Abstract
Acetaminophen is a widely used analgesic and antipyretic agent. It is also available in over the counter formulations, which has increased its wide use. There have been many studies to date that have aimed to evaluate the mechanism of the analgesic action of acetaminophen. Additional to the inhibition of the cyclooxygenase pathway in the central nervous system, the involvement of opioidergic, cannabinoidergic, dopaminergic, cholinergic, and nitrergic systems as well as the contribution of descending pain inhibitory systems like the bulbospinal serotonergic pathway has been proposed as possible mechanisms of the analgesic action of acetaminophen. In this review, we aimed to collect the data from studies revealing the contribution of the central serotonergic system and the role of central nervous system-located serotonergic receptor subtypes in the analgesic effect of acetaminophen. While doing this, we mainly focused on the research that has been performed in the last ten years and tried to link the previous data with the lately added results. In addition to serotonergic system involvement, we also reviewed the role of nitric oxide in the analgesic action of acetaminophen, especially with the new findings reported over the last decade.
Keywords: Acetaminophen, serotonin, pain, nitric oxide
INTRODUCTION
Although acetaminophen is one of the most commonly used medications, its exact analgesic mechanism of action is still a mystery. Not only has decreased prostaglandin production via cyclooxygenase (COX) enzyme inhibition (especially COX-2, and a central splice variant of COX-1, which is COX-3) been proposed as the primary mechanism of analgesic action,1,2,3 but also the contribution of cannabinoidergic4 and opioidergic5 systems has been shown. In addition to these main contributions, cholinergic6 and dopaminergic7 systems have also been shown to be involved in acetaminophen analgesia. Not only the above neuronal systems, but also the role of calcium channels (T-type voltage-gated calcium channels) has been proposed to be involved in the analgesic effect of acetaminophen.8
The aim of this review is to discuss the two other proposed mechanisms for the analgesic action of acetaminophen, namely the serotonergic system with its various receptor subtypes and nitric oxide (NO) systems. It is focused on the findings in the last decade regarding the contribution of these two systems in acetaminophen analgesia with the intention of comparing these new findings with the previous results and combining these novel findings.
The role of the central serotonergic system in acetaminophen analgesia
In 1991, the antinociceptive effect of acetaminophen in a formalin test was reduced following the chemical impairment of spinal serotonergic pathways (bulbospinal serotonergic pathway) by intrathecal 5,6-dihydroxytryptamine (5,6-DHT) administration in rats. That study indicated the contribution of the spinal serotonergic system in the analgesic action of acetaminophen.9 This was followed by the finding that the antinociceptive effect of systemic acetaminophen administration to rats was reduced by the administration of p-chlorophenylalanine, which was known to deplete the brain’s serotonin levels. Additionally, acetaminophen increased serotonin levels in the brain cortex and pons. As a result, these findings showed the involvement of the supra-spinal serotonergic system in acetaminophen analgesia.10
These previous results have been confirmed and also expanded with some additional studies performed in the last decade. In animal studies, central serotonergic system impairment with intrathecal and intracerebroventricular 5,7-DHT administration and assessment of brain serotonin levels were the most commonly used methods. These methods enabled the evaluation of serotonergic system involvement in acetaminophen analgesia. Among these studies, some differences were observed in the effects of chemical destruction of the central serotonergic system on the analgesic effect of acetaminophen between different animal pain models and doses of acetaminophen. Recent results are summarized in Table 1.
Table 1. Effect of the deterioration of the bulbospinal serotonergic pathway with 5,7-dihydroxytryptamine (5,7 DHT) in the antinociceptive effect of acetaminophen in different pain models in some studies performed in the last decade.
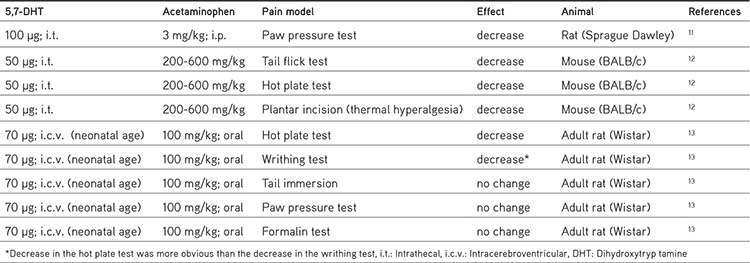
Acetaminophen-induced serotonin increases have also been confirmed in recent studies. Intraperitoneal acetaminophen administration (400 mg/kg) induced approximately 40% and 75% increases in serotonin levels in the pons and frontal cortex, respectively. These increases in serotonin levels have been found to be related to central (hydroxytryptamine) 5-HT2 receptors as well as opioid receptors (µ1 and κ).5 These data were confirmed in a study by Vijayakaran et al.14 in which acetaminophen (400 mg/kg; oral) caused increases in serotonin levels in the rat frontal cortex and brain stem. Serotonin increases were observed not only following acute applications but also following chronic acetaminophen applications. Subcutaneous acetaminophen in 10 and 50 mg/kg doses was administered to 3-month-old rats and serotonin levels in the prefrontal cortex, hippocampus, hypothalamus, and striatum were analyzed. Serotonin levels increased after the 10 mg/kg acetaminophen dose (not with 50 mg/kg) in the prefrontal cortex but not in the other brain regions analyzed in this study. Additionally, 5-HIAA levels decreased in the hypothalamus and striatum.15 All these recent studies have confirmed the idea that systemic acetaminophen administration increases serotonin levels in the brain cortex and brain stem (pons) and meet at a common point, which is that acute and chronic systemic administration of acetaminophen induces changes in central serotonergic neurotransmission. It can be concluded that, despite the involvement of 5-HT2-serotonergic and opioid receptors in acetaminophen-induced serotonin increases in some brain regions,5 apparently the exact mechanism (alterations in serotonin metabolism, release, or uptake) is still not clear and needs to be clarified.
The signs of serotonergic involvement in acetaminophen-induced analgesia in humans were also studied. However, despite some supportive results, serotonergic contribution seems still doubtful in human studies due to the challenges in studying pain in humans. Controversial data have been observed between the results in healthy volunteers and patients with pain.16 The findings of these studies will be discussed in the following sections.
Some metabolites of acetaminophen and the central serotonergic system
Acetaminophen, following its systemic administration, has been shown to be biotransformed to an amine compound, p-aminophenol, which occurs mainly in the liver. Enzymatic conversion of p-aminophenol to N-arachidonoyl-phenolamine (AM404), which is catalyzed by fatty acid amide hydrolase (FAAH) enzyme with the conjugation of arachidonic acid, occurs in the brain, spinal cord, and dorsal root ganglia.17 AM404 metabolite of acetaminophen has been shown to activate TRPV1 (transient receptor potential vanilloid-1, capsaicin receptor) channels and act as a CB1 (cannabinoid receptor type-1) ligand.17,18 Mallet et al.11 showed that CB1 receptors are vital for the analgesic action of orally administered acetaminophen, because CB1 receptor antagonism as well as gene deletion totally inhibited the analgesic action of acetaminophen in various pain models, i.e. thermal, mechanical, and chemical (formalin) painful stimuli in rats. CB1 receptor related activation of the descending serotonergic pathway has been suggested as the following step, because the antinociceptive effect of systemic acetaminophen was negated following the chemical impairment of the spinal serotonergic pathway. As a CB1 receptor ligand, AM404 metabolite of acetaminophen has been claimed to be responsible for this action. Spinal 5HT1A and 5HT3/4 receptors have been shown to contribute at the spinal cord level to the analgesic action of acetaminophen eventually. On the other hand, Ruggieri et al.5 claimed that AM404 can only partially contribute to the analgesic action of systemically administered acetaminophen, depending on the fact that the observed analgesic action of AM404 was approximately half of the analgesic action of acetaminophen. This AM404 contribution seems to be related to the central 5HT3 receptors, but not to 5HT1A or 5HT2 receptor subtypes. Interestingly, a central 5HT2 receptor subtype, but not 5HT3 or 5HT1A, has been found to be involved in the analgesic action of acetaminophen depending on the dose-dependent inhibition of acetaminophen’s analgesic action with systemic ketanserin. Another important finding of their study was the increase in serotonin levels in the pons and frontal cortex following the administration of acetaminophen, but not with AM404. All these results of this study pointed out that acetaminophen and its metabolite AM404 have both analgesic actions but the mechanisms that play a role in this analgesic action differ between these two compounds. These differences regarding the contribution of AM404 to acetaminophen analgesia and the involvement of different serotonergic receptor subtypes may be related to the use of different types and different administration routes of serotonergic receptor subtype antagonists as well as the dose of acetaminophen, which was different in the two studies.
Finally, Barrière et al.19 showed the contribution of a descending serotonergic antinociceptive pathway in the analgesic effect of 4-aminophenol, another metabolite of acetaminophen as mentioned above. The analgesic effect of intraperitoneally administered 4-aminophenol was reported to depend on AM404 formation in the brain, which is catalyzed by FAAH enzyme, and TRPV1 and CB1 receptor stimulation-induced descending antinociceptive serotonergic system activation and spinal 5-HT3 and 5-HT1A serotonergic receptor subtypes were claimed to play an important role.
As a result, studies showed that not only acetaminophen itself but also its metabolites like AM404 and 4-aminophenol may play an important role in the analgesic action of acetaminophen. It can be concluded that AM404 metabolite contributes to the analgesic action of systemic acetaminophen to some extent and activates the descending serotonergic antinociceptive pathway via the contribution of central TRPV1 and CB1 receptors. Spinal serotonergic receptor subtypes eventually play a role in the antinociceptive action, which may act differently to acetaminophen and its metabolites.
Role of 5-HT1 receptors
It is known that 5-HT1A and 5-HT1B serotonergic receptor subtypes are largely located at the supra-spinal level: 5-HT1A on the cell bodies and dendrites of serotonergic neurons and 5-HT1B mainly on the axon terminals. Both 5-HT1A and 5-HT1B serotonergic receptor subtypes have important effects on extracellular serotonin levels via modulation of nerve firing mainly for 5-HT1A receptors and by modification of serotonin release for mainly 5-HT1B serotonergic receptor subtypes.20 Blockade of 5-HT1A receptor subtypes has been shown to enhance the extracellular levels of serotonin.21 Involvement of 5-HT1 serotonergic receptor subtypes in the analgesic effect of acetaminophen has been studied in various studies with different animal pain models and with different ligands for 5-HT1A and 5-HT1B receptor subtypes. Incompatible results were obtained in earlier studies regarding the contribution of 5-HT1 serotonergic receptors in the analgesic effect of acetaminophen. An earlier study showed that pre-administered WAY-100635 (5HT1A receptor antagonist, 10 µg/rat; intrathecal) did not change the analgesic effect of intravenous acetaminophen (200 mg/kg) in a rat paw pressure test.22However; intrathecal administration of WAY-100635 (40 µg/rat) has been shown to block acetaminophen analgesia (3 mg/kg, i.p.) in both phase I and II of a rat formalin test.11 In addition, intraperitoneal administration of NAN-190 (5-HT1 serotonergic receptor antagonist, 1-5 mg/kg) did not change the acetaminophen analgesia in hot plate or paw pressure tests and did not show any blockage of acetaminophen-induced serotonin increases in the frontal cortex and pons.5 However, it should not be underestimated that NAN-190 could also block the α2-adrenergic receptors and this finding can raise some suspicions regarding NAN-190 when using it as a specific 5-HT1A receptor antagonist.23 Interestingly, another earlier study showed that systemic administration of 5-HT1A and 5-HT1B receptor antagonists enhanced the acetaminophen analgesic action, whereas stimulation of the same receptor subtypes blocked the acetaminophen analgesia in a hot plate test.24 This was in close agreement with the findings reported by Sandrini et al.25 in which systemic administration of CP 93129 (5-HT1B receptor agonist) prevented acetaminophen analgesia in hot plate and paw pressure tests. These two findings suggested that increased serotonin release and/or enhanced firing of serotonergic nerves, which liberate themselves from the suppressing effects of 5-HT1A and 5-HT1B receptors, augment the antinociceptive action of acetaminophen. The findings of a recent study also were in good accordance with those previous results. Oral buspirone as a 5HT1 serotonergic receptor agonist blocked the antinociceptive action of intraperitoneal acetaminophen (200 mg/kg) in a hot plate test and in the early phase of a formalin test in mice.26
As a result, when taken together it can be concluded that despite some negative results 5-HT1A and 5-HT1B serotonergic receptor subtypes are likely to contribute to acetaminophen analgesia. However, characteristics of this contribution seem to depend on the ligands and animal pain models tested as well as the location (spinal/supra-spinal or presynaptic/postsynaptic) of 5-HT1 serotonergic receptors and still needs to be evaluated.
Role of 5-HT2 receptors
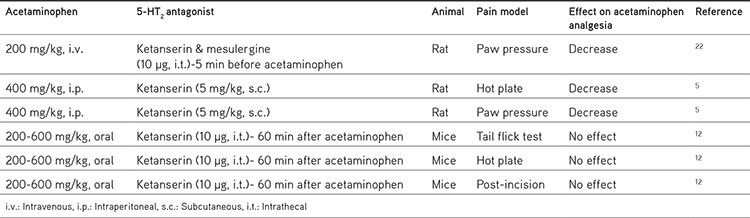
The possible involvement of 5-HT2 serotonergic receptor subtypes in the analgesic effect of acetaminophen has also been examined in recent studies. Ruggieri et al.5 showed a statistically significant reduction in the antinociceptive action of acetaminophen when ketanserin (5 mg/kg; subcutaneous) was administered systemically before acetaminophen (400 mg/kg; intraperitoneal), whereas it did not change the antinociceptive effect of AM404 in hot plate and paw pressure tests. In another study, Dogrul et al.12 showed that intrathecally administered ketanserin (10 µg) did not change the antinociceptive effect of acetaminophen (200-600 mg/kg; oral) in hot plate or tail flick tests or in thermal hyperalgesia after incision of the hind paw. This recent study seems to reveal findings opposite those from the study by Courade et al.22 due to the fact that intrathecally administered ketanserin (5-HT2A antagonist) as well as mesulergine (5-HT2C antagonist) decreased vocalization thresholds, which had been increased by intravenously administered propacetamol (water soluble prodrug form of acetaminophen). The differences in the study designs, like the animals (mice/rat), the animal pain models (tail flick-hot plate/paw pressure test) that were used, and the timing of ketamine administration (before or after acetaminophen), between these two studies should be considered. Even so, when these studies are considered together, although the involvement of spinally located 5-HT2 receptors in acetaminophen analgesia needs to be elucidated, it can be speculated that supra-spinal 5-HT2 serotonergic receptors may contribute to the analgesic effects of acetaminophen. Additionally, 5-HT2 receptors are likely to be involved in the antinociceptive effect of acetaminophen, not in the antinociceptive effect of its metabolite, AM404. Supporting this assumption, systemic ketanserin has also been shown to block acetaminophen-induced serotonin increases in the frontal cortex and pons.5 Acetaminophen administration has also been shown to increase the serotonin levels in supra-spinal structures and led to a down-regulation of 5-HT2A receptor subtypes in the frontal cortex and brain stem.14 In that study, the authors stated that an increase in serotonin release triggered by acetaminophen caused down-regulation of 5-HT2A receptors related to the long duration of stimulus by serotonin. This assertion was supported by the study by Srikiatkhachorn et al.27 claiming that 5-HT2A receptor down-regulation is important for the analgesic effect of acetaminophen. Thus, it may be speculated that supra-spinal located (most likely post-synaptic) 5-HT2 receptor stimulation by serotonin, which is enhanced following acetaminophen administration, contributes to the analgesic action of acetaminophen. Recent results are summarized in Table 2.
Table 2. Some studies on the effect on the role of 5-HT2 receptors on the analgesic effect of acetaminophen in different pain models.
Role of 5-HT3 receptors
The contribution of 5-HT3 receptors in the analgesic effect of acetaminophen has been tested in various animal pain models as well as in human studies. Different 5-HT3 receptor antagonists, like granisetron, ondansetron, and tropisetron, have been used to study the interaction of these receptor subtypes in acetaminophen analgesia. In 1996, the indirect contribution of spinal 5-HT3 serotonergic receptor subtypes was pointed out based on the findings of research. In that research, it was shown that spinal tropisetron totally inhibited the antinociceptive action of systemically and spinally administered acetaminophen in a rat paw pressure test.28 This finding had also been confirmed in inflammatory pain models.29 In the last decade, a study by Mallet et al.11 showed that intrathecal application of 0.5 µg of tropisetron pre-treatment blocked the increased vocalization thresholds by systemic administration of acetaminophen, which was in good accordance with the previous findings. However, studies with the other tested 5-HT3 receptor antagonists revealed mostly opposite results. Ondansetron administration (systemic as well as intrathecal) was shown not to alter the analgesic effect of acetaminophen significantly.25,30 Recent studies also confirmed this finding. Systemic ondansetron pretreatment (2 mg/kg; subcutaneous) did not alter the effect of acetaminophen in hot plate or paw pressure tests in rats,5 in good accordance with the finding that spinally administered ondansetron caused no change in the effect of orally administered acetaminophen-induced analgesia in hot plate or tail flick tests or in thermal hyperalgesia in a plantar-incision model.12 An exception is a study in which acetaminophen-induced analgesia was blocked by ondansetron in a mouse formalin test.31 Among these studies, differential involvement of 5-HT3 receptors in acetaminophen and AM404-induced analgesia (similar to ketanserin) has been shown in which ondansetron administration was able to block the analgesic effect of AM404.5 Another 5-HT3 receptor antagonist, granisetron, caused no significant changes in the analgesic effect of acetaminophen in a paw pressure test.22,30 As a result, when the animal studies over the last ten years are considered together with the previous data, we can conclude that administration of ondansetron and granisetron is not likely to alter the effect of acetaminophen-induced analgesia, whereas tropisetron inhibits the analgesic effect of acetaminophen in various animal pain models. These different contributions can be explained by the differences between these antagonists regarding their pharmacokinetical properties (especially primary responsible cytochrome p450 system in the liver for their metabolism), 5-HT3 receptor binding affinities, selectivity and specificity on 5-HT3 receptors, and their duration of action.32,33 However, another issue raised at this point was the examination of 5-HT3 receptor subtype contribution in the interaction between tropisetron- and acetaminophen-induced analgesia due to the finding that acetaminophen analgesia was not altered by other 5-HT3 receptor antagonists like ondansetron and granisetron. Additionally, spinal 5-HT3 receptor antisense oligodeoxynucleotide pre-treatment, which aimed to decrease the synthesis of 5-HT3 receptors, did not inhibit the antinociceptive action of acetaminophen.30 As a result, it has been started to be speculated that not the spinal 5-HT3 receptor subtypes but another tropisetron-sensitive receptor may play a role in the analgesic action of acetaminophen.30 Additionally, it has been indicated that tropisetron can also show affinity to other receptors like α7-nicotinic receptor subtypes.30,34 When all these are considered together, the role of central 5-HT3 serotonergic receptors in the analgesic effect of acetaminophen seems to be clarified with further studies.
The contribution of 5-HT3 receptors in the analgesic effect of acetaminophen has also been studied in humans using tropisetron, granisetron, and ondansetron. These studies had two important goals: to reveal the involvement of 5-HT3 serotonergic receptors in acetaminophen analgesia in humans and evaluate the possible drug interaction between 5-HT3 blockers and acetaminophen, which are used in cancer patients together for vomiting and pain management, respectively. The first report showed blockage of the analgesic effect of acetaminophen (1 g, oral) when administered after tropisetron (5 mg, i.v.) or granisetron (3 mg, i.v.) in healthy volunteers tested with electrically stimulated pain.35 The results of another study revealed that descending serotonergic inhibitory pathway stimulation by acetaminophen contributed to acetaminophen analgesia in healthy volunteers where central 5-HT3 receptors were involved.36 These data were confirmed by a randomized, double-blind, and placebo-controlled study conducted in 16 healthy volunteers in which the combination of 1 g intravenous acetaminophen with 5 mg of tropisetron exerted no analgesic action in electrically stimulated pain. In that study, tropisetron and acetaminophen alone both led to analgesic actions.37 The analgesic action of tropisetron administration alone was also confirmed by Tiippana et al.16 in healthy volunteers. Due to the fact that co-administration of acetaminophen with tropisetron in healthy volunteers did not lead to statistically significant changes in the blood levels of acetaminophen,35,37 it has been claimed that the interaction between acetaminophen and tropisetron was pharmacodynamic. However, studies performed in post-operative patients revealed confusing results that were not totally parallel with the results of healthy volunteers. Ondansetron (4 mg) did not change the analgesic action of acetaminophen in women who underwent laparoscopic hysterectomy.38 In a study performed in 36 patients who underwent ear surgery, those receiving a combination of tropisetron and acetaminophen reported higher pain scores but the increase was not statistically significant. However, patients who received tropisetron and acetaminophen needed more rescue analgesic agent.39 A randomized, double-blinded study showed that ondansetron (8 mg) reduced the analgesic effect of acetaminophen (1 g) in patients who had undergone abdominal hysterectomy; however, this reduction was in a short period of time.40
The results of those human studies indicate that there is a questionable interaction between acetaminophen analgesia and 5-HT3 blockers due to some conflicting results. Those conflicting results, showing a lack of obvious interaction, were mainly related to post-operative pain conditions.38,39 However, in healthy volunteers, the interaction between acetaminophen and 5-HT3 blockers (tropisetron and granisetron) seems more obvious and is likely to be a pharmacodynamic interaction.35,36,37 Apparently, studies with larger patient populations with different painful conditions are needed to clarify the interaction between 5-HT3 blockers and acetaminophen in humans.
Role of 5-HT7 receptors
5-HT7 receptors are G protein-coupled receptors linked with adenylyl cyclase and detected in the central nervous system regions that are involved in pain transmission, like the cerebral cortex, the thalamus, and the superficial lamina of the dorsal horn.41 Despite the fact that 5-HT7 receptors are one of the serotonergic receptor subtypes that have been studied less compared to the other subtypes (5-HT1, 5-HT2, and 5-HT3 subtypes),41 some studies pointed out the contribution of these receptors to the antinociceptive action of acetaminophen in the last decade. Dogrul et al.12 used SB-269970 as a selective 5-HT7 receptor antagonist to evaluate the role of these receptors in acetaminophen analgesia and administered intrathecally (10 µg) after the oral administration of 200-600 mg/kg acetaminophen in mice. Intrathecal administration of SB-269970 blocked the antinociceptive action of acetaminophen in tail flick and hot plate tests. Similarly, intrathecal SB-269970 blocked the antihyperalgesic action of oral acetaminophen in a plantar-incision model. This study was the first to reveal the contribution of spinal 5-HT7 receptors in the antinociceptive action of acetaminophen. A following study showed that an intrathecally administered lower dose of SB-269970 (3 µg) was again successful in reversing the analgesic action of systemic acetaminophen in phase II of a formalin test in mice. This finding was important to confirm the contribution of spinal 5-HT7 receptors in acetaminophen analgesia, but also revealed reduction in the reversing effect of SB-269970 administration on acetaminophen analgesia in mice lacking adenosine type-1 receptors, which additionally indicated a strong interaction between the adenosinergic system and 5-HT7 receptors in the analgesic action of acetaminophen.42
The role of nitric oxide in acetaminophen analgesia
NO is widely accepted as an important messenger molecule and neurotransmitter in the central nervous system that is involved in various physiological functions.43,44 NO plays important roles in pain transmission, either inducing hyperexcitability leading to hyperalgesia or exerting antinociceptive actions.45,46,47
Björkman et al.48 in 1994 showed that suppression of N-methyl-D-aspartate and substance P-induced pain related behaviors with acetaminophen administration was reversed by L-arginine administration to rats. Their study pointed out the involvement of neuronal NO systems in the analgesic action of acetaminophen. Additionally and in good accordance with that study, neuronal NO synthase was found to be involved in the analgesic effect of acetaminophen when acetaminophen was used in lower doses (especially with 100 mg/kg, oral) in the Randall-Selitto pain model, whereas both neuronal and inducible NO synthases were found to be involved in the analgesic action of acetaminophen in lower doses (especially 50 and 100 mg/kg, oral) in a writhing test. However, the involvement of NO systems was weak or nonexistent with the maximal doses of acetaminophen.49 It has also been shown that acetaminophen inhibited induced NO synthesis in spinal cord tissue.50 As a result, it can be concluded that NO systems are involved in acetaminophen analgesia and it is more likely that suppression of the central NO systems contributes to the central analgesic mechanisms of acetaminophen.
When focusing on the findings related to the interaction between acetaminophen and NO in the last decade, it might be appropriate not to underestimate the recent studies related to NO-acetaminophen (NCX-701). NO-acetaminophen is a novel compound with a combination of NO releasing moiety with acetaminophen.51 This novel compound has been shown to exert enhanced analgesic activity compared to the parent compound in non-inflamed, acetic-acid induced, and inflammatory pain models51,52,53 and was also analgesic in arthritis-related pain.54 Additionally, NO-acetaminophen had considerable anti-inflammatory activity and less hepatotoxic potential compared to acetaminophen.51,52 The mechanism of action of NO-acetaminophen has been suggested to be different from that of acetaminophen itself. It has been proposed that although NO-acetaminophen and acetaminophen may share some common mechanisms like COX inhibition, the sustained release of low amounts of NO when combined with specific pharmacological actions of acetaminophen may add different but not clearly understood pharmacological properties. Inhibition of the wind-up phenomenon indicating a mechanism of action in the central nervous system level, more probably in the spinal cord, and reduction in the amounts of some cytokines in the peripheral tissues has been proposed.53,55
Additional to the above accumulated data related to the promising effects of NO-acetaminophen, the antinociceptive effect of intravenously as well as intrathecally administered NO-acetaminophen has also been shown in a neuropathic pain model (partial ligation of the sciatic nerve) in rats, where acetaminophen alone was ineffective. In good accordance with the previous speculations, the spinal cord was claimed to be the anatomic region involved in this antihyperalgesic action of NO-acetaminophen. Addition of gabapentin to NO-acetaminophen showed a synergistic effect.56 Similar to gabapentin, lowered doses of NO-acetaminophen also have been shown to enhance the analgesic effect of an α2-adrenergic receptor agonist, medetomidine, when combined with the sub-effective doses of NO-acetaminophen in a carrageenan-induced inflammatory model in rats.57 These two recent studies with NO-acetaminophen pointed out the beneficial effects of this novel acetaminophen compound in neuropathic and inflammatory pain conditions. Additionally, it is important to note that NO-acetaminophen was effective in conditions in which acetaminophen alone did not show analgesic action or NO-acetaminophen enhanced the analgesic potency of α2-adrenergic receptor agonist when acetaminophen alone did not. As a result, these studies showed that NO-acetaminophen can be an effective analgesic in neuropathic and inflammatory painful conditions and also can lead to synergistic actions when used in combination with gabapentin or α2-adrenergic receptor agonists in related painful conditions.
CONCLUSION
Findings in the last decade related to the contribution of the serotonergic system and NO in the analgesic effect of acetaminophen confirmed and expanded the involvement of these systems in acetaminophen analgesia. Due to the finding that direct binding of acetaminophen has not been shown with 5-HT1, 5-HT2, or 5-HT3 serotonergic receptor subtypes,58 interactions between these serotonergic receptors and acetaminophen are likely to be indirect. Recent studies confirmed bulbospinal serotonergic pathway involvement in acetaminophen analgesia and acetaminophen-induced serotonin increases in the central nervous system. The metabolite of acetaminophen, AM404, contributes to the analgesic effect of acetaminophen; however, the serotonergic receptor subtypes that contribute to the antinociceptive actions of acetaminophen and AM404 may be different. The involvement of 5-HT1 receptors in acetaminophen analgesia is still not clear due to the conflicting results and requires to be evaluated with further studies. Despite the conflicting data, the contribution of 5-HT2 receptors has been shown in acetaminophen analgesia (but not in AM404), and the localization is most likely to be the supra-spinal centers of the central nervous system. In animal studies, the blockage of acetaminophen analgesia with tropisetron is more obvious compared to ondansetron and granisetron. It seems that the speculation regarding the involvement of tropisetron-sensitive receptors instead of 5-HT3 receptors in the analgesic action of acetaminophen is still valid and waiting to be confirmed and clarified with further studies. Recent studies showed the contribution of 5-HT7 serotonergic receptor subtypes as well. Despite the fact that there are some conflicting results between the studies in volunteers and post-operative patients, an important number of human studies expanded the data regarding the contribution of serotonergic receptors. Although there were not many additional findings related to the contribution of NO systems in the antinociceptive action of acetaminophen, the latest findings expanded the beneficial analgesic effects of the NO releasing derivative of acetaminophen, NO-acetaminophen.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Botting R, Ayoub SS. COX-3 and the mechanism of action of acetaminophen/acetaminophen. Prostaglandins Leukot Essent Fatty Acids. 2005;72:85–87. doi: 10.1016/j.plefa.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BJ. Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth. 2008;18:915–921. doi: 10.1111/j.1460-9592.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- 4.Păunescu H, Coman OA, Coman L, Ghiţă I, Georgescu SR, Drăghia F, Fulga I. Cannabinoid system and cyclooxygenases inhibitors. J Med Life. 2011;4:11–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggieri V, Vitale G, Pini LA, Sandrini M. Differential involvement of opioidergic and serotonergic systems in the antinociceptive activity of N-arachidonoyl-phenolamine (AM404) in the rat: comparison with paracetamol. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:219–229. doi: 10.1007/s00210-008-0284-9. [DOI] [PubMed] [Google Scholar]
- 6.Tariq SA, Khan H, Popalzai AJ, Niazi IU. Attenuation of erythrocytic acetyl cholinesterase by acetaminophen and chloroquine: evidence in an in vitro study. Pak J Pharm Sci. 2014;27:261–264. [PubMed] [Google Scholar]
- 7.Bhagyashree A, Manikkoth S, Sequeira M, Nayak R, Rao SN. Central dopaminergic system plays a role in the analgesic action of acetaminophen: Preclinical evidence. Indian J Pharmacol. 2017;49:21–25. doi: 10.4103/0253-7613.201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerckhove N, Mallet C, François A, Boudes M, Chemin J, Voets T, Bourinet E, Alloui A, Eschalier A. Ca(v)3.2 calcium channels: the key protagonist in the supraspinal effect of paracetamol. Pain. 2014;155:764–772. doi: 10.1016/j.pain.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Tjølsen A, Lund A, Hole K. Antinociceptive effect of paracetamol in rats is partly dependent on spinal serotonergic systems. Eur J Pharmacol. 1991;193:193–201. doi: 10.1016/0014-2999(91)90036-p. [DOI] [PubMed] [Google Scholar]
- 10.Pini LA, Sandrini M, Vitale G. The The antinociceptive action of paracetamol is associated with changes in the serotonergic system in the rat brain. Eur J Pharmacol. 1996;308:31–40. doi: 10.1016/0014-2999(96)00261-0. [DOI] [PubMed] [Google Scholar]
- 11.Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, Libert F, Eschalier A. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139:190–200. doi: 10.1016/j.pain.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Dogrul A, Seyrek M, Akgul EO, Cayci T, Kahraman S, Bolay H. Systemic paracetamol-induced analgesic and antihyperalgesic effects through activation of descending serotonergic pathways involving spinal 5-HT₇ receptors. Eur J Pharmacol. 2012;677:93–101. doi: 10.1016/j.ejphar.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Muchacki R, Szkilnik R, Malinowska-Borowska J, Żelazko A, Lewkowicz Ł, Nowak PG. Impairment in Pain Perception in Adult Rats Lesioned as Neonates with 5.7-Dihydroxytryptamine. Adv Clin Exp Med. 2015;24:419–427. doi: 10.17219/acem/23362. [DOI] [PubMed] [Google Scholar]
- 14.Vijayakaran K, Kesavan M, Kannan K, Sankar P, Tandan SK, Sarkar SN. Arsenic decreases antinociceptive activity of acetaminophen: possible involvement of serotonergic and endocannabinoid receptors. Environ Toxicol Pharmacol. 2014;38:397–405. doi: 10.1016/j.etap.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Blecharz-Klin K, Piechal A, Pyrzanowska J, Joniec-Maciejak I, Kiliszek P, Widy-Tyszkiewicz E. Paracetamol--the outcome on neurotransmission and spatial learning in rats. Behav Brain Res. 2013;253:157–164. doi: 10.1016/j.bbr.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Tiippana E, Hamunen K, Kontinen V, Kalso E. The effect of paracetamol and tropisetron on pain: experimental studies and a review of published data. Basic Clin Pharmacol Toxicol. 2013;112:124–131. doi: 10.1111/j.1742-7843.2012.00935.x. [DOI] [PubMed] [Google Scholar]
- 17.Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 18.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- 19.Barrière DA, Mallet C, Blomgren A, Simonsen C, Daulhac L, Libert F, Chapuy E, Etienne M, Högestätt ED, Zygmunt PM, Eschalier A. Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain. PLoS One. 2013;8:e70690. doi: 10.1371/journal.pone.0070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiger M, Varnäs K, Okubo Y, Lundberg J. The 5-HT(1B) receptor - a potential target for antidepressant treatment. Psychopharmacology (Berl). 2018;235:1317–1334. doi: 10.1007/s00213-018-4872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreshfield LJ, Wong DT, Perry KW, Engleman EA. Enhancement of fluoxetine-dependent increase of extracellular serotonin (5-HT) levels by (-)-pindolol, an antagonist at 5-HT1A receptors. Neurochem Res. 1996;21:557–562. doi: 10.1007/BF02527753. [DOI] [PubMed] [Google Scholar]
- 22.Courade JP, Chassaing C, Bardin L, Alloui A, Eschalier A. 5-HT receptor subtypes involved in the spinal antinociceptive effect of acetaminophen in rats. Eur J Pharmacol. 2001;432:1–7. doi: 10.1016/s0014-2999(01)01464-9. [DOI] [PubMed] [Google Scholar]
- 23.Foong JP, Bornstein JC. 5-HT antagonists NAN-190 and SB 269970 block alpha2-adrenoceptors in the guinea pig. Neuroreport. 2009;20:325–330. doi: 10.1097/WNR.0b013e3283232caa. [DOI] [PubMed] [Google Scholar]
- 24.Roca-Vinardell A, Ortega-Alvaro A, Gibert-Rahola J, Micó JA. The role of 5-HT1A/B autoreceptors in the antinociceptive effect of systemic administration of acetaminophen. Anesthesiology. 2003;98:741–747. doi: 10.1097/00000542-200303000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Sandrini M, Pini LA, Vitale G. Differential involvement of central 5-HT1B and 5-HT3 receptor subtypes in the antinociceptive effect of acetaminophen. Inflamm Res. 2003;52:347–352. doi: 10.1007/s00011-003-1185-5. [DOI] [PubMed] [Google Scholar]
- 26.Karandikar YS, Belsare P, Panditrao A. Effect of drugs modulating serotonergic system on the analgesic action of acetaminophen in mice. Indian J Pharmacol. 2016;48:281–285. doi: 10.4103/0253-7613.182874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srikiatkhachorn A, Tarasub N, Govitrapong P. Acetaminophen-induced antinociception via central 5-HT(2A) receptors. Neurochem Int. 1999;34:491–498. doi: 10.1016/s0197-0186(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 28.Pelissier T, Alloui A, Caussade F, Dubray C, Cloarec A, Lavarenne J, Eschalier A. Paracetamol exerts a spinal antinociceptive effect involving an indirect interaction with 5-hydroxytryptamine3 receptors: in vivo and in vitro evidence. J Pharmacol Exp Ther. 1996;278:8–14. [PubMed] [Google Scholar]
- 29.Alloui A, Chassaing C, Schmidt J, Ardid D, Dubray C, Cloarec A, Eschalier A. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur J Pharmacol. 2002;443:71–77. doi: 10.1016/s0014-2999(02)01578-9. [DOI] [PubMed] [Google Scholar]
- 30.Libert F, Bonnefont J, Bourinet E, Doucet E, Alloui A, Hamon M, Nargeot J, Eschalier A. Acetaminophen: a central analgesic drug that involves a spinal tropisetron-sensitive, non-5-HT(3) receptor-mediated effect. Mol Pharmacol. 2004;66:728–734. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 31.Girard P, Pansart Y, Coppé MC, Niedergang B, Gillardin JM. Modulation of paracetamol and nefopam antinociception by serotonin 5-HT(3) receptor antagonists in mice. Pharmacology. 2009;83:243–246. doi: 10.1159/000207448. [DOI] [PubMed] [Google Scholar]
- 32.Gan TJ. Selective serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting: are they all the same? CNS Drugs. 2005;19:225–238. doi: 10.2165/00023210-200519030-00004. [DOI] [PubMed] [Google Scholar]
- 33.Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2006;19:606–611. doi: 10.1097/01.aco.0000247340.61815.38. [DOI] [PubMed] [Google Scholar]
- 34.Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001;11:319–321. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- 35.Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79:371–378. doi: 10.1016/j.clpt.2005.12.307. [DOI] [PubMed] [Google Scholar]
- 36.Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84:47–51. doi: 10.1038/sj.clpt.6100403. [DOI] [PubMed] [Google Scholar]
- 37.Bandschapp O, Filitz J, Urwyler A, Koppert W, Ruppen W. Tropisetron blocks analgesic action of acetaminophen: a human pain model study. Pain. 2011;152:1304–1310. doi: 10.1016/j.pain.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Jokela R, Ahonen J, Seitsonen E, Marjakangas P, Korttila K. The influence of ondansetron on the analgesic effect of acetaminophen after laparoscopic hysterectomy. Clin Pharmacol Ther. 2010;87:672–678. doi: 10.1038/clpt.2009.281. [DOI] [PubMed] [Google Scholar]
- 39.Pickering G, Faure M, Commun F, de Boissy EC, Roche G, Mom T, Simen E, Dubray C, Eschalier A, Gilain L. Tropisetron and paracetamol association in post-operative patients. Fundam Clin Pharmacol. 2012;26:432–437. doi: 10.1111/j.1472-8206.2011.00933.x. [DOI] [PubMed] [Google Scholar]
- 40.Koyuncu O, Leung S, You J, Oksar M, Turhanoglu S, Akkurt C, Dolapcioglu K, Sahin H, Sessler DI, Turan A. The effect of ondansetron on analgesic efficacy of acetaminophen after hysterectomy: A randomized double blinded placebo controlled trial. J Clin Anesth. 2017;40:78–83. doi: 10.1016/j.jclinane.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 41.Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol Ther. 2011;129:120–148. doi: 10.1016/j.pharmthera.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Reid AR, Sawynok J. Antinociception by systemically-administered acetaminophen (acetaminophen) involves spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors. Neurosci Lett. 2013;536:64–68. doi: 10.1016/j.neulet.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 43.Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- 44.Džoljić E, Grbatinić I, Kostić V. Why is nitric oxide important for our brain? Funct Neurol. 2015;30:159–163. doi: 10.11138/FNeur/2015.30.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 46.Lothe A, Li P, Tong C, Yoon Y, Bouaziz H, Detweiler DJ, Eisenach JC. Spinal cholinergic alpha-2 adrenergic interactions in analgesia and hemodynamic control: role of muscarinic receptor subtypes and nitric oxide. J Pharmacol Exp Ther. 1994;270:1301–1306. [PubMed] [Google Scholar]
- 47.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 48.Björkman R, Hallman KM, Hedner J, Hedner T, Henning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain. 1994;57:259–264. doi: 10.1016/0304-3959(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 49.Bujalska M, Gumułka WS. Effect of cyclooxygenase and NO synthase inhibitors on antinociceptive action of acetaminophen. Pol J Pharmacol. 2001;53:341–350. [PubMed] [Google Scholar]
- 50.Godfrey L, Bailey I, Toms NJ, Clarke GD, Kitchen I, Hourani SM. Paracetamol inhibits nitric oxide synthesis in murine spinal cord slices. Eur J Pharmacol. 2007;562:68–71. doi: 10.1016/j.ejphar.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 51.Moore PK, Marshall M. Nitric oxide releasing acetaminophen (nitroacetaminophen) Dig Liver Dis. 2003;35(Suppl 2):49–60. doi: 10.1016/s1590-8658(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 52.al-Swayeh OA, Futter LE, Clifford RH, Moore PK. Nitroparacetamol exhibits anti-inflammatory and anti-nociceptive activity. Br J Pharmacol. 2000;130:1453–1456. doi: 10.1038/sj.bjp.0703509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romero-Sandoval EA, Mazario J, Howat D, Herrero JF. NCX-701 (nitroparacetamol) is an effective antinociceptive agent in rat withdrawal reflexes and wind-up. Br J Pharmacol. 2002;135:1556–1562. doi: 10.1038/sj.bjp.0704589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alfonso Romero-Sandoval E, Del Soldato P, Herrero JF. The effects of sham and full spinalization on the antinociceptive effects of NCX-701 (nitroparacetamol) in monoarthritic rats. Neuropharmacology. 2003;45:412–419. doi: 10.1016/s0028-3908(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 55.Romero-Sandoval EA, Curros-Criado MM, Gaitan G, Molina C, Herrero JF. Nitroacetaminophen (NCX-701) and pain: first in a series of novel analgesics. CNS Drug Rev. 2007;13:279–295. doi: 10.1111/j.1527-3458.2007.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curros-Criado MM, Herrero JF. Antinociceptive effects of NCX-701 (nitro-paracetamol) in neuropathic rats: enhancement of antinociception by co-administration with gabapentin. Br J Pharmacol. 2009;158:601–609. doi: 10.1111/j.1476-5381.2009.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molina C, Herrero JF. Subeffective doses of nitroparacetamol (NCX-701) enhance the antinociceptive activity of the α₂-adrenoceptor agonist medetomidine. Pharmacol Biochem Behav. 2011;99:385–390. doi: 10.1016/j.pbb.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 58.Raffa RB, Codd EE. Lack of binding of acetaminophen to 5-HT receptor or uptake sites (or eleven other binding/uptake assays) Life Sci. 1996;59:37–40. doi: 10.1016/0024-3205(96)00273-1. [DOI] [PubMed] [Google Scholar]


