Abstract
Enantioenriched α-aminoboronic acids play a unique role in medicinal chemistry and have emerged as privileged pharmacophores in proteasome inhibitors. Additionally, they represent synthetically useful chiral building blocks in organic synthesis. Recently, CuH-catalyzed asymmetric alkene hydrofunctionalization has become a powerful tool to construct stereogenic carbon centers. In contrast, applying CuH cascade catalysis to achieve reductive 1,1-difunctionalization of alkynes remains an important, but largely unaddressed, synthetic challenge. Herein, we report an efficient strategy to synthesize α-aminoboronates via CuH-catalyzed hydroboration/hydroamination cascade of readily available alkynes. Notably, this transformation selectively delivers the desired 1,1-heterodifunctionalized product in favor of alternative homodifunctionalized, 1,2-heterodifunctionalized, or reductively monofunctionalized byproducts, thereby offering rapid access to these privileged scaffolds with high chemo-, regio- and enantioselectivity.
Alkenes and alkynes are ideal starting materials in organic synthesis due to the fact that they can be readily prepared using a variety of convenient synthetic methods and are widely available from commercial suppliers. Catalytic functionalization of the π-bonds in these substrates leads to a valuable array of building blocks and in the case of alkenes offers a gateway to chiral products from planar achiral starting materials1-4. The myriad of options for alkene functionalization notwithstanding5,6, the development of a complementary platform for stereoselective multi-functionalization of alkynes that bypasses synthesis and isolation of an alkene intermediate would be enabling, as it would increase the scope of product structures that can be accessed, reduce step count, and improve atom economy.
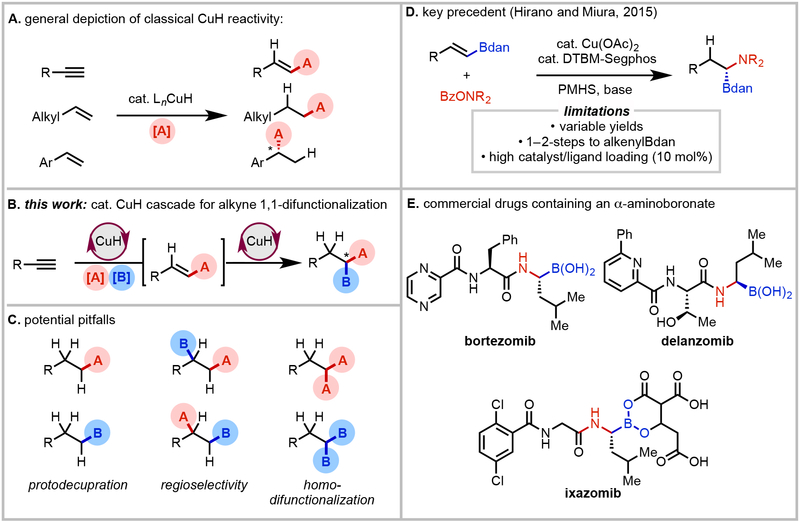
In light of the rapid rise of CuH catalysis for enabling hydrofunctionalization of carbon–carbon π-bonds during recent years (Fig. 1A)7,8, this type of system appeared to be a plausible means of achieving reductive alkyne difunctionalization. Specifically, we envisioned that a single CuH catalyst would play the dual role of converting the alkyne starting material into a functionalized alkene and subsequently hydrofunctionalize this alkene intermediate to the desired 1,1-difunctionalized product (Fig. 1B). Using a single CuH catalyst system rather than two separate catalysts for each of the steps offer the advantages of operational simplicity, reduced cost, and lower likelihood of cross-compatibility problems. The key challenge in this regard is to identify a catalyst that would exhibit the requisite levels of selectivity in each of the key steps and minimize side product formation. Indeed, several issues pertaining to reagent compatibility and catalyst selectivity could be anticipated during the development of this platform, including the possibility of side reactions stemming from homodifunctionalization9, reduction10,11, or non-selective hydrometalation (Fig. 1C)12. In parallel to the present study, Lalic and coworkers described a cascade CuH/Pd dual catalytic system to access racemic 1,1-borylarylated products from alkynes13.
Fig. 1.
Overview of proposed approach to CuH-catalyzed cascade reductive of difunctionalization of alkynes. A, General depiction of classical CuH reactivity in hydrofunctionalization of simple alkynes and alkenes. B, C, Design of CuH-catalyzed cascade process for alkyne 1,1-difunctionalization and potential pitfalls. D, Key precedent of CuH-catalyzed hydroamination of alkenylBdan. E, Commercial drugs containing an α-aminoboronate.
Several groups have previously disclosed relevant CuH-catalyzed hydrofunctionalization reactions of functionalized alkenes14-20. We were particularly inspired by a seminal publication by Hirano and Miura describing a procedure for enantioselective CuH-catalyzed hydroamination of alkenylBdan substrates (dan = naphthalene-1,8-diaminato) to access chiral 1,1-aminoboronates (Fig. 1D)17, which are widely used in commercial proteasome inhibitor therapeutics21-24. Though highly enabling in its own right, Hirano and Miura’s method has notable drawbacks, including variable yields across different alkene substrates, the need to prepare the alkenylBdan starting material (which is then used in excess, 1.2 equiv) through a 1–2 step sequence, and the relative high loading of catalyst and ligand loading (10 mol%).
Herein, we report an efficient strategy to synthesize enantioenriched α-aminoboronates via a cascade process in which a single CuH catalyst mediates sequential hydroboration and hydroamination of readily available alkyne substrates. The transformation offers practical value in streamlining access to the important aminoboronates substructure in a manner that complements existing methods 25-34. This system takes advantage on in situ formation of the key terminal alkenylBdan intermediate, a process that had only been described for aliphatic alkynes with HBpin (pin = pinacolato) using a Cu•NHC catalyst (NHC = N-heterocyclic carbene) prior to this work35-37. This reaction is compatible with aliphatic and aryl alkynes, as well as acyclic and cyclic O-benzoyl hydroxylamine electrophiles, including those containing an C=C bond. The cascade transformation can be performed in an intramolecular fashion to generate heterocyclic boronates, namely azetidine-2-ylboronates and pyrrolidin-2-ylboronates. Additionally, this strategy facilitates the efficient synthesis of the aminoboronic acid drug, bortezomib. Mechanistic studies including reaction kinetics and DFT calculations shed light on the origin of the experimentally observed chemoselectivity and enantioselectivity.
Results
Optimization studies
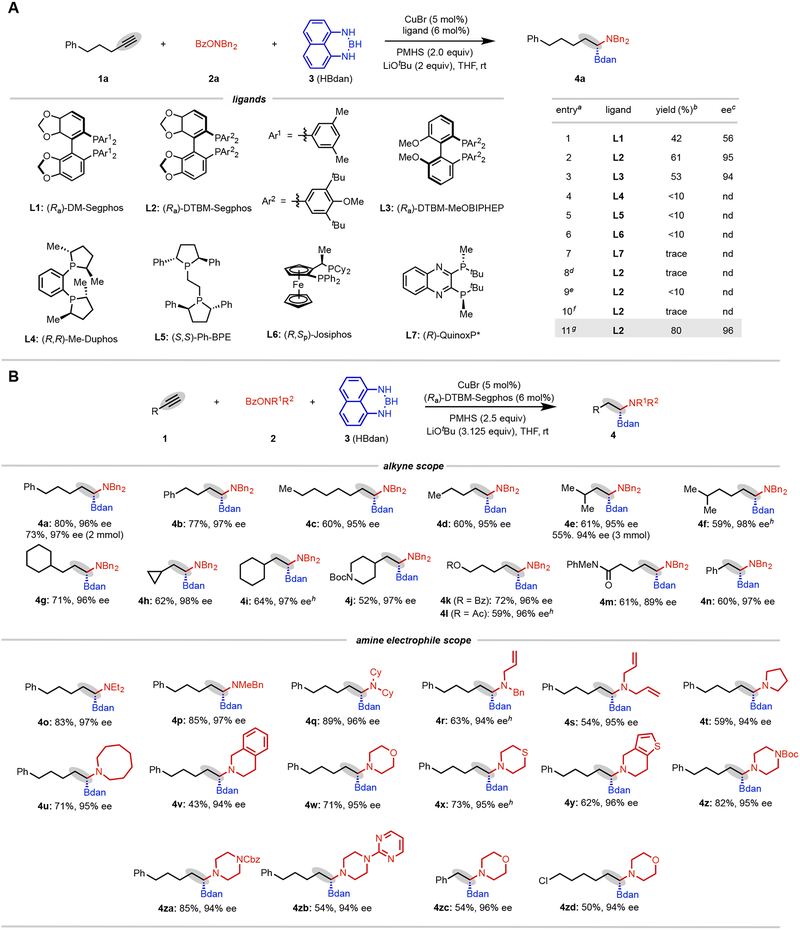
We first examined possible reaction conditions by using 5-phenyl-1-pentyne (1a) as the model substrate, O-benzoyl-N,N-dibenzylhydroxylamine as the amine electrophile, HBdan as the boron reagent, and polymethylhydrosiloxane (PMHS) as the hydride source. As summarized in Fig. 2A, a diverse array of bisphosphine ligands were examined for their efficiency to promote the cascade reaction. The desired product 4a could be isolated in 42% yield and 56% ee by initially choosing (Ra)-DM-Segphos as the ligand (entry 1). We were delighted to find that sterically bulky (Ra)-DTBM-Segphos was a suitable ligand, providing α-aminoboronic ester 4a in 61% yield and 95% ee without any observation of homo-difunctionalized byproducts (entries 2–3). A series of ligands bearing alternative backbones led to the desired product 4a in low yields (entries 4–7). Further screening of boron and base sources did not lead to any improvement (entries 8–10). The optimal reaction conditions were found by slightly tuning the amount of PMHS and LiOtBu (80%, 96% ee, entry 11).
Fig. 2.
Discovery and evaluation of CuH cascade process.A, Reaction optimization. B, Substrate scope. (see Supplementary Information Section 1.3.2)
aReaction conditions for optimization experiments: 1a (0.2 mmol), 2a (0.24 mmol), 3 (0.24 mmol), PMHS (0.4 mmol), LiOtBu (0.4 mmol), CuBr (5 mol%), and ligand (6 mol%) in THF (1.0 mL) at room temperature for 16 h. nd = not determined. bIsolated yield. cThe enantioselectivity was determined by SFC analysis. dHBPin instead of HBdan. eNaOtBu as the base. fKOtBu as the base. gPMHS (2.5 equiv) and LiOtBu (3.125 equiv). hUsing PMHS (3.0 equiv) and LiOtBu (3.75 equiv).
Scope of the reaction
After obtaining the optimized reaction conditions, we systematically investigated the substrate scope of terminal alkynes (Fig. 2B). A wide range of alkynes could be successfully transformed into the target products in good yields, high regioselectivity, and excellent enantioselectivity. Alkynes containing different primary alkyl chains all readily underwent efficient sequential hydroborylation/hydroamination (4a–d). Reactions of alkynes featuring sterically hindered isopropyl and cycloalkyl groups proceeded smoothly to deliver the corresponding products in synthetically useful yields with high levels of stereoinduction (4e–i). Of particular note, the alkyne with a pendant piperidine, a common heterocycle found in medicinally relevant molecules, was also a competent coupling partner (4j). Ester and amide functional groups did not show any deleterious effect on the efficiency of this reaction (4k–m). The cascade reductive 1,1-difunctionalization of phenylacetylene delivers the enantioenriched product 4n in 60% yield and 97% ee. Gratifyingly, this reaction could be scaled up to 2- or 3-mmol scale without notable erosion of yield and enantioselectivity (4a and 4e).
The generality of this reaction was further evaluated by exploring different hydroxylamine electrophiles, as shown in Fig 2B. Acyclic hydroxylamine derivatives were subjected to the reaction conditions, affording α-amidoboronates 4o–q in 83–89% yields and 96–97% ee. N-benzyl-N-allyl- and N,N-diallylhydroxylamine electrophiles (2r–s) were competent partners, allowing highly chemo-, regio- and enantioselective synthesis of 4r and 4s with preservation of the alkene moieties, a somewhat surprising result given that alkenes display a high degree of reactivity with hydroxylamine electrophiles and boron hydride reagents in the presence of copper hydrides. This strategy also allows for the straightforward and expedient synthesis of α-aminoboronates containing saturated aza-heterocycles (4t–zd). It is worth noting that the desired products containing morpholine (4w, 4zc–zd), thiomorpholine (4x), tetrahydrothienopyridine (4y) and piperazine (4z–zb) are important for drug development considering the prevalence of these heterocyclic motifs in medicinal chemistry. Across these different experiments, the only product generally observed was the desired 1,1-aminoborylated compound, with the remainder of the material typically being unreacted alkyne substrate, hydroborylated intermediate, or decomposition byproducts. The exquisite selectivity is noteworthy given the three-component nature of the reaction and the possibility for multiple competing hydroamination and hydroboration processes. In cases where it is a possible to make a direct head-to-head comparison with Hirano and Miura’s procedure for hydroboration of pre-formed alkenylBdan reagents17, this cascade procedure typically provides similar or higher yields (see Supplementary Figure 6 for details), illustrating the synergistic nature of the two catalytic cycles involved (see below).
Synthetic utility
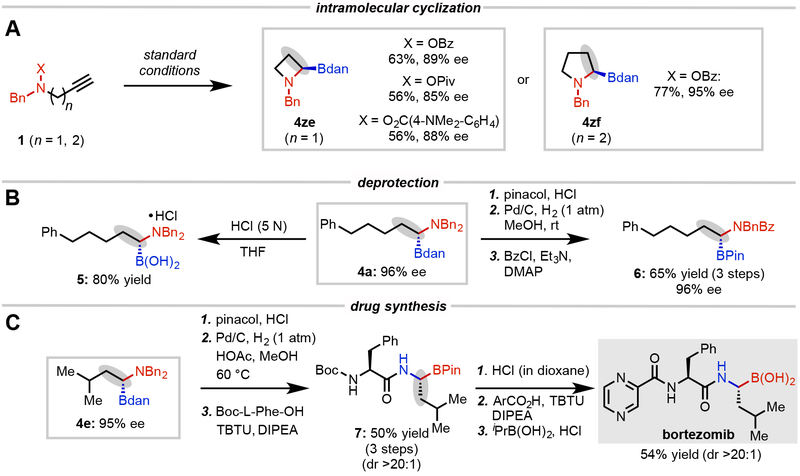
We hypothesized that an intramolecular cascade hydroboration and hydroamination might occur if the hydroxylamine electrophile and alkyne were tethered together38. To our delight, the benzoyl-protected amine derivative resulted in the formation of the desired azetidin-2-ylboronate, which represents another class of pharmaceutically important heterocycles, in 63% yield and 89% ee (Fig. 3A). Furthermore, the incorporation of pivaloate and electron-rich p-N,N-dimethylaminobenzoate39 generate 4ze in slightly reduced yield and enantioselectivity. Notably, pyrrolidin-2-ylboronate could be conveniently accessed in a highly enantioselective manner through the same intramolecular hydrofunctionalization strategy (4zf).
Fig. 3. Synthetic applications of enantioselective 1,1-aminoboration method.
A, CuH-catalyzed intramolecular cascade hydroboration and hydroamination. B, Deprotection of Bn and Bdan group. C, Synthesis of bortezomib.
To demonstrate the utility of this method in enabling synthesis of diverse chiral building blocks, we next sought to established robust methods for cleaving the protecting groups on nitrogen and boron and to apply this strategy to a representative product in a multi-step synthetic sequence (Fig. 3B). Product 4a could undergo hydrolysis of the Bdan group to reveal chiral α-aminoboronic acid 5 in 80% yield. α-Aminoboronic ester 6 could be efficiently prepared in three steps with a 65% isolated yield and 96% ee by conversion of Bdan to Bpin under acidic conditions followed by selective hydrogenolysis of one benzyl group and protection of the secondary amine. Moreover, this convenient approach to accessing α-aminoboronates provides a facile tool for the construction of proteasome inhibitors, which will expand access to these compounds and potentially reduce the cost of research and development. As an example, α-aminoboronate 4e could undergo conversion of Bdan to Bpin, hydrogenolysis of both benzyl groups, and condensation with Boc-L-Phe-OH to furnish the peptide 7 in 50% yield (dr >20:1) over three steps. Hydrolysis of 7 with 4 N HCl provided hydrochloride salt 8, which was coupled with 2-pyrazine carboxylic acid. Upon subsequent hydrolysis of the Bpin group, the asymmetric total synthesis of Bortezomib was accomplished (Fig. 3C).
Mechanistic studies
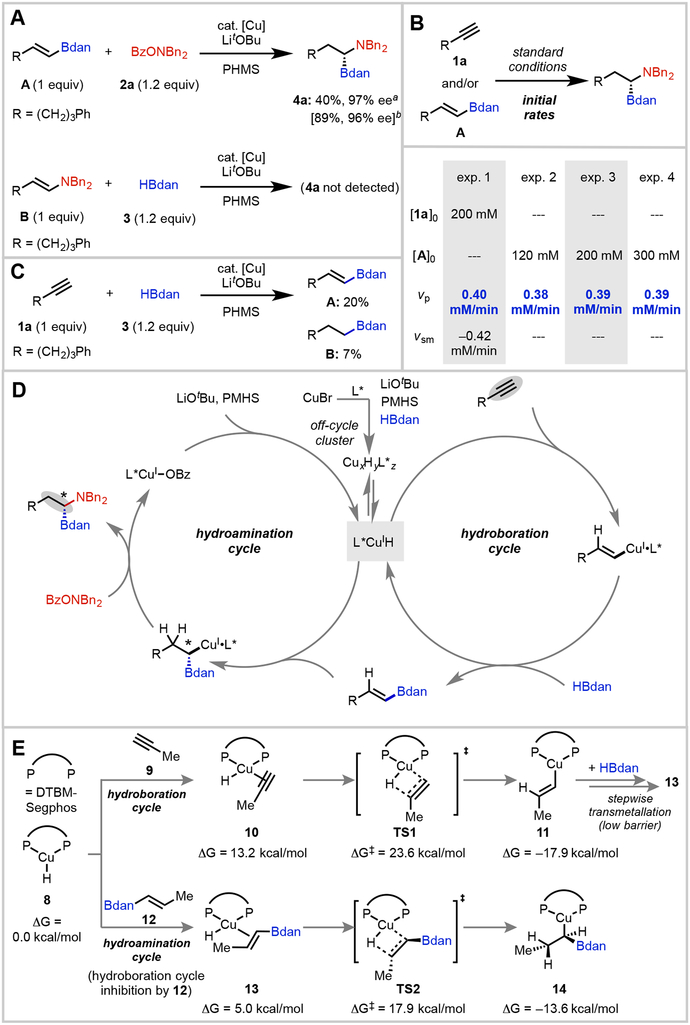
The remarkable selectivity of this reaction for a single enantiomeric product out of the myriad of possibilities prompted us to examine the reaction mechanism. There are two feasible reaction pathways, depending on the order of events: either a hydroboration-first sequence (Path A) or a hydroamination-first sequence (Path B) (see Supplementary Figure 5 for detailed depictions of these possible mechanisms). To probe which was operable, we subjected each of the putative intermediates, alkenylBdan A (Path A) and substituted enamine B (Path B), to the reaction conditions (Fig. 4A). We found that alkenylBdan A could be converted to the desired product in similar ee to in the cascade sequence, while enamine B only led to decomposition. These results show that alkenylBdan A is a competent intermediate and that enamine B is not, ruling out Path B. Furthermore, during kinetics experiments (vide infra), we observed free alkenylBdan in solution, which corroborates the conclusion that Path A is operative. Notably, the addition of HBdan to a hydroamination-only reaction of alkenylBdan A significantly improves the yield.
Fig. 4.
Mechanistic Overview. A, Experiments to establish the competency of plausible intermediates. B, Initial rate measures revealing that the hydroamination cycle is turnover-limiting. C, Experiment to gain insight into the nature of the hydroboration-only cycle. D, Proposed catalytic cycle. E, Computational studies.
a Yield determined by 1H NMR. b The bracketed values correspond to an experiment with added HBdan (1.2 equiv).
We next moved on to examine the reaction kinetics. First we performed initial rate experiments on the cascade process with 1a and 2a as representative reactants. From in situ monitoring, we found that alkenylBdan A built up quickly to [A] = 17 mM and then leveled off. Alkyne 1a was gradually consumed and converted to 4a, indicating that hydroboration of 1a still takes place in the presence of alkenylBdan A. Concentration dependencies on individual components were as follows: zero-order in [1a] and [2a], fractional-negative-order in [PMHS], and fractional-positive-order in [CuBr] catalyst, [LiOtBu], and [HBDan]. We found that the kinetic behavior of the cascade process was essentially identical to that of a hydroamination-only cycle using reaction of alkenylBdan A as starting material (Fig. 4B, see Supplementary Figure 8 for additional data), suggesting that hydroamination is the turnover-limiting cycle. Furthermore, both the cascade and hydroamination-only reactions were zero-order in [A]. Within the hydroamination cycle, zero-order dependencies on [A] and [2a] and fractional-positive-order dependency on [LiOtBu] rule out hydrocupration and electrophilic amination, as turnover-limiting steps, pointing to a base-mediated catalyst regeneration process as turnover-limiting39-41. The complex pattern of rate dependencies on [LiOtBu], [PMHS], and [HBDan] point to an intricate network of interactions. Though the mechanistic details of these steps are somewhat unclear, the data are consistent with a scenario in which LiOtBu promotes regeneration of LCuH catalyst from LCuOBz via the intermediacy of LCuOtBu. The negative rate order of [PMHS] may be attributed to deactivating interactions between PMHS and LiOtBu42, which may reduce the concentration of active base, thereby inhibiting formation of LCuOtBu. The observation of positive order of [HBdan] indicates that some percentage of active LCuH may also be generated from reaction of HBdan with LCuOtBu or LCuOBz.
To understand the origins of selectivity, the hydroboration-only cycle was also examined (Fig. 4C). In this case, we observed a maximum of 10–20% of alkenylBdan A formation. At extended reaction times, we noticed the appearance of reduced alkylBdan B (7%). Upon spiking a hydroboration-only experiment with the amine electrophile, the 1,1-aminoboronate product could be formed catalytically (see Supplementary Figure 5). These experiments suggest that CuH reacts rapidly with alkenylBdan to form an α-boryl-alkyl-Cu species13,43,44, which is off-cycle in the hydroboration-only process but on-cycle for the hydroamination cycle in the overall cascade. This is further supported by 31P NMR analysis of the hydroboration-only cycle; upon mixing a catalytic amount of in situ-generated LCuH and A, a new major resting state, presumably the α-boryl-alkyl-Cu intermediate, was observed at –8.4 ppm (see Supplementary Figure 11). The regioselectivity of CuH addition is controlled by electronic polarization of the alkene due to the Bdan substituent15. We next performed density functional theory (DFT) calculations to investigate the feasibility of forming the off-cycle α-boryl-alkyl-Cu species under the hydroboration-only conditions. Our calculations indicated that the hydrocupration of alkyne (TS1) is the rate-limiting step in the hydroboration-only cycle. The subsequent transmetallation of the alkenyl–copper intermediate 11 occurs via a stepwise mechanism and requires lower barriers (see Supplementary Figure 12 for details). The hydrocupration of alkenylBdan (12) via TS2 requires a 5.7 kcal/mol lower barrier than that of alkyne via TS1. Therefore, in the presence of alkenylBdan (12), the LCuH catalyst 8 is consumed to form α-boryl-alkyl-Cu 14 as an off-cycle intermediate. Consequently, the alkyne hydroboration is suppressed due to catalyst inhibition by the alkenylBdan product.
In summary, we have developed a cascade CuH-catalyzed method to access enantioenriched 1,1-aminoboronates from terminal alkynes, thereby simplifying the preparation of this family of biomedically important products. It is expected that this general blueprint to enantioselective reductive 1,1-difunctionalization will be applicable to array of different transformations, thereby allowing alkynes to serve as direct progenitors to chiral scaffolds of broad interest.
Methods
General procedure
To a flame-dried reaction tube equipped with a Teflon-coated magnetic stir bar were added CuBr (1.4 mg, 0.01 mmol, 0.05 equiv), (Ra)-DTBM-Segphos (14.2 mg, 0.012 mmol, 0.06 equiv), and LiOtBu (50.0 mg, 0.625 mmol, 3.125 equiv) under N2 atmosphere, and the reaction vessel was evacuated under high vacuum for 10 min then back-filled with N2. Anhydrous THF (1.0 mL) was added, and the mixture was stirred at room temperature for 10 min. Next, polymethylhydrosiloxane (PMHS, 111.3 mg, 0.5 mmol, 2.5 equiv) and HBdan (40.3 mg, 0.24 mmol, 1.2 equiv) were added successively, and the resultant mixture was stirred at room temperature for an additional 10 min. Finally, alkyne 1 (0.2 mmol, 1.0 equiv) and BzONR1R2 (0.24 mmol, 1.2 equiv) were added in succession. The reaction solution was allowed to stir at room temperature for 16 h, at which point it was filtered through a pad of celite and concentrated in vacuo. After removal of volatiles under reduced pressure, the resulting residue was purified by silica gel flash column chromatography to afford the desired product 4.
Data availability
Most of the data that support the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments:
Prof. Donna G. Blackmond, David E. Hill, and Prof. Jeffrey S. Bandar are acknowledged for helpful discussion regarding reaction mechanism.
Funding: This work was financially supported by Scripps Research, Pfizer, Inc., Bristol-Myers Squibb (Unrestricted Grant), and the National Institutes of Health (5R35GM125052-02; R35GM128779). We gratefully acknowledge the Nankai University College of Chemistry for a Summer Project Scholarship (T.-Z.Q.) and the China Scholarship Council for supporting a visiting studentship (X.W.). Calculations were performed at the Center for Research Computing at the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the NSF.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Competing interests
The authors declare no competing interests.
References:
- 1.Kolb HC, VanNieuwenhze MS & Sharpless KB Catalytic asymmetric dihydroxylation. Chem. Rev 94, 2483–2547 (1994). [Google Scholar]
- 2.McDonald RI, Liu G & Stahl SS Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev 111, 2981–3019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derosa J, Tran VT, van der Puyl VA & Engle KM Carbon–carbon π-bonds as conjunctive reagents in cross-coupling. Aldrichimica Acta 51, 21–32 (2018). [Google Scholar]
- 4.Sauer GS & Lin S An electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal. 8, 5175–5187 (2018). [Google Scholar]
- 5.Nelson HM, Williams BD, Miró J & Toste FD Enantioselective 1,1-Arylborylation of Alkenes: Merging Chiral Anion Phase Transfer with Pd Catalysis. J. Am. Chem. Soc 137, 3213–3216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann AM, Dorn SK, Smith KB, Logan KM & Brown MK Catalyst-Controlled 1,2- and 1,1-Arylboration of α-Alkyl Alkenyl Arenes. Angew. Chem. Int. Ed 58, 1719–1723 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirnot MT, Wang Y-M & Buchwald SL Copper hydride catalyzed hydroamination of alkenes and alkynes. Angew. Chem. Int. Ed 55, 48–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan AJ, Lalic G & Sadighi JP Coinage Metal Hydrides: Synthesis, Characterization, and Reactivity. Chem. Rev 116, 8318–8372 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Lin S, Wang L, Aminoleslami N, Lao Y, Yagel C & Sharma A A modular and concise approach to MIDA acylboronates via chemoselective oxidation of unsymmetrical geminal diborylalkanes: unlocking access to a novel class of acylborons. Chem. Sci 10, 4684–4691 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S-L & Buchwald SL Copper-catalysed selective hydroamination reactions of alkynes. Nat. Chem 7, 38–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L-J & Mankad NP Cu-catalyzed hydrocarbonylative C–C coupling of terminal alkynes with alkyl iodides. J. Am. Chem. Soc 139, 10200–10203 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Shi S-L, Niu D, Liu P & Buchwald SL Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 349, 62–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong MK & Lalic G Differential dihydrofunctionalization of terminal alkynes: synthesis of benzylic alkyl boronates through reductive three-component coupling. J. Am. Chem. Soc 141, 6173–6179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JCH, McDonald R & Hall DG Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nat. Chem 3, 894–899 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Jeon H & Yun J Regio- and enantioselective copper(I)-catalyzed hydroboration of borylalkenes: asymmetric synthesis of 1,1-diborylalkanes. Angew. Chem. Int. Ed 52, 3989–3992 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Niljianskul N, Zhu S & Buchwald SL Enantioselective synthesis of α-aminosilanes by copper-catalyzed hydroamination of vinylsilanes. Angew. Chem. Int. Ed 54, 1638–1641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa D, Hirano K & Miura M Asymmetric synthesis of α-aminoboronic acid derivatives by copper-catalyzed enantioselective hydroamination. J. Am. Chem. Soc 137, 15620–15623 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Hirano K & Miura M Synthesis of β-boryl-α-aminosilanes by copper-catalyzed aminoboration of vinylsilanes. Angew. Chem. Int. Ed 55, 14400–14404 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Han JT, Jang WJ, Kim N & Yun J Asymmetric synthesis of borylalkanes via copper-catalyzed enantioselective hydroallylation. J. Am. Chem. Soc 138, 15146–15149 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Torker S & Hoveyda AH Versatile homoallylic boronates by chemo-, SN2’-, diastereo- and enantioselective catalytic sequence of Cu−H addition to vinyl-B(pin)/allylic substitution. Angew. Chem. Int. Ed 56, 821–826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee S-L, Leighton J, Liang CY, Lostritto RT, McGuinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang Y-C & Pazdur R Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Cancer Res. 10, 3954–3964 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Matteson DS α-Amido boronic acids: a synthetic challenge and their properties as serine protease inhibitors. Med. Res. Rev 28, 233–246 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Touchet S, Carreaux F, Carboni B, Bouillon A & Boucher J-L Aminoboronic acids and esters: from synthetic challenges to the discovery of unique classes of enzyme inhibitors. Chem. Soc. Rev. 40, 3895–3914 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Rentsch A, Landsberg D, Brodmann T, Bülow L, Girbig A-K, Kalesse M, Synthesis and Pharmacology of Proteasome Inhibitors. Angew. Chem. Int. Ed 52, 5450–5488 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Ohmura T, Awano T & Suginome M Stereospecific suzuki–miyaura coupling of chiral α-(acylamino)benzylboronic esters with inversion of configuration. J. Am. Chem. Soc 132, 13191–13193 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Shiro T, Schuhmacher A, Jackl MK & Bode JW Facile synthesis of α-aminoboronic acids from amines and potassium acyltrifluoroborates (KATs) via trifluoroborate-iminiums (TIMs). Chem. Sci 9, 5191–5196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matteson DS, Sadhu KM & Lienhard GE (R)- l-Acetamido-2-phenylethaneboronic acid. A specific transition-state analogue for chymotrypsin. J. Am. Chem. Soc 103, 5241–5242 (1981). [Google Scholar]
- 28.He Z, Zajdlik A, St. Denis JD, Assem N & Yudin AK Boroalkyl group migration provides a versatile entry into α-aminoboronic acid derivatives. J. Am. Chem. Soc 134, 9926–9929 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Zajdlik A, Wang Z, Hickey JL, Aman A, Schimmer AD & Yudin AK α-Boryl isocyanides enable facile preparation of bioactive boropeptides. Angew. Chem. Int. Ed 52, 8411–8415 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Beenen MA, An C & Ellman JA Asymmetric copper-catalyzed synthesis of α-amino boronate esters from N-tert-butanesulfinyl aldimines. J. Am. Chem. Soc 130, 6910–6911 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Li C, Wang J, Barton LM, Yu S, Tian M, Peters DS, Kumar M, Yu AW, Johnson KA, Chatterjee AK, Yan M & Baran PS Decarboxylative borylation. Science 356, 1045–1054 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong K & Morken JP Catalytic enantioselective one-pot aminoborylation of aldehydes: a strategy for construction of nonracemic α-amino boronates. J. Am. Chem. Soc 135, 9252–9254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Cao P, Wang B, Jia T, Lou Y, Wang M & Liao J Copper(I)-catalyzed asymmetric pinacolboryl addition of N-boc-imines using a chiral sulfoxide–phosphine ligand. Org. Lett. 17, 2420–2423 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Schwamb CB, Fitzpatrick KP, Brueckner AC, Richardson HC, Cheong PH-Y & Scheidt KA Enantioselective synthesis of α-amidoboronates catalyzed by planar-chiral NHC-Cu(I) complexes. J. Am. Chem. Soc 140, 10644–10648 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Jang H, Zhugralin AR, Lee Y & Hoveyda AH Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC−Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J. Am. Chem. Soc 133, 7859–7871 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Jang WJ, Lee WL, Moon JH, Lee JY & Yun J Copper-catalyzed trans-hydroboration of terminal aryl alkynes: stereodivergent synthesis of alkenylboron compounds. Org. Lett 18, 1390–1393 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Bai T, Yang Y & Han C Isolation and characterization of hydrocarbon soluble NHC copper(I) phosphoranimide complex and catalytic application for alkynehydroboration reaction. Tetrahedron Lett. 58, 1523–1527 (2017). [Google Scholar]
- 38.Wang H, Yang JC & Buchwald SL CuH-catalyzed regioselective intramolecular hydroamination for the synthesis of alkyl-substituted chiral aziridines. J. Am. Chem. Soc 139, 8428–8431 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandar JS, Pirnot MT & Buchwald SL Mechanistic studies lead to dramatically improved reaction conditions for the Cu-catalyzed asymmetric hydroamination of olefins. J. Am. Chem. Soc 137, 14812–14818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y & Hartwig JF Mechanistic Studies of Copper-Catalyzed Asymmetric Hydroboration of Alkenes. J. Am. Chem. Soc 139, 12758–12772 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Radomkit S, Torker S, d Pozo J & Hoveyda AH Mechanism-based enhancement of scope and enantioselectivity for reactions involving a copper-substituted stereogenic carbon center. Nat. Chem 10, 99–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revunova K & Nikonov GI Base-catalyzed hydrosilylation of ketones and esters and insight into the mechanism. Chem. Eur. J 20, 839–845 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Laitar DS, Tsui EY & Sadighi JP Copper(I) β-Boroalkyls from Alkene Insertion: Isolation and Rearrangement. Organometallics 25, 2405–2408 (2018). [Google Scholar]
- 44.Jang WJ, Han JT & Yun J NHC-Copper-Catalyzed Tandem Hydrocupration and Allylation of Alkenyl Boronates. Synthesis 49, 4753–4758 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the data that support the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding author upon reasonable request.