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Disclosure of potential conflicts of interest
The authors declare that their research is conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.WHO . Leishmaniasis Fact Sheet. World Health Organization; [cited 2019. April 1]. Published 2017. Available from: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis [Google Scholar]
- 2.Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME.. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016;34(26):2992–95. doi: 10.1016/j.vaccine.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 3.de Vries HJC, Reedijk SH, Schallig HDFH. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16(2):99–109. doi: 10.1007/s40257-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson RD, Sousa ADQ. Clinical spectrum of leishmaniasis. Clin Infect Dis. 2010;22(1):1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Mayrink W, De Carvalho Botelho AC, Magalhães PA, Batista SM, Lima ADO, Genaro O, Costa CAD, Melo MND, Michalick MSM, Williams P, et al. Immunotherapy, immunochemotherapy and chemotherapy for American cutaneous leishmaniasis treatment. Rev Soc Bras Med Trop. 2006;39(1):14–21. doi: 10.1590/S0037-86822006000100003. [DOI] [PubMed] [Google Scholar]
- 6.Brelaz MCA, De Oliveira AP, De Almeida AF, de Assis Souza M, Medeiros ÂC, de Brito ME, Pereira VR. Antigenic fractions of Leishmania (Viannia) braziliensis: the immune response characterization of patients at the initial phase of disease. Parasite Immunol. 2012;34(4):236–39. doi: 10.1111/j.1365-3024.2012.01351.x. [DOI] [PubMed] [Google Scholar]
- 7.Reis LDC, Edileuza M, De Brito F, De Assis M. Mecanismos Imunologicos na resposta celular e humoral na leishmaniose tegumentar Americana. Revista de Patologia Tropical. 2006;35(81):103–15. [Google Scholar]
- 8.Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 2012;8(2):e1002536. doi: 10.1371/journal.ppat.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott P, Novais FO. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. 2016;16:581–92. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 10.Alexander J, Brombacher F. T helper1/T helper2 cells and resistance/susceptibility to Leishmania infection: is this paradigm still relevant? Front Immunol. 2012;3(APR):1–13. doi: 10.3389/fimmu.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza MDA, Castro MCABD, Oliveira APD, Oliveira BC, Almeida AF, Almeida TMD, Pereira VRA. Immunity against leishmaniasis. New York: Nova Science Publishers; 2013. [Google Scholar]
- 12.Lainson R, Shaw JJ. Leishmaniasis in Brazil: XII. Observations on cross-immunity in monkeys and man infected with Leishmania mexicana mexicana, L. m. amazonensis, L. braziliensis braziliensis, L. b. guyanensis and L. b. panamensis. J Trop Med Hyg. 1977;80:29–35. [PubMed] [Google Scholar]
- 13.Porrozzi R, Teva A, Amaral V, Da Costa MV, Grimaldi G. Cross-immunity experiments between different species or strains of Leishmania in rhesus macaques (Macaca Mulatta). Am J Trop Med Hyg. 2018;71(3):297–305. doi: 10.4269/ajtmh.2004.71.297. [DOI] [PubMed] [Google Scholar]
- 14.Brito ME, Mendonça M, Gomes Y, Jardim M, Abath FG. Dynamics of the antibody response in patients with therapeutic or spontaneous cure of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95(2):203–06. doi: 10.1016/S0035-9203(01)90168-3. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho EM, Filho DC, Bacellar O, Almeida RP, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 2017;53(3):273–77. doi: 10.4269/ajtmh.1995.53.273. [DOI] [PubMed] [Google Scholar]
- 16.Peters NC, Pagán AJ, Lawyer PG, Hand TW, Henrique Roma E, Stamper LW, Romano A, Sacks DL. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog. 2014;10:12. doi: 10.1371/journal.ppat.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Luca PM, Macedo ABB. Cutaneous leishmaniasis vaccination: A matter of quality. Front Immunol. 2016. doi: 10.3389/fimmu.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostka L, Dinges S, Griewank K, Iwakura Y. Udey MC von SE. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2010;182(5):3039–46. doi: 10.4049/jimmunol.0713598.IL-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 20.Bahrami F, Harandi AM, Rafati S. Biomarkers of cutaneous leishmaniasis. Front Cell Infect Microbiol. 2018;8. doi: 10.3389/fcimb.2018.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glennie ND, Scott P. Memory T cells in cutaneous leishmaniasis. Cell Immunol. 2016;309:50–54. doi: 10.1016/j.cellimm.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva RF, Ferreira LFGR, Hernandes MZ, de Brito MEF, de Oliveira BC, da Silva AA, de-Melo-Neto OP, Rezende AM, Pereira VRA . Combination of in silico methods in the search for potential CD4+ and CD8+ T cell epitopes in the proteome of leishmania braziliensis. Front Immunol. 2016;7(AUG). doi: 10.3389/fimmu.2016.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brito RCF, Guimarães FG, Velloso JPL, Corrêa-Oliveira R, Ruiz J, Reis A, Resende D. Immunoinformatics features linked to Leishmania vaccine development: data integration of experimental and in silico studies. Int J Mol Sci. 2017;18(2):1–18. doi: 10.3390/ijms18020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava S, Shankar P, Mishra J, Singh S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasites Vectors. 2016;9(1):1–15. doi: 10.1186/s13071-016-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcondes M, Day MJ. Current status and management of canine leishmaniasis in Latin America. Res Vet Sci. 2019;123(January):261–72. doi: 10.1016/j.rvsc.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Parra LE, Borja-Cabrera GP, Santos FN, Souza LOP, Palatnik-de-Sousa CB, Menz I. Safety trial using the Leishmune® vaccine against canine visceral leishmaniasis in Brazil. Vaccine. 2007;25(12):2180–86. doi: 10.1016/j.vaccine.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 27.Starita C, Gavazza A, Lubas G. Hematological, biochemical, and serological findings in healthy canine blood donors after the administration of CaniLeish® vaccine. Vet Med Int. 2016;2016:1–6. doi: 10.1155/2016/4601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palatnik-de-Sousa CB. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine. 2008;26(14):1709–24. doi: 10.1016/j.vaccine.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Seyed N, Peters NC, Rafati S. Translating observations from leishmanization into non-living vaccines: the potential of dendritic cell-based vaccination strategies against Leishmania. Front Immunol. 2018;9(JUN):1–10. doi: 10.3389/fimmu.2018.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Luca P, Mayrink W, Pinto J, Coutinho SG, Santiago MA, Toledo VP, Costa CA, Genaro O, Reis AB, Mendonça SCF, et al. A randomized double-blind placebo-controlled trial to evaluate the immunogenicity of a candidate vaccine against American tegumentary leishmaniasis. Acta Trop. 2001;80(3):251–60. doi: 10.1016/S0001-706X(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 31.Mayrink W, Mendonça-Mendes A, de Paula JC, Siqueira LMV, Marrocos SDR, Dias ES, de Andrade HM, Machado-Coelho GLL. Cluster randomised trial to evaluate the effectiveness of a vaccine against cutaneous leishmaniasis in the caratinga microregion, south-east brazil. Trans R Soc Trop Med Hyg. 2013;107(4):212–19. doi: 10.1093/trstmh/trt006. [DOI] [PubMed] [Google Scholar]
- 32.Vélez ID, Gilchrist K, Martínez S, Ramírez-Pineda JR, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28(2):329–37. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 33.Gomes R, Teixeira C, Oliveira F, Lawyer PG, Elnaiem D-E, Meneses C, Goto Y, Bhatia A, Howard RF, Reed SG, et al. KSAC, a defined Leishmania antigen, plus adjuvant protects against the virulence of L. major transmitted by its natural vector Phlebotomus duboscqi. PLoS Negl Trop Dis. 2012;6(4):e1610. doi: 10.1371/journal.pntd.0001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kocourkova A, Honegr J, Kuca K, Danova J. Vaccine ingredients: components that influence vaccine efficacy. Mini-Reviews Med Chem. 2017;17(5):451–66. doi: 10.2174/1389557516666160801103303. [DOI] [PubMed] [Google Scholar]
- 35.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Raman VS, Duthie MS, Fox CB, Matlashewski G, Reed SG. Adjuvants for Leishmania vaccines: from models to clinical application. Front Immunol. 2012;3(JUN):1–15. doi: 10.3389/fimmu.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter D, Fox CB, Day TA, Guderian JA, Liang H, Rolf T, Vergara J, Sagawa ZK, Ireton G, Orr MT, et al. A structure-function approach to optimizing TLR4 ligands for human vaccines. Clin Transl Immunol. 2016;5(11):e108. doi: 10.1038/cti.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, Persing D. Taking a toll on human disease: toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4(7):1129–38. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 39.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6(1):1–12. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30(2):134–41. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coler RN, Skeiky YAW, Bernards K, Greeson K, Carter D, Cornellison CD, Modabber F, Campos-Neto A, Reed SG. Immunization with a polyprotein vaccine consisting of the T-cell antigens thiol-specific antioxidant, leishmania major stress-inducible protein 1, and leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70(8):4215–25. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alcolea PJ, Alonso A, Larraga V. Rationale for selection of developmentally regulated genes as vaccine candidates against leishmania infantum infection. Vaccine. 2016;34(46):5474–78. doi: 10.1016/j.vaccine.2016.08.081. [DOI] [PubMed] [Google Scholar]
- 43.Seyed N, Taheri T, Rafati S. Post-genomics and vaccine improvement for leishmania. Front Microbiol. 2016;7(APR):1–13. doi: 10.3389/fmicb.2016.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rappuoli R. Reverse vaccinology. Curr Opin Microbiol. 2000;3:445–50. doi: 10.1016/S1369-5274(00)00119-3. [DOI] [PubMed] [Google Scholar]
- 45.De Groot AS. Exploring the immunome: A brave new world for human vaccine development. Hum Vaccin. 2009;5(12):790–93. doi: 10.4161/hv.10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doolan DL, Weiss WR, Sette A, Felgner PL, Regis DP, Quinones-Casas P, Yates JR, Blair PL, Richie TL, Hoffman SL. Utilization of genomic sequence information to develop malaria vaccines. J Exp Biol. 2003;206(21):3789–802. doi: 10.1242/jeb.00615. [DOI] [PubMed] [Google Scholar]
- 47.John L, John GJ, Kholia T. A reverse vaccinology approach for the identification of potential vaccine candidates from leishmania spp. Appl Biochem Biotechnol. 2012;167(5):1340–50. doi: 10.1007/s12010-012-9649-0. [DOI] [PubMed] [Google Scholar]
- 48.Agallou M, Koutsoni O, Dotsika E, Karagouni E. In silico prediction of promiscuous Leishmania infantum KMP-11, H1, LeIF, CPA, CPB peptides and experimental validation of eliciting CD4+ and CD8+ T-cell specific responses. 15th International Congress of Immunology (ICI), 2013 Aug 22–27; Milan, Italy..
- 49.Agallou M, Margaroni M, Athanasiou E, Toubanaki DK, Kontonikola K, Karidi K, Kammona O, Kiparissides C, Karagouni E. Identification of BALB/c immune markers correlated with a partial protection to Leishmania infantum after vaccination with a rationally designed multi-epitope cysteine protease a peptide-based nanovaccine. PLoS Negl Trop Dis. 2017;11(1):1–27. doi: 10.1371/journal.pntd.0005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn P, Fühner V, Unkauf T, Moreira GMSG, Frenzel A, Miethe S, Hust M. Recombinant antibodies for diagnostics and therapy against pathogens and toxins generated by phage display. Proteomics Clin Appl. 2016;10(9–10):922–48. doi: 10.1002/prca.201600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carvalho GB, Costa LE, Lage DP, Ramos FF, Santos TTO, Ribeiro PAF, Dias DS, Salles BCS, Lima MP, Carvalho LM, et al. High-through identification of T cell-specific phage-exposed mimotopes using PBMCs from tegumentary leishmaniasis patients and their use as vaccine candidates against Leishmania amazonensis infection. Parasitology. 2019;146(3):322–32. doi: 10.1017/S0031182018001403. [DOI] [PubMed] [Google Scholar]
- 52.Salvador I, Carlos SJ, María RJ, Manuel S. Vaccine candidates against leishmania under current research. Expert Rev Vaccines. 2018;17(4):323–34. doi: 10.1080/14760584.2018.1459191. [DOI] [PubMed] [Google Scholar]
- 53.Saljoughian N, Taheri T, Rafati S. Live vaccination tactics: possible approaches for controlling visceral leishmaniasis. Front Immunol. 2014;5(MAR):1–11. doi: 10.3389/fimmu.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Convit J, Ulrich M, Zerpa O, Borges R, Aranzazu N, Valera M, Villarroel H, Zapata Z, Tomedes I. Immunotherapy of American cutaneous leishmaniasis in Venezuela during the period 1990–99. Trans R Soc Trop Med Hyg. 2003;97(4):469–72. [DOI] [PubMed] [Google Scholar]
- 55.Convit J, Ulrich M, Polegre MA, Avila A, Rodríguez N, Mazzedo MI, Blanco B. Therapy of venezuelan patients with severe mucocutaneous or early lesions of diffuse cutaneous leishmaniasis with a vaccine containing pasteurized Leishmania promastigotes and bacillus calmette-guerin - preliminary report. 2004;99(February):57–62. doi: 10.1590/s0074-02762004000100010. [DOI] [PubMed] [Google Scholar]
- 56.Ghorbani M, Farhoudi R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des Devel Ther. 2018;12:25–40. doi: 10.2147/DDDT.S146521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modabber F. Leishmaniasis vaccines: past, present and future. Int J Antimicrob Agents. 2010;36(SUPPL. 1):S58–S61. doi: 10.1016/j.ijantimicag.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Ginsberg AM. Designing tuberculosis vaccine efficacy trials – lessons from recent studies. Expert Rev Vaccines. 2019;18(5):423–32. doi: 10.1080/14760584.2019.1593143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romano A, Doria NA, Mendez J, Sacks DL, Peters NC. Cutaneous infection with Leishmania major mediates heterologous protection against visceral infection with Leishmania infantum. J Immunol. 2015;195(8):3816–27. doi: 10.4049/jimmunol.1500752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breton M, Tremblay MJ, Ouellette M, Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect Immun. 2005;73(10):6372–82. doi: 10.1128/IAI.73.10.6372-6382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okwor I, Mou Z, Liu D, Uzonna J. Protective immunity and vaccination against cutaneous leishmaniasis. Front Immunol. 2012;3(MAY). doi: 10.3389/fimmu.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa CHN, Peters NC, Maruyama SR, de Brito EC Jr, de Miranda Santos IK. Vaccines for the leishmaniases: proposals for a research Agenda. PLoS Negl Trop Dis. 2011;5(3):1–9. doi: 10.1371/journal.pntd.0000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whyte DC, Zufferey R. Cutaneous Leishmaniasis: update on vaccine development. Hum Parasit Dis. 2018. doi: 10.4137/hpd.s16588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okwor I, Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res. 2008;41(2):123–36. doi: 10.1007/s12026-008-8016-2. [DOI] [PubMed] [Google Scholar]
- 65.Viana SM, Celes FS, Ramirez L, Kolli B, DK Ng, KP Chang, De Oliveira CI. Photodynamic vaccination of BALB/c mice for prophylaxis of cutaneous leishmaniasis caused by Leishmania amazonensis. Front Microbiol. 2018;9(FEB):1–10. doi: 10.3389/fmicb.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart J, Curtis J, Spurck TP, Ilg T, Garami A, Baldwin T, Courret N, McFadden GI, Davis A, Handman E, et al. Characterisation of a Leishmania mexicana knockout lacking guanosine diphosphate-mannose pyrophosphorylase. Int J Parasitol. 2005;35:861–73. doi: 10.1016/j.ijpara.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Heravi Shargh V, Jaafari MR, Khamesipour A, Jalali SA, Firouzmand H, Abbasi A, Badiee A. Cationic liposomes containing soluble Leishmania antigens (SLA) plus CpG ODNs induce protection against murine model of leishmaniasis. Parasitol Res. 2012;111(1):105–14. doi: 10.1007/s00436-011-2806-5. [DOI] [PubMed] [Google Scholar]
- 68.Rostamian M, Bahrami F, Niknam HM. Vaccination with whole-cell killed or recombinant leishmanial protein and toll-like receptor agonists against Leishmania tropica in BALB/c mice. PLoS One. 2018;13:e0204491. doi: 10.1371/journal.pone.0204491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pratti JES, da Fonseca Martins AM, da Silva JP, Ramos TD, Pereira JC, Firmino-Cruz L, Oliveira-Maciel D, Vieira TSDS, Lacerda LL, Vale AM, et al. The role of TLR9 on Leishmania amazonensis infection and its influence on intranasal LaAg vaccine efficacy. PLoS Negl Trop Dis. 2019;13(2):e0007146. doi: 10.1371/journal.pntd.0007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ilg T, Demar M, Harbecke D. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J Biol Chem. 2001;276(7):4988–97. doi: 10.1074/jbc.M008030200. [DOI] [PubMed] [Google Scholar]
- 71.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol. 2014;172(6):3793–97. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 72.Elikaee S, Mohebali M, Rezaei S, Eslami H, Khamesipour A, Keshavarz H, Eshraghian MR. Development of a new live attenuated Leishmania major p27 gene knockout: safety and immunogenicity evaluation in BALB/c mice. Cell Immunol. 2018;332(July):24–31. doi: 10.1016/j.cellimm.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell GF, Handman E, Spithill TW. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Immunol Cell Biol. 1984;62(2):145–53. doi: 10.1038/icb.1984.14. [DOI] [PubMed] [Google Scholar]
- 74.Gorczynski R. Immunization of Susceptible BALB/c Mice against Leishmania braziliensis. Cell Immunol. 1985;94(1):11–20. doi: 10.1016/0008-8749(85)90081-4. [DOI] [PubMed] [Google Scholar]
- 75.Kimsey PB, Theodos CM, Mitchen TK, Turco SJ, Titus RG. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect Immun. 1993;61:5205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rivier D, Bovay P, Shah R, Didisheim S, Mauël J. Vaccination against Leishmania major in a CBA mouse model of infection: role of adjuvants and mechanism of protection. Parasite Immunol. 1999;21(9):461–73. doi: 10.1046/j.1365-3024.1999.00244.x. [DOI] [PubMed] [Google Scholar]
- 77.Daneshvar H, Hagan P, Phillips RS. Leishmania mexicana H-line attenuated under pressure of gentamicin, potentiates a Th1 response and control of cutaneous leishmaniasis in BALB/c mice. Parasite Immunol. 2003;25(11–12):589–96. doi: 10.1111/j.0141-9838.2004.00671.x. [DOI] [PubMed] [Google Scholar]
- 78.Duncan SM, Jones NG, Mottram JC. Recent advances in Leishmania reverse genetics: manipulating a manipulative parasite. Mol Biochem Parasitol. 2017;216(April):30–38. doi: 10.1016/j.molbiopara.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 79.Amaral VF, Teva A, Oliveira-Neto MP, Silva AJ, Pereira MS, Cupolillo E, Porrozzi R, Coutinho SG, Pirmez C, Beverley SM, et al. Study of the safety, immunogenicity and efficacy of attenuated and killed Leishmania (Leishmania) major vaccines in a rhesus monkey (Macaca mulatta) model of the human disease. Mem Inst Oswaldo Cruz. 2002;97(7):1041–48. doi: 10.1590/S0074-02762002000700019. [DOI] [PubMed] [Google Scholar]
- 80.Titus RG, Gueiros-Filho FJ, de Freitas LA, Beverley SM. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci. 2006;92(22):10267–71. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silvestre R, Cordeiro-Da-Silva A, Santarém N, Vergnes B, Sereno D, Ouaissi A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. J Immunol. 2007;179(5):3161–70. doi: 10.4049/jimmunol.179.5.3161. [DOI] [PubMed] [Google Scholar]
- 82.Buxbaum LU. A detrimental role for IgG and FcgammaR in Leishmania mexicana infection. Immunol Res. 2008;42(1–3):197–209. doi: 10.1007/s12026-008-8074-5. [DOI] [PubMed] [Google Scholar]
- 83.Shaw J. Ecological and evolutionary pressures on leishmanial parasites. Brazilian J Genet. 1997;20(1). doi: 10.1590/S0100-84551997000100021. [DOI] [Google Scholar]
- 84.Taylor VM, Muñoz DL, Cedeño DL, Vélez ID, Jones MA, Robledo SM. Leishmania tarentolae: utility as an in vitro model for screening of antileishmanial agents. Exp Parasitol. 2010;126(4):471–75. doi: 10.1016/j.exppara.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 85.Abdossamadi Z, Seyed N, Zahedifard F, Taheri T, Taslimi Y, Montakhab-Yeganeh H, Badirzadeh A, Vasei M, Gharibzadeh S, Rafati S, et al. Human Neutrophil Peptide 1 as immunotherapeutic agent against Leishmania infected BALB/c mice. PLoS Negl Trop Dis. 2017;11(12):1–20. doi: 10.1371/journal.pntd.0006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montakhab-Yeganeh H, Abdossamadi Z, Zahedifard F, Taslimi Y, Badirzadeh A, Saljoughian N, Taheri T, Taghikhani M, Rafati S. Leishmania tarentolae expressing CXCL-10 as an efficient immunotherapy approach against Leishmania major-infected BALB/c mice. Parasite Immunol. 2017;39:10. doi: 10.1111/pim.12461. [DOI] [PubMed] [Google Scholar]
- 87.Katebi A, Gholami E, Taheri T, Zahedifard F, Habibzadeh S, Taslimi Y, Shokri F, Papadopoulou B, Kamhawi S, Valenzuela JG, et al. Leishmania tarentolae secreting the sand fly salivary antigen PpSP15 confers protection against Leishmania major infection in a susceptible BALB/c mice model. Mol Immunol. 2015;67(2):501–11. doi: 10.1016/j.molimm.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Sundar S, Singh B. Identifying vaccine targets for anti-leishmanial vaccine development. Expert Rev Vaccines. 2014;13(4):489–505. doi: 10.1586/14760584.2014.894467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duarte MC, Lage DP, Martins VT, Costa LE, Carvalho AMRS, Ludolf F, Santos TTDO, Vale DL, Roatt BM, Menezes-Souza D, et al. A vaccine composed of a hypothetical protein and the eukaryotic initiation factor 5a from Leishmania braziliensis cross-protection against Leishmania amazonensis infection. Immunobiology. 2017;222(2):251–60. doi: 10.1016/j.imbio.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 90.Graumann K, Premstaller A. Manufacturing of recombinant therapeutic proteins in microbial systems. Biotechnol J. 2006;1(2):164–86. doi: 10.1002/biot.200500051. [DOI] [PubMed] [Google Scholar]
- 91.Josefsberg JO, Buckland B. Vaccine process technology. Biotechnol Bioeng. 2012;109(6):1443–60. doi: 10.1002/bit.24493. [DOI] [PubMed] [Google Scholar]
- 92.Bacon KM, Hotez PJ, Kruchten SD, Kamhawi S, Bottazzi ME, Valenzuela JG. The potential economic value of a cutaneous leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine. 2014;31(3):480–86. doi: 10.1016/j.vaccine.2012.11.032.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salay G, Dorta ML, Santos NM, Mortara RA, Brodskyn C, Oliveira CI, Barbieri CL, Rodrigues MM. Testing of four Leishmania vaccine candidates in a mouse model of infection with Leishmania(Viannia) braziliensis, the main causative agent of cutaneous leishmaniasis in the NewWorld. Clin Vaccine Immunol CVI. 2007;14:1173–81. doi: 10.1128/CVI.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell K, Diao H, Ji J, Soong L. DNA immunization with the gene encoding P4 nuclease of Leishmania amazonensis protects mice against cutaneous Leishmaniasis. Infect Immun. 2003;71:6270–78. doi: 10.1128/IAI.71.11.6270-6278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fedeli CE, Ferreira JH, Mussalem JS, Longo-Maugeri IM, Gentil LG, Dos Santos MR, Katz S, Barbiéri CL. Partial protective responses induced by a recombinant cysteine proteinase from Leishmania (Leishmania) amazonensis in a murine model of cutaneous leishmaniasis. Exp Parasitol. 2010;124:153–58. doi: 10.1016/j.exppara.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Montalvo-Alvarez AM, Folgueira C, Carrion J, Monzote-Fidalgo L, Canavate C, Requena JM. The Leishmania HSP20 is antigenic during natural infections, but, as DNA vaccine, it does not protect BALB/c mice against experimental L. amazonensis infection. J Biomed Biotechnol. 2008;2008:695432. doi: 10.1155/2008/695432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988;56:3272–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez CR, Noriega FR, Huerta S, Santiago A, Vega M, Paniagua J, Ortiz-Navarrete V, Isibasi A, Levine MM. Immunogenicity of a Salmonella typhi CVD 908 candidate vaccine strain expressing the major surface protein gp63 of Leishmania Mexicana mexicana. Vaccine. 1998;16:1043–52. doi: 10.1016/S0264-410X(97)00267-3. [DOI] [PubMed] [Google Scholar]
- 99.Mendez S, Gurunathan S, Kamhawi S, Belkaid Y, Moga MA, Skeiky YA, Campos-Neto A, Reed S, Seder RA, Sacks D, et al. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J Immunol. 2001;166:5122–28. doi: 10.4049/jimmunol.166.8.5122. [DOI] [PubMed] [Google Scholar]
- 100.Rivier D, Bovay P, Shah R, Didisheim S, Mauel J. Vaccination against Leishmania major in a CBA mouse model of infection: role of adjuvants and mechanism of protection. Parasite Immunol. 1999;21:461–73. doi: 10.1046/j.1365-3024.1999.00244.x. [DOI] [PubMed] [Google Scholar]
- 101.Sjolander A, Baldwin TM, Curtis JM, Bengtsson KL, Handman E. Vaccination with recombinant Parasite Surface Antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine. 1998;16:2077–84. doi: 10.1016/S0264-410X(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 102.Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, Reed SG. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solioz N, Blum-Tirouvanziam U, Jacquet R, Rafati S, Corradin G, Mauel J, Fasel N. The protective capacities of histone H1 against experimental murine cutaneous leishmaniasis. Vaccine. 1999;18:850–59. doi: 10.1016/S0264-410X(99)00340-0. [DOI] [PubMed] [Google Scholar]
- 104.Soussi N, Milon G, Colle JH, Mougneau E, Glaichenhaus N, Goossens PL. Listeria monocytogenes as a short-lived delivery system for the induction of type 1 cell-mediated immunity against the p36/LACK antigen of Leishmania major. Infect Immun. 2000;68:1498–506. doi: 10.1128/IAI.68.3.1498-1506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mougneau E, Altare F, Wakil A, Zheng S, Coppola T, Wang Z, Waldmann R, Locksley R, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268(5210):563–66. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 106.Gurunathan S, Sacks DL, Brown DR, Reiner SL, Charest H, Glaichenhaus N, Seder RA. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186(7):1137–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9314562%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2199076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chenik M, Louzir H, Ksontini H, Dilou A, Abdmouleh I, Dellagi K. Vaccination with the divergent portion of the protein histone H2B of Leishmania protects susceptible BALB/c mice against a virulent challenge with Leishmania major. Vaccine. 2006;24(14):2521–29. doi: 10.1016/j.vaccine.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 108.Masina S, Gicheru M, Demotz SO, Fasel NJ. Protection against cutaneous leishmaniasis in outbred vervet monkeys, using a recombinant histone H1 antigen. J Infect Dis. 2003;188(8):1250–57. doi: 10.1086/378677. [DOI] [PubMed] [Google Scholar]
- 109.Olobo JO, Anjili CO, Gicheru MM, Mbati PA, Kariuki TM, Githure JI, Koech DK, McMaster WR. Vaccination of vervet monkeys against cutaneous leishmaniosis using recombinant Leishmania “major surface glycoprotein” (gp63). Vet Parasitol. 1995;60(3–4):199–212. doi: 10.1016/0304-4017(95)00788-6. [DOI] [PubMed] [Google Scholar]
- 110.Abdelhak S, Louzir H, Timm J, Blel L, Benlasfar Z, Lagranderie M, Gheorghiu M, Dellagi K, Gicquel B. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immunity against. Microbiology. 1995;141:1585–92. doi: 10.1099/13500872-141-7-1585. [DOI] [PubMed] [Google Scholar]
- 111.González CR, Noriega FR, Huerta S, Santiago A, Vega M, Paniagua J, Ortiz-Navarrete V, Isibasi A, Levine MM. Immunogenicity of a Salmonella typhi CVD 908 candidate vaccine strain expressing the major surface protein gp63 of Leishmania mexicana mexicana. Vaccine. 1998;16(9–10):1043–52. doi: 10.1016/S0264-410X(97)00267-3. [DOI] [PubMed] [Google Scholar]
- 112.Goto Y, Bhatia A, Raman VS, Vidal SEZ, Bertholet S, Coler RN, Howard RF, Reed SG. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27(21):2884–90. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alves-Silva MV, Nico D, Morrot A, Palatnik M, Palatnik-de-Sousa CB. A chimera containing CD4+ and CD8+ T-cell epitopes of the Leishmania donovani nucleoside hydrolase (NH36) optimizes cross-protection against Leishmania amazonesis infection. Front Immunol. 2017;8(FEB). doi: 10.3389/fimmu.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alves-Silva MV, Nico D, de Luca PM, Palatnik De-sousa CB. The F1F3 recombinant chimera of Leishmania donovani-Nucleoside Hydrolase (NH36) and its epitopes induce cross-protection against Leishmania (V.) braziliensis infection in mice. Front Immunol. 2019;10(April):1–21. doi: 10.3389/fimmu.2019.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramírez L, Santos DM, Souza AP, Coelho EAF, Barral A, Alonso C, Escutia MR, Bonay P, de Oliveira CI, Soto M, et al. Evaluation of immune responses and analysis of the effect of vaccination of the Leishmania major recombinant ribosomal proteins L3 or L5 in two different murine models of cutaneous leishmaniasis. Vaccine. 2013;31(9):1312–19. doi: 10.1016/j.vaccine.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 116.Lestinova T, Rohousova I, Sima M, de Oliveira CI, Volf P. Insights into the sand fly saliva: blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl Trop Dis. 2017;11(7):1–26. doi: 10.1371/journal.pntd.0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martin-Martin I, Chagas AC, Guimaraes-Costa AB, Amo L, Oliveira F, Moore IN, DeSouza-Vieira TS, Sanchez EE, Suntravat M, Valenzuela JG, et al. Immunity to LuloHya and Lundep, the salivary spreading factors from Lutzomyia longipalpis, protects against Leishmania major infection. PLoS Pathog. 2018;14(5):1–26. doi: 10.1371/journal.ppat.1007006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290(5495):1351–54. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 119.Chagas AC, Oliveira F, Debrabant A, Valenzuela JG, Ribeiro JMC, Calvo E. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog. 2014;10(2):e1003923. doi: 10.1371/journal.ppat.1003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2014;167(9):5226–30. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 121.Cunha JM, Abbehusen M, Suarez M, Valenzuela J, Teixeira CR. Acta tropica immunization with LJM11 salivary protein protects against infection with Leishmania braziliensis in the presence of Lutzomyia longipalpis saliva. Acta Trop. 2018;177(October 2017):164–70. doi: 10.1016/j.actatropica.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skeiky YAW, Coler RN, Brannon M, Stromberg E, Greeson K, Crane RT, Campos-Neto A, Reed SG. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL® adjuvant. Vaccine. 2002;20(27–28):3292–303. doi: 10.1016/S0264-410X(02)00302-X. [DOI] [PubMed] [Google Scholar]
- 123.Peters NC, Bertholet S, Lawyer PG, Charmoy M, Romano A, Ribeiro-Gomes FL, LW S, Sacks DL. Evaluation of recombinant Leishmania poly-protein plus GLA-SE vaccines against sand fly-transmitted Leishmania major in C57Bl/6 mice. J Immunol. 2013;18(9):1199–216. doi: 10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zadeh-Vakili A, Taheri T, Taslimi Y, Doustdari F, Salmanian AH, Rafati S. Immunization with the hybrid protein vaccine, consisting of Leishmania major cysteine proteinases Type I (CPB) and Type II (CPA), partially protects against leishmaniasis. Vaccine. 2004;22(15–16):1930–40. doi: 10.1016/j.vaccine.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 125.Soong L, Duboise SM, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang DC, Devit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356(6365):152–54. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 127.Lechardeur D, Lukacs GL. Nucleocytoplasmic transport of plasmid DNA: a perilous journey from the cytoplasm to the nucleus. Hum Gene Ther. 2006;17(9):882–89. doi: 10.1089/hum.2006.17.882. [DOI] [PubMed] [Google Scholar]
- 128.Myhr AI. DNA vaccines: regulatory considerations and safety aspects. Curr Issues Mol Biol. 2017;22:79–88. doi: 10.21775/cimb.022.079. [DOI] [PubMed] [Google Scholar]
- 129.Kumar A, Samant M. DNA vaccine against visceral leishmaniasis: A promising approach for prevention and control. Parasite Immunol. 2016;38(5):273–81. doi: 10.1111/pim.12315. [DOI] [PubMed] [Google Scholar]
- 130.Xu D, Liew FY. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–76. [PMC free article] [PubMed] [Google Scholar]
- 131.Xu D, McSorley SJ, Chatfield SJ, Dougan G, Liew FY. Protection against Leishmania major infection in genetically susceptible BALB/c mice by GP63 delivered orally in attenuated Salmonella typhimurium (AroA- AroD-). Immunology. 1995;85:1–7. doi: 10.1016/S0378-7788(02)00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walker PS, Scharton-Kersten T, Rowton ED, Hengge U, Bouloc A, Udey MC, Vogel JC. Genetic immunization with glycoprotein 63 cDNA results in a helper T Cell type 1 immune response and protection in a murine model of leishmaniasis. Hum Gene Ther. 2008;9(13):1899–907. doi: 10.1089/hum.1998.9.13-1899. [DOI] [PubMed] [Google Scholar]
- 133.Zanin FHC, Coelho EAF, Tavares CAP, Marques-da-Silva EA, Silva Costa MM, Rezende SA, Gazzinelli RT, Fernandes AP. Evaluation of immune responses and protection induced by A2 and nucleoside hydrolase (NH) DNA vaccines against Leishmania chagasi and Leishmania amazonensis experimental infections. Microbes Infect. 2007;9(9):1070–77. doi: 10.1016/j.micinf.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 134.Tabatabaie F, Mahdavi M, Faezi S, Dalimi A, Sharifi Z, Akhlaghi L, Ghaffarifar F. Th1 platform immune responses against Leishmania major induced by thiol-specific antioxidant-based DNA vaccines. Jundishapur J Microbiol. 2014;7(2):1–8. doi: 10.5812/jjm.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Campos BLS, Silva TN, Ribeiro SP, Carvalho KIL, KallÁs EG, Laurenti MD, Passero LFD. Analysis of iron superoxide dismutase-encoding DNA vaccine on the evolution of the Leishmania amazonensis experimental infection. Parasite Immunol. 2015;37(8):407–16. doi: 10.1111/pim.12206. [DOI] [PubMed] [Google Scholar]
- 136.Carrión J. Mechanisms of immunity to Leishmania major infection in mice: the contribution of DNA vaccines coding for two novel sets of histones (H2A-H2B or H3-H4). Comp Immunol Microbiol Infect Dis. 2011;34(5):381–86. doi: 10.1016/j.cimid.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 137.Carneiro MW, Santos DM, Fukutani KF, Clarencio J, Miranda JC, Brodskyn C, Barral A, Barral-Netto M, Soto M, de Oliveira CI, et al. Vaccination with L. infantum chagasi nucleosomal histones confers protection against New World cutaneous leishmaniasis caused by leishmania braziliensis. PLoS One. 2012;7(12). doi: 10.1371/journal.pone.0052296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iborra S, Soto M, Carrión J, Alonso C, Requena J. Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis. Vaccine. 2004;22(29–30):3865–76. doi: 10.1016/j.vaccine.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 139.Maspi N, Ghaffarifar F, Sharifi Z, Dalimi A, Khademi SZ. DNA vaccination with a plasmid encoding LACK-TSA fusion against leishmania major infection in BALB/c mice. Malays J Pathol. 2017;39:267–75. [PubMed] [Google Scholar]
- 140.Hekele A, Bertholet S, Archer J, Gibson DG, Palladino G, Brito LA, Otten GR, Brazzoli M, Buccato S, Bonci A, et al. Rapidly produced SAM ® vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect. 2013;2(July):1–7. doi: 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bogers WM, Oostermeijer H, Mooij P, Koopman G, Verschoor EJ, Davis D, Ulmer JB, Brito LA, Cu Y, Banerjee K, et al. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis. 2015;211(6):947–55. doi: 10.1093/infdis/jiu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci. 2012;109(36):14604–09. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen K-J, Stitz L, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30(12):1210–16. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 144.Erasmus JH, Khandhar AP, Guderian J, Granger B, Archer J, Archer M, Gage E, Fuerte-Stone J, Larson E, Lin S, et al. A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol Ther. 2018;26:2507–22. doi: 10.1016/j.ymthe.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Das A, Ali N. Vaccine prospects of killed but metabolically active Leishmania against visceral leishmaniasis. Expert Rev Vaccines. 2012;11(7):783–85. doi: 10.1586/erv.12.50. [DOI] [PubMed] [Google Scholar]
- 146.Mortazavidehkordi N, Fallah A, Abdollahi A, Kia V, Khanahmad H, Najafabadi ZG, Hashemi N, Estiri B, Roudbari Z, Najafi A, et al. A lentiviral vaccine expressing KMP11-HASPB fusion protein increases immune response to Leishmania major in BALB/C. Parasitol Res. 2018;117(7):2265–73. doi: 10.1007/s00436-018-5915-6. [DOI] [PubMed] [Google Scholar]
- 147.Kedzierska K, Curtis JM, Valkenburg SA, Hatton LA, Kiu H, Peter C. Induction of Protective CD4 + T Cell-Mediated Immunity by a Leishmania Peptide Delivered in Recombinant Influenza Viruses. 2012;7(3):1–10. doi: 10.1371/journal.pone.0033161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, Wiggins R, Di Marco S, Colloca S, Siani L, et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl Trop Dis. 2017;11(5). doi: 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schroeder J, Aebischer T. Vaccines for leishmaniasis: from proteome to vaccine candidates. Hum Vaccine. 2011;7(10):10–15. doi: 10.4161/hv.7.0.14556. [DOI] [PubMed] [Google Scholar]
- 150.Duthie MS, Van Hoeven N, MacMillen Z, Picone A, Mohamath R, Erasmus J, Hsu F-C, Stinchcomb DT, Reed SG. Heterologous immunization with defined RNA and subunit vaccines enhances T cell responses that protect against leishmania donovani. Front Immunol. 2018;9. doi: 10.3389/fimmu.2018.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]


