Abstract
Simultaneously targeting multiple energy balance control systems is a promising direction for the development of obesity pharmacotherapies. Here, we explore the interaction between the GLP-1 and melanocortin system within the dorsal vagal complex (DVC) of the caudal brainstem. Using a pharmacological approach, we demonstrate that the full anorectic potential of liraglutide, an FDA-approved GLP-1 analog for the treatment of obesity, requires DVC melanocortin 3/4 receptor (MC3/4R) signaling. Specifically, the food intake and body weight suppressive effects of liraglutide were attenuated by DVC administration of the MC3/4R antagonist SHU9119. In contrast, the anorectic effects of liraglutide were enhanced by combined activation of DVC MC3/4Rs using the agonist MTII. Our findings highlight the modulation of liraglutide-induced anorexia by DVC MC3/4R signaling, thereby suggesting a site of action at which two important energy balance control systems interact.
Keywords: POMC, NTS, MSH, obesity, brainstem, area postrema
1. Introduction
For the nearly 100 million Americans considered obese or overweight [1], current options for weight loss include behavioral modifications (e.g. diet and exercise), bariatric surgery and pharmacotherapies. Unfortunately, the initial weight loss consequent of lifestyle changes and bariatric surgery is followed by weight regain in most patients [2–5]. In order to sustain clinically meaningful weight loss over time, weight loss pharmacotherapies are being prescribed, often in conjunction with other approaches, to encourage a sustained suppression of food intake and body weight.
The development of therapeutics for weight loss has been informed by basic science research and the characterization of peptides and hormones that contribute to the neural control of food intake. The success of such efforts is evident by the FDA-approved status of the glucagon-like peptide-1 receptor (GLP-1R) agonist liraglutide for treating obesity. Liraglutide targets endogenous GLP-1Rs in both the periphery and the brain to cause anorexia and body weight loss in both preclinical and clinical models [see [6] for review]. However, there is still progress to be made, as liraglutide and all other approved weight loss drugs cause a limited magnitude of weight loss and do so by producing adverse gastrointestinal side effects (e.g. nausea and emesis) [7–11]. One approach to improving the therapeutic potential of weight loss drugs has been combination therapies, which in contrast to traditional monotherapies, simultaneously target multiple hormone systems [12–14]. Indeed, a wealth of neuroendocrine research has suggested that anorectic neural and endocrine signals interact with each other to suppress food intake and consequently body weight [15–22].
Work by Clemmenson et al. [23] in mice has demonstrated that the intake suppressive effects of liraglutide can be improved by simultaneously targeting the melanocortin system, an essential neuropeptide system for energy balance control (see [24] for review). The melanocortin-3 and melanocortin-4 receptors (MC3/4R), in particular, regulate food intake by integrating anorectic and orexigenic signals provided by the endogenous agonist α-melanocyte-stimulating hormone (α-MSH) and antagonist agouti-related protein (AGRP), respectively (see [25] for review). Experimental inactivation of the MC4R in mice leads to hyperphagia and obesity [26] and MC4R loss-of-function mutations are the most common known monogenetic cause of obesity in humans [27,28]. While the work of Clemmenson has shown that systemic co-administration of liraglutide and an MC4R agonist enhances the weight loss efficacy of either drug alone [23], the mechanism and sites-of-action within the CNS by which the GLP-1 and melanocortin systems interact is still unclear.
The MC4R is densely expressed within DVC of the brainstem [29], where it has a functional role for energy balance control. Indeed, hindbrain delivery of the MC3/4R agonist melanotan II (MTII) or antagonist SHU9119 suppresses or augments feeding and body weight, respectively [30–36]. A distinct role for hindbrain MC4Rs in the control of meal size has been postulated to involve modulation of incoming satiation signals [31–35,37]. Support for this hypothesis comes from the fact that DVC MC4Rs [38], are anatomically well-positioned to interact with vagally-transmitted and blood borne energy balance signals that control meal size (i.e. native GLP-1 and liraglutide) [39,40]. Here, we examine the requirement of DVC MC3/4Rs for the full anorectic potential of liraglutide via DVC administration of the MC3/4R antagonist Shu9119. Next, we test the ability of DVC MC3/4R agonism, via MTII, to augment the food intake and body weight suppression by liraglutide. The bidirectional modulation of liraglutide-induced feeding suppression by DVC MC3/4Rs observed in our studies highlights a critical population of melanocortin receptors within the DVC that contribute to the food intake and body weight suppressive effects of liraglutide.
2. Materials and Methods
2.1. Animals and Diet
Adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, Massachusetts), weighing between 300–400g at the start of experimentation, were individually housed in hanging wire-bottom metal cages. Rats were maintained on a 12-h light, 12-h dark cycle in a climate-controlled environment (22–24°C) with ad libitum access to standard rat chow (LabDiet 5001, Lab Diet, St. Louis, MO) and water except where otherwise noted. All procedures were performed in accordance with NIH Guidelines for the Care and Use of Laboratory Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
2.2. Stereotaxic cannula implantation
Rats were anesthetized with an intramuscular injection of a ketamine (90 mg/kg; Butler Animal Health Supply, Dublin, OH)/xylazine (2.8 mg/kg; Anased, Shenandoah, IA)/acepromazine (0.72 mg/kg; Butler Animal Health Supply) cocktail, prepped for surgery, and placed in a stereotaxic apparatus. A bilateral infusion guide cannula (11-mm projection, 1.5-mm spacing, 26-gauge; Plastics One, Roanoke, VA) was implanted above the nucleus tractus solitarius (NTS) of the DVC according to the following stereotaxic coordinates of Paxinos and Watson 2007 [41]: −1.0-mm posterior to the occipital ridge, ± 0.75-mm medial/lateral, −5.9-mm dorsal/ventral from the skull surface. Jeweler screws and dental cement was used to affix guide cannula to the skull. All rats were given subcutaneous analgesia (Metacam; 2 mg/kg, Boehringer Ingelheim Vetmedica, St Joseph, MO) immediately following surgery and for three additional postoperative days. Placements of cannula within the DVC were confirmed postmortem by injection of blue dye (100 nl, 2% Chicago sky blue ink) through the guide cannula. Animals with dye confined to the DVC were included in the analyses.
2.3. Pharmacological testing
Rats (n=9, 15, 13 for Experiments 1, 2, 3, respectively) were handled and habituated to injections prior to testing. All experiments consisted of a within-subjects counterbalanced design with drug treatments separated by 72 hours. At the time of or 1-hr prior to DVC drug injection for Shu9119 and MTII experiments, respectively, food was removed from the animal’s cage. A micropump-depressed (PHD 2000; Harvard Apparatus, Holliston, MA) Hamilton syringe attached to tubing that terminated in a 33-gauge injector extending 2.0 mm beyond the cannula was used to bilaterally deliver either artificial cerebral spinal fluid (aCSF; vehicle control), MTII (1 or 5 pmol) or Shu9119 (20 pmol) to the parenchyma of the DVC. All DVC delivered infusions were administered 1-hr before the onset of the dark cycle at a volume of 100 nl/hemisphere and a flow rate of 1.2 μl/min. Injectors were left in place for 30 seconds to allow for diffusion of the drug. Just prior to the onset of the dark cycle, rats were treated with an intraperitoneal (IP) injection of 0.9% saline (vehicle control; 1 ml/kg) or liraglutide (50 or 100 μg/ml; 1ml/kg, gift of Novo Nordisk). Doses of liraglutide (100 μg/ml for Experiment 1 and 50 μg/ml for Experiment 2) were chosen in order to allow for attenuation of food intake and body weight by Shu9119 or further suppression of intake by MTII. Food was returned at the onset of the dark cycle. Food intake measurements were performed by calculating the cumulative change in the weight of the food hopper (+/− 0.1g) from the time of injection to the indicated time point (1, 4, 24, 48 hrs). Food spillage was accounted for by subtracting the weight of crumbs, collected via papers placed beneath each hanging wire-cage, from the weight change of the food hopper. Animals were weighed prior to drug treatment and at 24 and 48 hrs post treatment for the calculation of body weight change.
2.4. Statistical Analysis
All data are expressed as means ± SEM. Analysis of variance (ANOVA) were performed using the GraphPad Prism 7.0 Software (GraphPad Software Inc., San Diego, CA, USA). The dose response analysis for MTII was performed using a repeated measures one-way ANOVA with Tukey’s post hoc test for multiple comparisons. All comparisons of the effects of either MTII or SHU9119 on liraglutide or vehicle- induced food intake and body weight were analyzed using a repeated-measures two-way ANOVA with Tukey’s post hoc test for multiple comparisons. For all statistical tests, the α level for significance was 0.05.
3. Results
Experiment 1: Shu9119 attenuates the food intake and body weight suppressive effects of liraglutide
Two-way repeated measures ANOVAs for each measured time-point (1, 4, 24, 48) with Tukey’s post hoc tests were used to examine the effects of Shu9119 or liraglutide alone and in combination. There was no main effect of liraglutide on food intake revealed by ANOVA at 1 hour [F(1,8)=3.147]. Significant main effects of liraglutide occurred at 4, 24, and 48 hour time points [F(1,8)=24.77, p=0.001; F(1,8)=227.1, p<0.0001; F(1,8)=54.96, p<0.0001, respectively]. At the 1 and 4 hour time points, there were no main effects of Shu9119 [F(1,8)=0.292, p=0.603 and F(1,8)= 1.098, p=0.325]. However, at 24 and 48 hours there were both main effects of Shu9119 [F(1,8)=73.74, p<0.0001 and F(1,8)=3.459, p=0.100, respectively] and interactions [F(1,8)=227.1, p<0.0001 and F(1,8)=10.49, p=0.011, respectively]. Post hoc tests at 24 and 48 hours revealed that vehicle/liraglutide treated rats consumed significantly less than vehicle/vehicle (p<0.0001 and p=0.0001) and Shu9119/vehicle (p<0.0001and p=0.0002) treated rats. At 24 hours, a suppression of food intake was not observed for Shu9119/liraglutide treated rats compared to vehicle/vehicle (p=0.999) or Shu9119/vehicle (p>0.999) treated rats. At 48 hours, Shu9119/liraglutide intake was significantly different than vehicle/vehicle (p=0.0187), Shu9119/vehicle (p=0.0219) and vehicle/liraglutide (p=0.009) treated rats. As previously reported [42] and intended by our experimental design, the chosen dose of Shu9119 (20 pmol) was indeed subthreshold for food intake-suppressive effects at every measured time point. As such, post hoc tests did not reveal differences between vehicle/vehicle and Shu9119/vehicle treated groups at 1 (p=0.9987), 4 (p>0.9999), 24 (p=0.999) or 48-hour (p=0.9994) time points (Fig. 1A).
Fig 1.
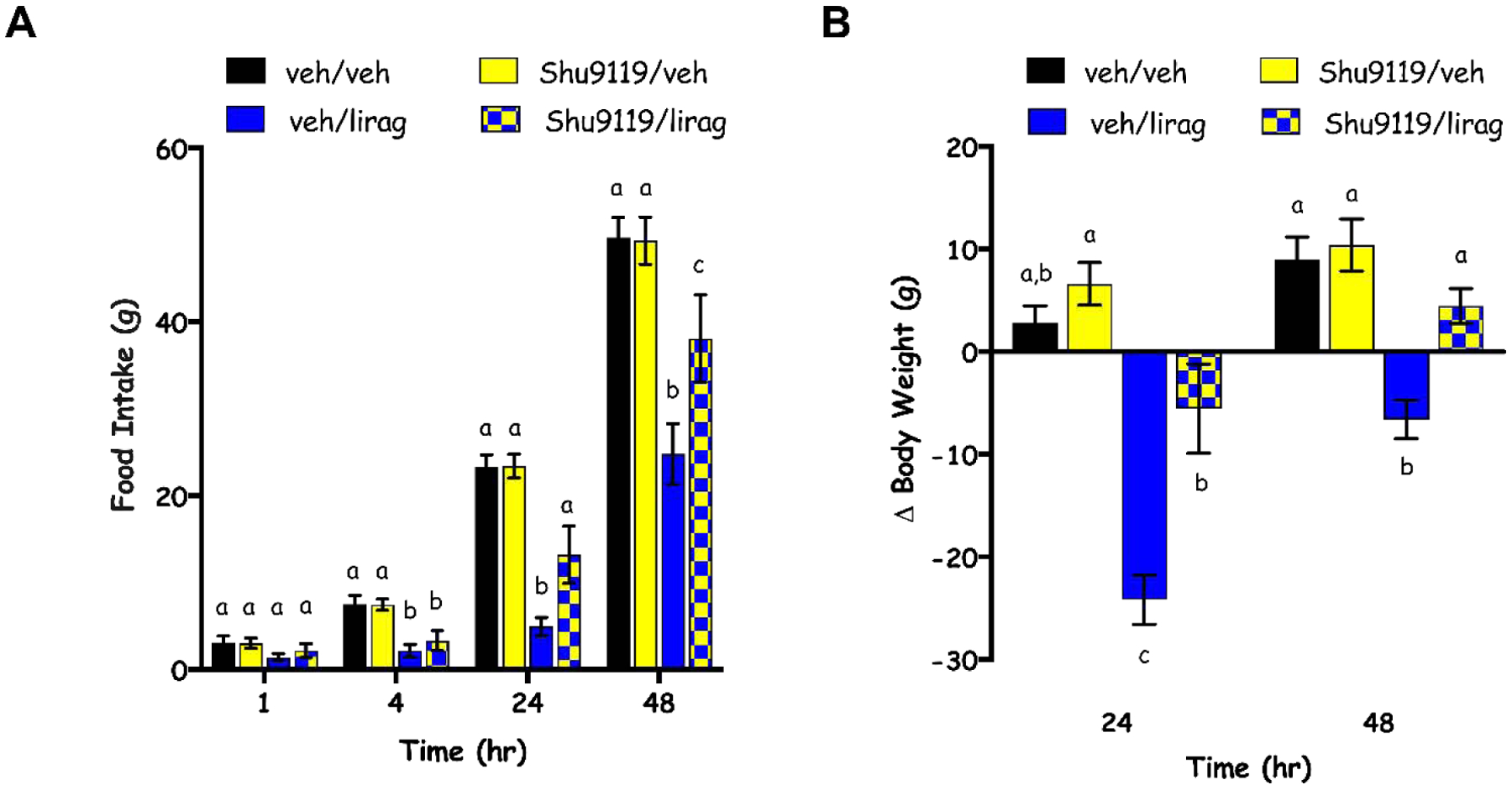
Cumulative chow intake (A) and change in body weight (B) at 1, 4, 24, 48 hours (hr) post-administration of vehicle (veh) or Shu9119 (20 pmol; intra-DVC) followed by vehicle or liraglutide (lirag; 100 μg/kg; IP). Data are expressed as mean ± SEM. Different letters indicate significant differences between groups (p<0.05).
Similarly for body weight, while there were main effects of liraglutide [F(1,8)=76.59, p<0.0001 and F(1,8)=37.32, p=0.0003], Shu9119 [F(1,8)=11.01, p=0.0106 and F(1,8)=6.319, p=0.0362] and interactions between the drugs [F(1,8)=9.806, p=0.0140 and F(1,8)=9.673, p=0.0144] at 24 and 48 hours, respectively, post hoc tests revealed no effects of Shu9119 alone on body weight when compared to vehicle/vehicle treated rats at either 24 (p=0.6840) or 48 hour time points (p=0.9073). Instead, vehicle/liraglutide suppressed body weight compared to both control groups (vehicle/vehicle and Shu9119/vehicle) and Shu9119/liraglutide treated rats at 24 (p=0.0002, p<0.0001, p=0.0024) and 48 hours (p=0.0004, p=0.0002, p=0.0043). Body weight for Shu9119/liraglutide treated rats was different than Shu9119/vehicle at 24 (p=0.0277) but not 48 hours (p=0.0900; Fig. 1B).
Experiment 2: MTII Dose Response
A dose response experiment was performed in order to determine a dose of MTII that when delivered intra-DVC was subthreshold for food intake and body weight suppressive effects. One-way repeated measures ANOVAs for each measured time point (1, 4, 24, 48) revealed no main effects of dose on food intake at the 1 [F(2,28)=1.017, p=0.3747] or 48 [F(2,28)=1.283, p=0.2931] hour time point. In contrast, there was a main effect of dose on food intake at 4 [F(2,28)=4.876, p=0.0152] and 24 hours [F(2,28)=155.5, p<0.0001] hours. At both 4 and 24 hours, Tukey’s post hoc tests revealed significant differences between vehicle and the 5 pmol dose (p=0.0147 and p<0.0001, respectively) but not vehicle and the 1 pmol dose (p=0.7137 and p=0.4359, respectively). At 24 hours, there was also a difference between the 1 pmol and 5 pmol dose (p<0.0001) that was not significant at 4 hours (p=0.0846; Fig 2A).
Fig 2.
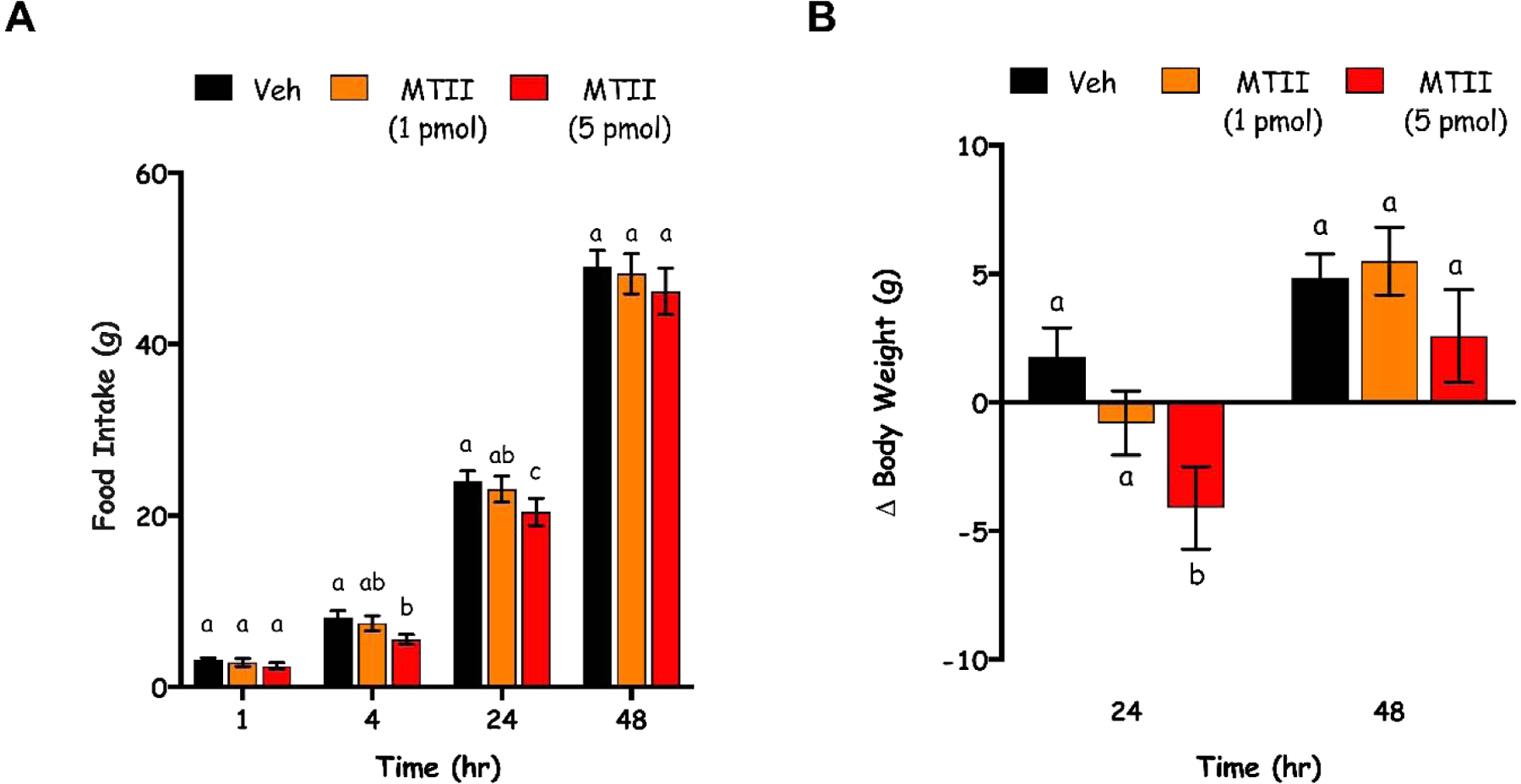
Dose-dependent effects of DVC-directed MTII on food intake (A) and body weight (B) at 1, 4, 24, 48 hours (hr) post-administration of vehicle (veh) or MTII (1 or 5 pmol; intra-DVC). Data are expressed as mean ± SEM. Different letters indicate significant differences between groups (p<0.05).
There was a main effect of dose on body weight at 24 [F(2,28)=5.207, p=0.0119] but not 48 hours [F(2,28)=1.327, p=0.2814; Fig. 2B]. Tukey’s post hoc tests revealed that at 24 hours, vehicle treatment was different than 5 mol (p=0.0087), but not 1 mol (p=0.3242). There was not a difference in body weight between rats treated with 1 and 5 pmol at 24 hours (p=0.2012; Fig. 2B).
Experiment 3: MTII augmentation of the food intake and body weight suppressive effects of liraglutide
Two-way repeated measures ANOVAs for each measured time-point (1, 4, 24, 48) were used to examine the effects of MTII or liraglutide alone or in combination. There was no main effect of liraglutide on food intake revealed by ANOVA at 1 hour [F(1,12)=1.106]. Significant main effects of liraglutide occurred at 4, 24, and 48 hour time points [F(1,12)=11.58, p=0.0052; F(1,12)=75.91, p<0.0001; F(1,12)=26.65, p=0.0002, respectively]. At the 1 hour time point, there was no main effect of MTII [F(1,12)=2.508, p=0.1392]. However, at 4, 24 and 48 hours there were main effects of MTII on food intake [F(1,12)=11.77, p<0.0050; F(1,12)=21.17, p=0.0006; F(1,12)=5.375, p=0.0389, respectively] but no interactions [F(1,12)=0.4529, p=0.5137; F(1,12)=1.6, p=0.2300; F(1,12)= 0.09474, p=0.7635, respectively]. Post hoc tests at 4, 24 and 48 hours revealed only significant differences between groups at 24 and 48 hours. At 24 hours, liraglutide suppressed food intake relative to vehicle/vehicle treated rats (p=0.0308). At this time point, the MTII/liraglutide group was significantly different from all three other treatment groups (p=0.0001 relative to vehicle/vehicle, p=0.0014 relative to MTII/vehicle, p=0.0301 relative to MTII/liraglutide). At 48 hours, liraglutide treatment alone did not suppress food intake relative to vehicle/vehicle treated animals (p=0.0850), however, when MTII/liraglutide did suppress food intake relative to both vehicle/vehicle (p=0.0061) and MTII/vehicle (p=0.0403), but not vehicle/liraglutide (p=0.4564), treated rats (Fig. 3A). As informed by our dose response (Fig. 2), MTII was indeed subthreshold for food intake effects at all measured time points. As such, post hoc tests did not reveal differences between vehicle/vehicle and MTII/vehicle treated groups at 1 (p=0.9925), 4 (p=0.8780), 24 (p=0.4827) or 48-hour time points (p=0.7050; Fig. 3A).
Fig 3.
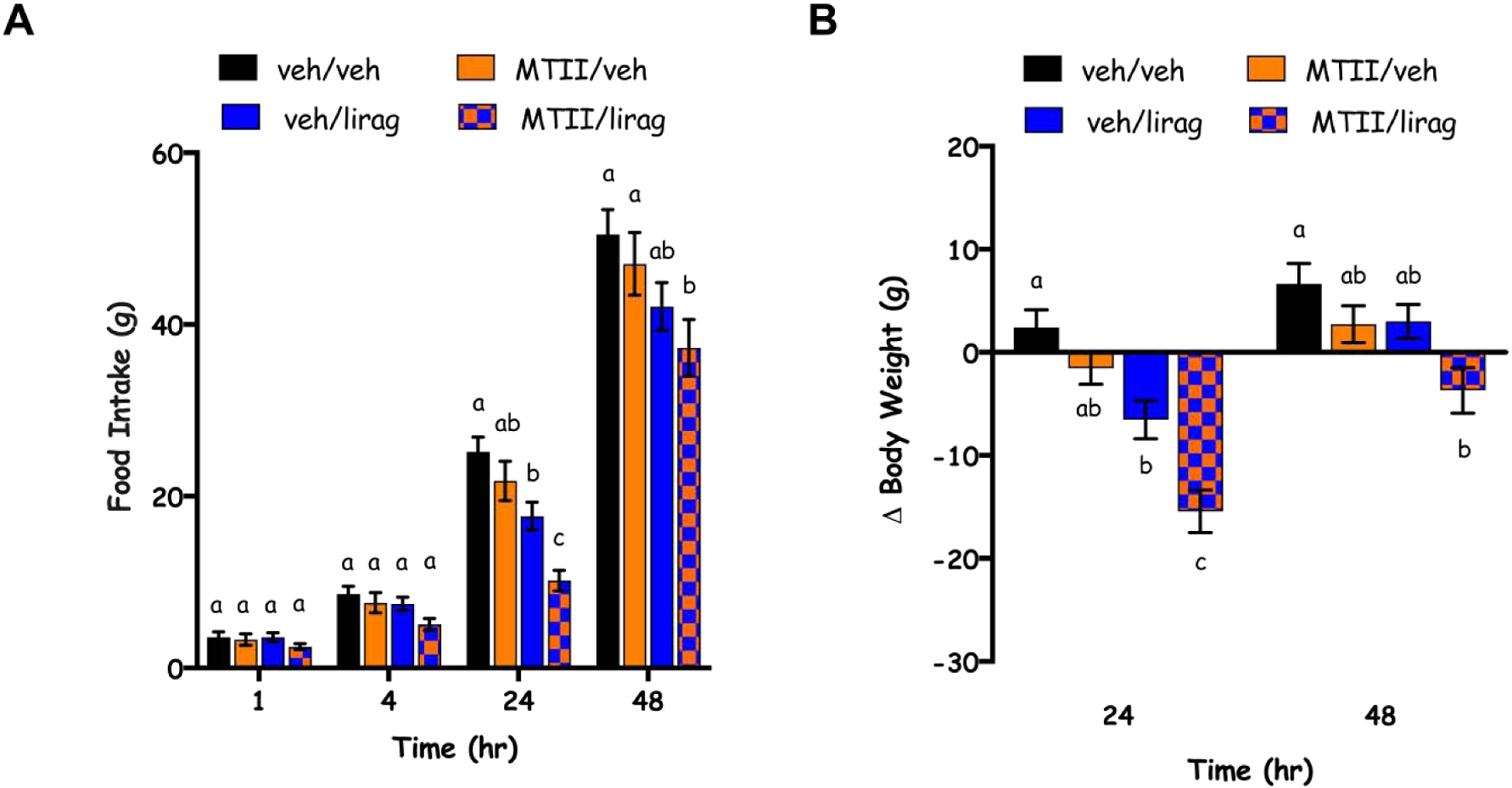
Cumulative chow intake (A) and change in body weight (B) at 1, 4, 24, 48 hours (hr) post-administration of vehicle (veh) or MTII (1 pmol; intra-DVC) followed by vehicle (veh) or liraglutide (lirag; 50 μg/kg; IP). Data are expressed as mean ± SEM. Different letters indicate significant differences between groups (p<0.05).
Similarly for body weight, there were main effects of liraglutide [F(1,12)=52.16, p<0.0001 and F(1,12)=8.349, p=0.0136] and MTII [F(1,12)=26.36, p=0.0002 and F(1,12)=12.81, p=0.0038], but not interactions between the drugs [F(1,12)=1.53, p=0.2398 and F(1,12)=0.4536, p=0.5134], at 24 and 48 hours, respectively. Post hoc tests revealed no effects of MTII alone on body weight when compared to vehicle/vehicle treated rats at either 24 (p=0.5248) or 48-hour time points (p=0.5516). At 24 hours, there was an effect of liraglutide alone on body weight (p=0.0359 for vehicle/vehicle compared to vehicle/liraglutide) that was enhanced by MTII such that body weight for the MTII/liraglutide treated animals was significantly different than vehicle/vehicle (p=0.0002), MTII/vehicle (p=0.0018) and vehicle/liraglutide (p=0.0368) treated rats. At 48 hours, the only significantly different groups (p<0.05) were the vehicle/vehicle compared to the MTII/liraglutide groups (p=0.0180; Fig. 3B).
4. Discussion
The suppression of food intake and body weight by liraglutide undoubtedly involves the modulation of multiple CNS energy balance regulatory systems. Each of these regulatory systems therefore represent a potential target for enhancing the food intake and body weight suppressive effects of liraglutide. Here, we provide evidence that DVC MC3/4Rs play a modulatory role for liraglutide’s anorectic effects and can be targeted to augment the of food intake and body weight suppression by liraglutide. Specifically, blockade of DVC MC3/4Rs partially attenuated the intake and body weight suppression by liraglutide. Further, activation of MC3/4R by MTII augmented the food intake and body weight suppressive effect of liraglutide at 24 and 48-hour time-points.
The dynamic modulation of liraglutide signaling by MC3/4Rs that we observe in our studies is not surprising given [1] the abundance of endogenous feeding signals that are known to synergize at the level of the DVC to modulate food intake [15,40], including but not limited to: leptin and CCK [16,17,43,44], amylin and leptin [18], leptin and GLP-1 [19,45–47] and CCK and GLP-1 [48] and [2] the well-supported role for central melanocortin receptors as potentiators of both short-term gut-derived and long-term adipostatic energy balance signals. Indeed, agonism of melanocortin receptors augments the intake-inhibitory effects of exogenous CCK, whereas genetic deletion or antagonism of central MC4Rs, both within the hypothalamus and hindbrain, blocks the suppression of intake by CCK [34,49,50]. The integration of more long-term signals is evidenced by the disruption of leptin-induced feeding suppression by MC3/4R antagonism [51] or POMC deficiency in obese mice [52]. Our work adds GLP-1R signaling (specifically that produced by liraglutide) to the list of neuromodulatory signaling roles of MC3/4Rs by demonstrating that the full intake-suppressive effects of liraglutide requires DVC MC3/4R activation.
While a site at which GLP-1 and melanocortin receptor signaling converge to modulate food intake has been identified here, further research is necessary to identify the mechanism by which DVC MC3/4Rs are tuning liraglutide signaling. An attractive hypothesis is that the interaction is occurring within the DVC on cells that express both the MC3/4R and GLP-1R. Indeed, the DVC expresses both GLP-1R and MC3/4Rs [32,38,53–58]. Numerous studies have shown that liraglutide crosses the blood brain barrier and permeates the DVC [11,59,60]. Additional evidence supporting liraglutide and MC3/4R action directly within the DVC comes from pharmacological studies showing that intra-DVC delivery of either MC3/4R or GLP-1R agonists suppresses food intake [30,58,61,62]. As our pharmacological studies do not restrict analysis to subnuclei of the DVC, MC3/4R populations in the area postrema, nucleus of the solitary tract and dorsal motor nucleus of the vagus are all possibilities for GLP-1R and MC3/4R co-expression. Future in situ hybridization work is required to inform a site of action. Additionally, in-depth analyses are required to determine whether the MC3/4R and/or GLP-1R are expressed on post-synaptic cells or pre-synaptically on incoming fibers, of vagal afferent origin or otherwise (see [15] for review). Indeed, the critical population of MC4Rs, either pre- or postsynaptic, that mediates food intake control within the NTS remains controversial [31–33].
Our data suggest that liraglutide is engaging POMC neurons, either directly or indirectly, and that the endogenous release of α-MSH is potentiating liraglutide-induced food intake and body weight suppression. The expression of GLP-1Rs on POMC neurons has been demonstrated in the hypothalamus [60] but whether there is a local endemic source of α-MSH from NTS POMC neurons downstream of liraglutide remains to be tested. It is also possible that liraglutide is engaging hypothalamic POMC neurons [60] which potentially project to DVC MC3/4R expressing neurons for tuning of a liraglutide-induced signal at the level of the DVC. Studies which selectively silence either hypothalamic or NTS GLP-1R populations expressed on either POMC neurons of the arcuate nucleus of the hypothalamus or the NTS will be necessary to fully understand the mechanism at play. It is important to note, however, that the DVC is likely not the only site in the CNS at which liraglutide signaling is modulated by MC3/4Rs to affect food intake. The incomplete rescue of food and body weight suppression by Shu9119 delivered to the DVC suggests the involvement of other CNS-mediated mechanisms that have yet to be identified.
A complete understanding of the mechanism and sites-of-action where GLP-1 and MTII signals converge may help inform the development of future targeted therapies with improved efficacy and reduced side effects. Our work suggests that targeting DVC MC3/4Rs improves the weight loss potential of liraglutide. This population of MC3/4Rs should also be examined in the regulation of glucose metabolism by liraglutide as melanocortin receptor ligands and liraglutide have independent effects on glucose metabolism [63,64] that are improved when liraglutide and MC4R agonists are combined systemically [23]. Future studies will also be necessary to test whether liraglutide and MTII combination therapies can minimize the negative side effects of each drug alone while preserving their anorectic effects. Unfortunately, liraglutide induces nausea and malaise in both rodent and human studies [7,11,65] and MTII has adverse effects on body temperature, heart rate and blood pressure [36,37,66]. In addition, MTII has reported side effects of nausea and penile erection in humans [67,68], which together with the cardiovascular effects, have limited MC3/4R agonists’ potential as therapeutics for obesity. It remains possible, however, that the side effects of both drugs can be minimized by co-administering each individual drug at lower doses, as we have chosen to do here. Our results support further investigation of the mechanism by which DVC MC3/4Rs regulate liraglutide-induced signaling and anorexia given that DVC MC3/4Rs are required for the full intake suppressive effects of liraglutide and that liraglutide-induced anorexia is augmented with agonism of the MC3/4R.
Highlights:
The full anorectic potential of liraglutide requires DVC MC3/4Rs
Agonism of DVC MC3/4Rs potentiates the food intake and body weight suppressive effects of liraglutide
Acknowledgements and Conflicts of Interest:
The authors would like to thank Rinzin Lhamo for technical assistance and experimental advice. We thank Novo Nordisk for generously supplying the liraglutide used in these studies. Funding: This work was supported by NIH-DK021397 (MRH) and NIH-DK120211 (SMF). Author contributions: SMF and MRH conceived and designed the experimental approach. SMF and JC performed experiments. SMF analyzed the data and SMF and MRH prepared the manuscript. Competing interests: MRH also receives research funding from Zealand Pharma, Eli Lilly & Co., Novo Nordisk and Boehringer Ingelheim that was not used in support of these studies. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Hales CM, Carroll MD, Fryar CD, Ogden CL, Prevalence of Obesity Among Adults and Youth: United States, 2015–2016 Key findings Data from the National Health and Nutrition Examination Survey, 2015. [Google Scholar]
- [2].Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR, Long-term maintenance of weight loss: current status., Health Psychol. 19 (2000) 5–16. [DOI] [PubMed] [Google Scholar]
- [3].Christiansen T, Bruun JM, Madsen EL, Richelsen B, Weight Loss Maintenance in Severely Obese Adults after an Intensive Lifestyle Intervention: 2- to 4-Year Follow-Up*, Obesity. 15 (2007) 413–420. doi: 10.1038/oby.2007.530. [DOI] [PubMed] [Google Scholar]
- [4].Nguyen N, Champion JK, Ponce J, Quebbemann B, Patterson E, Pham B, Raum W, Buchwald JN, Segato G, Favretti F, A Review of Unmet Needs in Obesity Management, Obes. Surg 22 (2012) 956–966. doi: 10.1007/s11695-012-0634-z. [DOI] [PubMed] [Google Scholar]
- [5].Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF, Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials., BMJ. 348 (2014) g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kanoski SE, Hayes MR, Skibicka KP, GLP-1 and weight loss: unraveling the diverse neural circuitry, Am. J. Physiol. Integr. Comp. Physiol 310 (2016) R885–R895. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lean MEJ, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rössner S, Van Gaal L, Astrup A, NN8022–1807 Investigators, Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults, Int. J. Obes 38 (2014) 689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mehta A, Marso SP, Neeland IJ, Liraglutide for weight management: a critical review of the evidence, Obes. Sci. Pract 3 (2017) 3–14. doi: 10.1002/osp4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM, Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults, Int. J. Obes 38 (2014) 784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van Gaal L, Dirinck E, Pharmacological approaches in the treatment and maintenance of weight loss, Diabetes Care. 39 (2016) S260–S267. doi: 10.2337/dcS15-3016. [DOI] [PubMed] [Google Scholar]
- [11].Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR, The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide., Neuropharmacology. 62 (2012) 1916–27. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Greenway FL, Bray GA, Combination Drugs for Treating Obesity, Curr. Diab. Rep 10 (2010) 108–115. doi: 10.1007/s11892-010-0096-4. [DOI] [PubMed] [Google Scholar]
- [13].Rodgers RJ, Tschöp MH, Wilding JPH, Anti-obesity drugs: past, present and future., Dis. Model. Mech 5 (2012) 621–6. doi: 10.1242/dmm.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sadry SA, Drucker DJ, Emerging combinatorial hormone therapies for the treatment of obesity and T2DM., Nat. Rev. Endocrinol 9 (2013) 425–33. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- [15].Grill HJ, Hayes MR, The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake, Int. J. Obes 33 (2009) S11–S15. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- [16].Wang L, Barachina MD, Martínez V, Wei JY, Taché Y, Synergistic interaction between CCK and leptin to regulate food intake., Regul. Pept 92 (2000) 79–85. [DOI] [PubMed] [Google Scholar]
- [17].Yuan CS, Attele AS, Dey L, Xie JT, Gastric effects of cholecystokinin and its interaction with leptin on brainstem neuronal activity in neonatal rats., J. Pharmacol. Exp. Ther 295 (2000) 177–82. [PubMed] [Google Scholar]
- [18].Trevaskis JL, Parkes DG, Roth JD, Insights into amylin–leptin synergy, Trends Endocrinol. Metab 21 (2010) 473–479. doi: 10.1016/J.TEM.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [19].Huo L, Gamber KM, Grill HJ, Bjørbæk C, Divergent Leptin Signaling in Proglucagon Neurons of the Nucleus of the Solitary Tract in Mice and Rats, Endocrinology. 149 (2008) 492–497. doi: 10.1210/en.2007-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR, Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner, Int. J. Obes 36 (2012) 1522–1528. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kanoski SE, Ong ZY, Fortin SM, Schlessinger ES, Grill HJ, Liraglutide, leptin and their combined effects on feeding: additive intake reduction through common intracellular signalling mechanisms, Diabetes, Obes. Metab 17 (2015) 285–293. doi: 10.1111/dom.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Trevaskis JL, Sun C, Athanacio J, D’Souza L, Samant M, Tatarkiewicz K, Griffin PS, Wittmer C, Wang Y, Teng C-H, Forood B, Parkes DG, Roth JD, Synergistic metabolic benefits of an exenatide analogue and cholecystokinin in diet-induced obese and leptin-deficient rodents, Diabetes, Obes. Metab 17 (2015) 61–73. doi: 10.1111/dom.12390. [DOI] [PubMed] [Google Scholar]
- [23].Adan RAH, Tiesjema B, Hillebrand JJG, la Fleur SE, Kas MJH, de Krom M, The MC4 receptor and control of appetite., Br. J. Pharmacol 149 (2006) 815–27. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F, Targeted disruption of the melanocortin-4 receptor results in obesity in mice., Cell. 88 (1997) 131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- [25].Vaisse C, Clement K, Guy-Grand B, Froguel P, A frameshift mutation in human MC4R is associated with a dominant form of obesity, Nat. Genet 20 (1998) 113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- [26].Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S, A frameshift mutation in MC4R associated with dominantly inherited human obesity., Nat. Genet 20 (1998) 111–2. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- [27].Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD, Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain., Mol. Endocrinol 8 (1994) 1298–308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- [28].Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM, Brainstem Application of Melanocortin Receptor Ligands Produces Long-Lasting Effects on Feeding and Body Weight, J. Neurosci 18 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Williams DL, Kaplan JM, Grill HJ, The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation., Endocrinology. 141 (2000) 1332–7. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- [30].Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud H-R, Travagli RA, Presynaptic Melanocortin-4 Receptors on Vagal Afferent Fibers Modulate the Excitability of Rat Nucleus Tractus Solitarius Neurons, J. Neurosci 28 (2008) 4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Campos CA, Shiina H, Ritter RC, Central vagal afferent endings mediate reduction of food intake by melanocortin-3/4 receptor agonist., J. Neurosci 34 (2014) 12636–45. doi: 10.1523/JNEUROSCI.1121-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fan W, Ellacott KLJ, Halatchev IG, Takahashi K, Yu P, Cone RD, Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system, Nat. Neurosci 7 (2004) 335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- [33].Sutton GM, Duos B, Patterson LM, Berthoud H-R, Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus., Endocrinology. 146 (2005) 3739–47. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- [34].Skibicka KP, Grill HJ, Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins., Endocrinology. 150 (2009) 5351–61. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng H, Patterson LM, Phifer CB, Berthoud H-R, Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections., Am. J. Physiol. Regul. Integr. Comp. Physiol 289 (2005) R247–58. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- [36].Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK, Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter., J. Neurosci 23 (2003) 7143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Berthoud H-R, Sutton GM, Townsend RL, Patterson LM, Zheng H, Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size, Physiol. Behav 89 (2006) 517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- [38].Grill HJ, Hayes MR, Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance., Cell Metab. 16 (2012) 296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paxinos G, Watson C, The rat brain in stereotaxic coordinates, 2007. [DOI] [PubMed]
- [40].Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y, Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Emond M, Schwartz GJ, Ladenheim EE, Moran TH, Central leptin modulates behavioral and neural responsivity to CCK., Am. J. Physiol 276 (1999) R1545–9. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- [42].Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR, Hindbrain leptin and glucagon-like- peptide-1 receptor signaling interact to suppress food intake in an additive manner., Int. J. Obes. (Lond) 36 (2012) 1522–8. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Goldstone AP, Mercer JG, Gunn I, Moar KM, Edwards CMB, Rossi M, Howard JK, Rasheed S, Turton MD, Small C, Heath MM, O’Shea D, Steere J, Meeran K, Ghatei MA, Hoggard N, Bloom SR, Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents, FEBS Lett. 415 (1997) 134–138. doi: 10.1016/S0014-5793(97)01103-4. [DOI] [PubMed] [Google Scholar]
- [44].Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW, Baskin DG, Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8, Am. J. Physiol. Integr. Comp. Physiol 296 (2009) R476–R484. doi: 10.1152/ajpregu.90544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sutton GM, Duos B, Patterson LM, Berthoud H-R, Melanocortinergic Modulation of Cholecystokinin-Induced Suppression of Feeding through Extracellular Signal-Regulated Kinase Signaling in Rat Solitary Nucleus, Endocrinology. 146 (2005) 3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- [46].Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, Van Dijk G, Baskin DG, Schwartz MW, Melanocortin receptors in leptin effects [7], Nature. 390 (1997) 349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- [47].Challis BG, Coll AP, Yeo GSH, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O’Rahilly S, Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY3–36, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK, Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat, J. Comp. Neurol 457 (2003) 213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- [49].Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J, Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions., J. Comp. Neurol 518 (2010) 6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Merchenthaler I, Lane M, Shughrue P, Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system., J. Comp. Neurol 403 (1999) 261–80. [DOI] [PubMed] [Google Scholar]
- [51].Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N, Grove KL, Expression and Distribution of Glucagon-Like Peptide-1 Receptor mRNA, Protein and Binding in the Male Nonhuman Primate (Macaca mulatta) Brain, Endocrinology. 156 (2015) 255. doi: 10.1210/EN.2014-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S, Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain., Mol. Metab 4 (2015) 718–31. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC, Hayes MR, Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance., J. Neurosci 36 (2016) 3531–40. doi: 10.1523/JNEUROSCI.3579-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Salinas CBG, Lu TT-H, Gabery S, Marstal K, Alanentalo T, Mercer AJ, Cornea A, Conradsen K, Hecksher-Sørensen J, Dahl AB, Knudsen LB, Secher A, Integrated Brain Atlas for Unbiased Mapping of Nervous System Effects Following Liraglutide Treatment, Sci. Rep 8 (2018) 10310. doi: 10.1038/s41598-018-28496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L, The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss, J. Clin. Invest 124 (2014) 4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hayes MR, Skibicka KP, Grill HJ, Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation., Endocrinology. 149 (2008) 4059–68. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK, Intracellular Signals Mediating the Food Intake-Suppressive Effects of Hindbrain Glucagon-like Peptide-1 Receptor Activation, Cell Metab. 13 (2011) 320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME, NN8022–1807 Study Group, Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study, Lancet. 374 (2009) 1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- [59].Fani L, Bak S, Delhanty P, van Rossum EFC, van den Akker ELT, The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options, Int. J. Obes 38 (2014) 163–169. doi: 10.1038/ijo.2013.80. [DOI] [PubMed] [Google Scholar]
- [60].Wessells H, Levine N, Hadley ME, Dorr R, Hruby V, Melanocortin receptor agonists, penile erection, and sexual motivation: Human studies with Melanotan II, Int. J. Impot. Res 12 (2000) S74–S79. doi: 10.1038/sj.ijir.3900582. [DOI] [PubMed] [Google Scholar]
- [61].Fani L, Bak S, Delhanty P, van Rossum EFC, van den Akker ELT, The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options, Int. J. Obes 38 (2014) 163–169. doi: 10.1038/ijo.2013.80. [DOI] [PubMed] [Google Scholar]
- [62].Savontaus E, Breen TL, Kim A, Yang LM, Chua SC, Wardlaw SL, Metabolic effects of transgenic melanocyte-stimulating hormone overexpression in lean and obese mice, Endocrinology. 145 (2004) 3881–3891. doi: 10.1210/en.2004-0263. [DOI] [PubMed] [Google Scholar]
- [63].Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JPH, SCALE Obesity and Prediabetes NN8022–1839 Study Group, A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management, N. Engl. J. Med 373 (2015) 11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- [64].Gutiérrez-Juárez R, Obici S, Rossetti L, Melanocortin-independent effects of leptin on hepatic glucose fluxes, J. Biol. Chem 279 (2004) 49704–49715. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]


