ABSTRACT
Introduction:
Early-onset epileptic encephalopathies are among the most severe early-onset epilepsies, leading to progressive neurodegeneration. An increasing number of novel genetic causes continue to be uncovered as the primary etiology.
Results:
We report a girl infant of Semitic (Saudi Arabian) descent who presented with multifocal seizures and later developed intractable infantile spasms and myoclonic seizures. Her clinical features and electroencephalography were consistent with early-onset epileptic encephalopathy. Whole exome sequence analysis showed homozygous novel pathogenic variant (variant p.Q287PfsX27; coding DNA c.858_862delACAAA) in the SYNJ1 gene.
Conclusion:
This is a newly described early-onset epileptic encephalopathy secondary to a critical reduction of the dual phosphatase activity of SYNJ. Clinical features include early-onset intractable focal, myoclonic seizures, infantile spasms, and hypotonia progressing to spastic quadriparesis, opisthotonus, dystonia, profound developmental delay, and a progressive neurodegenerative course. Brain magnetic resonance imaging is usually normal. Electroencephalography shows diffuse slowing with multifocal epileptiform discharges or modified hypsarrhythmia. These findings further expand the clinical spectrum of synaptic dysregulation in patients with severe epilepsy and emphasize the importance of this biological pathway in seizure pathophysiology.
KEYWORDS: Early-onset epilepsy, neurodegenerative disorder, recessive disorder, SYNJ1, SYNJ1 dual phosphatase activity
INTRODUCTION
Whole exome sequencing and comprehensive disease-specific massively parallel gene sequencing are increasingly becoming important in the diagnosis and understanding of neurobiology. Homozygous SYNJ1 mutation-related phenotypes are insufficiently characterized and have been reported to include movement disorder and early infantile epileptic encephalopathy-53 (EIEE53) [OMIM #617389]. Previously, EIEE53 has been described in four unrelated families. We report a patient with SYNJ1 homozygous novel pathogenic mutation with early-onset epileptic encephalopathy, hypotonia progressing to spastic quadriparesis, cortical visual impairment, opisthotonus, and dystonia and summarize the electroclinical syndrome of this rare epileptic encephalopathy.
CASE DESCRIPTION
The patient was born at 39 weeks of gestation to a healthy 22-year-old G2 P1 woman after an unremarkable pregnancy and delivery. Birth weight was 3160g and head circumference was 35.5 cm (35th percentile). Parents were first cousins of Saudi Arabian descent with no family history of neurodegenerative disease. The girl infant presented at 2 days of age with episodes of eye twitching, body stiffening, and bicycling movements of legs. No striking facial dysmorphism was observed, but she had profound hypotonia. Prolonged video electroencephalography captured multifocal seizures [Figure 1]. Seizures were initially controlled with phenobarbital, but it was switched to levetiracetam because of excessive sedation. She had also opisthotonic posturing that responded to baclofen. She stayed in the intensive care unit for 2 months because of intermittent recurrence of seizures and poor feeding. She was started on oxcarbazepine, topiramate, and clonazepam during that time. She received a gastrostomy tube before discharge. Her brain magnetic resonance imaging (MRI) was normal. Chromosomal microarray showed multiple long continuous stretches of homozygosity. Sequencing and deletion/duplication analysis of 22 epilepsy genes did not reveal any pathogenic variant. Whole exome sequence analysis showed a homozygous pathogenic variant (variant p.Q287PfsX27; coding DNA c.858_862delACAAA) in the SYNJ1 gene. At the age of 8 months, she started to have episodes of epileptic spasms and interictal electroencephalogram (EEG) showed modified hypsarrhythmia [Figure 2]. Her spasms remained intractable to adrenocorticotropic hormone, vigabatrin, cannabidiol oil, ketogenic diet, and vagus nerve stimulation. She also developed myoclonic seizures. During her last follow-up at the age of 28 months, she was noted to have a profound developmental delay with the developmental milestone of 2-month level. She had a severe cortical visual impairment, and her hypotonic muscle tone was evolved to severe spastic quadriparesis. Head circumference was 46.5 cm (16th percentile). She had brisk deep tendon reflexes, but plantars were mute. She had also persistent dystonic posturing of upper extremities (partially responded to clonazepam) and intermittent episodes of spasms and myoclonic seizures.
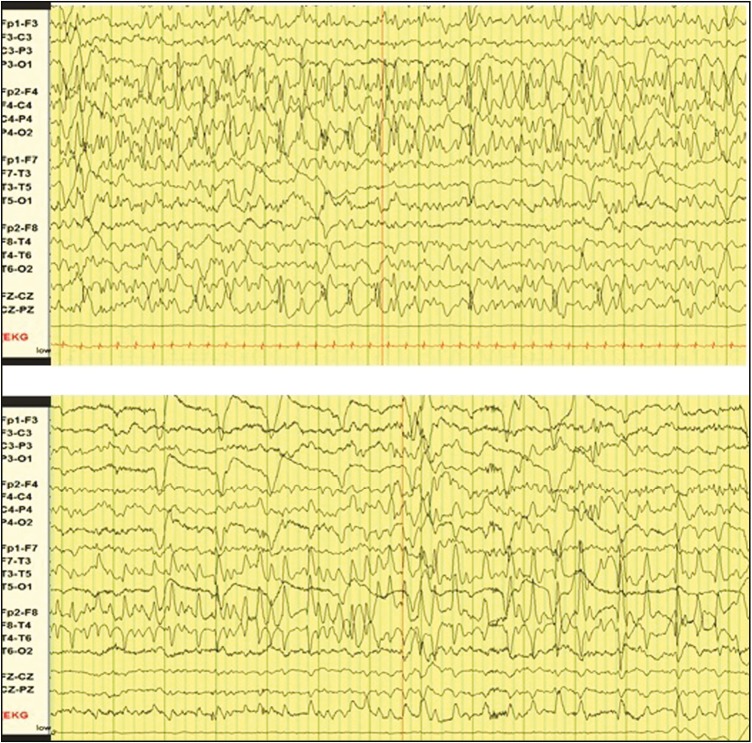
Figure 1.
Ictal electroencephalography during the neonatal period: focal seizure involving bilateral central chains (top) and focal seizure involving right more than the left temporal chain (bottom)
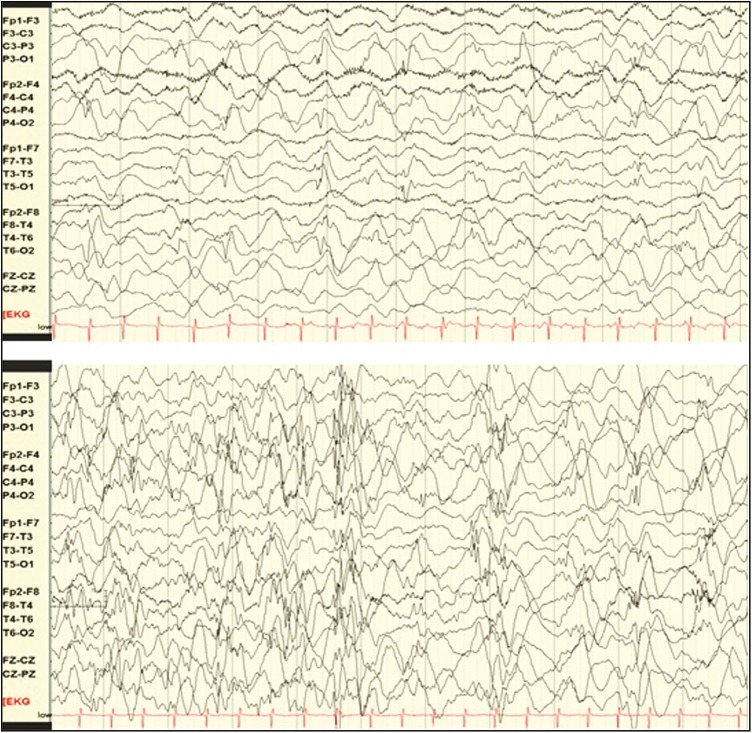
Figure 2.
Ictal electroencephalography during the neonatal period: focal seizure involving bilateral central chains (top) and focal seizure involving right more than the left temporal chain (bottom)
DISCUSSION
The c.858_862delACAAA pathogenic variant in the SYNJ1 gene has not been reported previously. This variant causes a frameshift starting with codon glutamine 287, changes this amino acid to a proline residue, and creates a premature stop codon at position 27 of the new reading frame, denoted p.Gln287ProfsX27. This variant is predicted to cause loss of normal protein function either through protein truncation or nonsense-mediated messenger ribonucleic acid (mRNA) decay. This variant was not observed in the NHLBI Exome Sequencing Project, which indicates that it is not a common benign variant.
SYNJ1 gene encodes a polyphosphoinositide phosphatase that plays an important role in clathrin-coated pit-mediated endocytosis.[1] Biallelic genetic disruption of SYNJ1 results in impaired synaptic vesicle recycling dynamics and accumulation of clathrin-coated vesicles in knockout mice and zebra fish models.[2]
Krebs et al.[3] identified a homozygous missense mutation of the Sac1 domain of SYNJ1 in a consanguineous Iranian family with early‐onset progressive parkinsonism with generalized seizures. This mutation selectively abolishes the phosphatase function of the domain. Affected patients showed progressive movement disorder in their second or third decade of life with or without cognitive deterioration, dystonia, and cerebral cortical atrophy. Quadri et al.[4] reported similar early-onset parkinsonism in an Italian family. Some of these patients had a developmental delay or rare seizures prior to the onset of progressive movement disorder. However, Dyment et al.[5] reported a novel phenotype with intractable early-onset epilepsy and profound intellectual impairment from a homozygous nonsense variant (p.Arg136*) resulting in loss of protein formation. Furthermore, children from three unrelated family with early-onset refractory seizure were described by Hardies et al.[6] because of the homozygous or compound heterozygote nonsense variants causing a critical reduction of the dual phosphate activity and loss of mRNA transcript rather than a selective decrease of Sac1 function seen in phenotypes associated with early-onset parkinsonism. Al Zaabi et al.[7] later reported two related children with a homozygous mutation in SYNJ1 (c.709C>T, p.Gln237*) from a consanguineous Emirati family of Omani origin, who presented with neonatal-onset pharmacoresistant seizures and a neurodegenerative disease course. Early hypotonia with later development of spastic quadriparesis, dystonia, and vision impairment are typical findings of this neurodegenerative condition as noted in our patient. Most affected children died in childhood, and one child was noted to have tau-positive neurofibrillary tangles in the substantia nigra.
Seizure onset was described from day 1 of life to as late as 6 months of age. Tonic-clonic, myoclonic, tonic, and epileptic spasms were described [Table 1]. All patients are uniformly treatment resistant and status epilepticus had been reported. Most commonly reported seizure is the myoclonic seizure. Interestingly, two patients had high lactate, liver disease, and combined deficiency of complex III and IV activity in liver and fibroblasts. Mitochondrial DNA screening was negative. Our patient also had an extensive evaluation for mitochondrial disease; however, metabolic and genetic studies were unremarkable for mitochondrial disease. Dystonia has been reported in one family. Our case is the second reported case and had improvement with the use of clonazepam, which indicates a possible use for this indication in these patients. No patients with homozygous nonsense mutation developed a parkinsonian feature, which is a key finding in association with a homozygous missense mutation. EEG showed the following variable findings: slow background, multifocal epileptiform discharges, hypsarrhythmia, and ictal findings associated with focal and myoclonic seizures. Our patient had normal brain MRI findings including spectroscopy, which is consistent with previously reported patients except inconsistent finding of decreased N-acetyl acetate to choline ratio, large creatine peak, and mild cerebral atrophy. Inconsistently creatine kinase elevation and decreased complex I activity and mild variation in fiber sizes were noted.
Table 1:
Phenotypic spectrum of SYNJ1 epileptic encephalopathy (early infantile epileptic encephalopathy-53)
| Our reported patient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Consanguineous family from Pakistan5 | Siblings from a consanguineous family of Moroccan origin6 | Consanguineous family from Moroccan origin6 | Non-consanguineous Caucasian couple. Only one affected had genetic testing.6 | Consanguineous family from Emirati family of Omani origin7 | Consanguineous family from Saudi Arabian origin | |||||
| homozygous c.406C>T, p.Arg136 | homozygous c.2663A>G, p.Tyr888Cys | homozygous c.2528G>A, p.Trp843 | Compound heterozygous c.1938delT, p.Gln647Argfs*6 and c.3365-2A>G, p.Ser1122Thrfs*6 | homozygous c.709C>T, p.Gln237 | homozygous c.858_862delACAAA | |||||
| Boy | Girl | Boy | Girl | Girl | Boy | Boy | Girl | Boy | Girl | |
| Intellectual impairment | Profound delay | Profound delay | Profound delay | Profound delay | Profound delay | Profound delay | Profound delay | Severe delay | Profound delay | Profound delay |
| Seizure | Clusters of tonic seizures, tonic- clonic seizures from day 9 of life | Flexion spasm at 10 months (may have started at 2.5 months) Developed myoclonic and tonic seizures | Epileptic spasms at 6 months Developed myoclonic and tonic seizures | Clonic seizure during day 1 of life and then developed myoclonic and tonic-clonic seizure | Clonic seizure during day 1 of life and then developed myoclonic and tonic-clonic seizure | Eye blinking and shoulder movement from Day 12 of life and then evolved into myoclonic and tonic-clonic type | Eye deviation from Day 1 of life and then refractory GTC and myoclonic seizures | Lip smacking and tonic seizures from D2 of life | Epileptic spasms, tonic clonic seizures, and myoclonus | Multifocal seizures from 2 days of age. Later developed epileptic spasms and myoclonic seizures |
| EEG | Modified hypsarrhythmia | modified hypsarrhythmia or multifocal epileptic activity on a slow background | modified hypsarrhythmia or multifocal epileptic activity on a slow background | modified hypsarrhythmia or focal spikes on a slow background | modified hypsarrhythmia or focal spikes on a slow background | Multifocal epileptiform activity in a slow background | Multifocal epileptiform activity in a slow background | Hypsarrhythmia | Hypsarrhythmia | modified hypsarrhythmia or multifocal epileptic activity on a slow background |
| Brain MRI | Mild cerebral atrophy at 5 years of age | normal | normal | normal | normal | normal | thin corpus callosum and limited gliosis and atrophy of the periventricular white matter | normal | Mild dilatation of ventricles and subarachnoid spaces | normal |
| Dystonia | Not available | no | no | no | no | yes | Yes | no | no | Yes |
| Parkinsonism | Not available | no | no | no | no | no | no | no | no | no |
| Other clinical features | Progressive neurodegenerative course, feeding intolerance, and G tube dependence, hypotonia progressed to multiple contractures, cortical blindness, Death at age 6 years | Progressive neurodegenerative course, feeding intolerance, and G tube dependence, hypotonia progressed to spastic quadriparesis, cortical visual impairment | Progressive neurodegenerative course, feeding intolerance, and G tube dependence, hypotonia progressed to spastic quadriparesis, cortical visual impairment | Progressive spastic quadriplegia and feeding problem | Progressive spastic quadriplegia and feeding problem | Progressive neurodegenerative course, feeding intolerance, and G tube dependence, hypotonia progressed to hypetonia to opisthotonus, Death at the age of 2.5 years | Progressive neurodegenerative course with death at the age of 8 years, spastic quadriparesis, cotical visual impairment, feeding dysfunction | Both developed acquired microcephaly and axial hypotnia and girl child had scoliosis | Hypotonia progressed to spastic quadriparesis, feeding intolerance and G tube dependence | |
| Other features | Decreased complex I activity and predominance of type 2 fibers in the muscle biopsy, tau- immunoreactive neurofibrillary degeneration in the substantia nigra | high lactate, combined deficiency in complex III and IV activity in liver and fibroblasts | Increased creatine kinase |
We described a girl with developmental and epileptic encephalopathy with a novel homozygous frameshift variant in the SYNJ1 gene. This case highlights a strong genotype–phenotype correlation in this early-onset epileptic encephalopathy with a homozygous nonsense variant in the SYNJ1. However, phenotype, neuroimaging, and EEG features are not sufficient to diagnose SYNJ1 encephalopathy and all genes (>67 genes) responsible for early-onset epileptic encephalopathy should be included in the differential diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cremona O, Di Paolo G, Wenk MR, Lüthi A, Kim WT, Takei K, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–88. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 2.Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, et al. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24:8641–50. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, et al. The sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive parkinsonism with generalized seizures. Hum Mutat. 2013;34:1200–7. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, et al. Mutation in the SYNJ1 gene associated with autosomal recessive SYNJ1 variants and severe epilepsy. Brain. 2016;139:2420–30. [Google Scholar]
- 5.Dyment DA, Smith AC, Humphreys P, Schwartzentruber J, Beaulieu CL, Bulman DE, et al. FORGE Canada Consortium Homozygous nonsense mutation in SYNJ1 associated with intractable epilepsy and tau pathology. Neurobiol Aging. 2015;36:1222.e1–5. doi: 10.1016/j.neurobiolaging.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Hardies K, Cai Y, Jardel C, Jansen AC, Cao M, May P, et al. AR Working Group of the EuroEPINOMICS RES Consortium Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain. 2016;139:2420–30. doi: 10.1093/brain/aww180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Zaabi N, Al Menhali N, Al-Jasmi F. SYNJ1 gene associated with neonatal onset of neurodegenerative disorder and intractable seizure. Mol Genet Genomic Med. 2018;6:109–13. doi: 10.1002/mgg3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]