Abstract
Objectives:
In recent years, studies on oral use have increased rapidly due to the restrictive aspects of parenteral administration of indispensable peptide-structured insulin in the rapidly growing worldwide treatment of diabetes. The aim of the study was to examine the development of a novel insulin-loaded LCS-NP complex, and its characterization and efficacy on pancreatic cells responsible for insulin release.
Materials and Methods:
Blank liposomes and insulin-loaded LCS-NPs were prepared using dry film hydration and ionotropic gelation methods, respectively. The LCS-NP complex was prepared by mixing liposomes/NPs in a 2:1 (w/w) ratio. The cytotoxic effects of the various concentrations of insulin and formulation components on the pancreatic cell line were determined using a 3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay and quantities to be used in the formulation were determined. Particle size, zeta potential, encapsulation efficiency, in vitro release profile and release kinetics, and transport properties of the prepared complex were investigated.
Results:
The newly developed insulin-loaded LCS-NP complex had a particle size of 2.85±0.035 μm and zeta potential of 8.11±1.025 mV. The encapsulation yield was found as 48±1.1%. In vitro insulin release from the complex was 80.9±2.71%. Insulin transport from β Tc cells was 30.50%. Permeability coefficients (log k) were calculated as -1.280±0.070 for the insulin solution and -1.020±0.062 for the insulin-loaded complex.
Conclusion:
This study suggests that insulin could be successfully loaded into the newly developed LCS-NP complex, and it is thought that this complex carries an effective formulation potential for long-term efficacy in the treatment of diabetes.
Keywords: Insulin, diabetes, chitosan nanoparticle, liposome-chitosan-nanoparticle complex
INTRODUCTION
Diabetes mellitus is a metabolic disorder that results in hyperglycemia associated with abnormalities in carbohydrate, fat, and protein metabolism, and insulin deficiency due to beta cell loss.1,2 Insulin, a peptide hormone produced by pancreatic β-cells, is used for the treatment of diabetes by regulating glucose concentration in blood. Although insulin therapy is the oldest and most effective in diabetes, some limitations have occurred. Insulin is commonly used via the parenteral route, which provides immediate action. However, there are many disadvantages of the parenteral route including pain, discomfort, and hypoglycemic episodes associated with multi-dose injections, which cause poor patient compliance.3 Administration of therapeutic peptide drugs such as insulin via the oral route, especially the gastrointestinal tract, represents one of the greatest challenges. Colloidal drug carriers have been developed for controlled drug release and represent an exciting approach to increase the uptake and transport of orally-administered peptide drugs such as insulin.4 In addition, these systems have many advantages including a decrease in multi-dose injections, improved patient compliance, decrease in drug plasma level fluctuations in the blood and total drug use, increased bioavailability of some drugs, and minimize drug toxicity.5 Liposomes and polymeric nanoparticles (NP) are suitable colloidal carriers for insulin delivery and many investigations have been performed for administration routes e.g., parenteral, ocular, nasal, pulmonary, transdermal, oral and buccal. Liposomes are spherical vesicles consisting of cholesterol (CHOL) and nontoxic phospholipids that are biodegradable, biocompatible, and non-immunogenic colloidal carriers. They increase peptide stability with protecting bioactive agents from digestion in the stomach and show significant levels of absorption in the gastrointestinal tract.6 Polymeric NPs are solid colloidal nanocarriers that provide controlled release of peptides depending on surface modifications by biodegradable polymers.Chitosan (CS), a hydrophilic natural polymer, has been used in protein and peptide encapsulated NP formulation for its unique characteristics including biocompatibility, biodegradability and mucoadhesivity.7,8
Cell culture is a laboratory process based on the survival of cells under controlled conditions while preserving their viability and shows in vivo-in vitro characteristics. The major advantages of using cell culture are the consistency and reproducibility of results that can be obtained from using a batch of cells and high in vivo correlation.9 (Yücel Ç. Development and investigation of efficiency of embryonic stem cell and insulin-loaded liposome, NP and cochleate formulations. Ph.D. Thesis, Gazi University, Institute of Health Sciences, Ankara, 2015:2-3).
In the present study, insulin was encapsulated in a liposome-CS-NP (LCS-NP) complex and in vitro characterization studies were performed on complexes including particle size, zeta potential, surface morphology, release and transport studies through pancreatic beta Tc (β Tc). Furthermore, in vitro cytotoxicities of insulin solution with different concentrations and formulation components on β Tc cells were evaluated.
EXPERIMENTAL
Materials
Insulin (Humulin R), (Humulin® R 100 IU/mL), CS hydrochloride (Protasan CL 110) and pentasodium tripolyphospate, (TPP) were purchased from Lilly (USA), FMC Biopolymers (Norway) and Kimetsan (Turkey), respectively. Distearoylphosphatidylcholine, (DSPC) and CHOL were supplied by Sigma (St. Louis, USA). All other chemicals used were analytical grade. β Tc cell lines were obtained from the American Type Culture Collection (CRL-11506). Cell culture flasks surface area 25 cm2 and 75 cm2 and 6-well cell culture plates were purchased from Corning®. Fetal Bovine Serum, Trypsin-ethylenediaminetetraacetic acid solution, dimethyl sulfoxide for cell culture, penicillin-streptomycin solution and MTT [3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide] were purchased from Sigma (St. Louis, USA). Cedex Smart Slides and Trypan Blue solution were purchased from Roche (Switzerland). The insulin mouse enzyme-linked immunosorbent assay (ELISA) kit was obtained from Sunredbio. Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Capricorn Scientific, Germany.
Analytical method and calibration
Drug content in formulations was measured using ultraviolet-spectrophotometry (Shimadzu-UV 1800). Initially, the absorbance of insulin was determined at respective wavelength. Stock solution of insulin (100 µg/mL) was diluted from an insulin market preparation with distilled water. Working standard solutions were prepared by diluting the stock solution in the concentration range of 0.5-100 µg/mL for calibration curves. Spectrophotometric determination was carried out at 756 nm. The method was found to be linear (r2=0.9992) and reproducible.
Preparation of chitosan nanoparticles (CS-NPs)
Blank and insulin-loaded CS NPs were prepared according to the ionotropic gelation process as described by Aktaş et al.10 The ratio of CS/TPP was established as 1:1 according to the preliminary studies. Blank nanoparticles were obtained upon the addition of a TPP aqueous solution (0.4 mg/mL) to a CS solution (1.75 mg/mL) stirred at room temperature on a magnetic stirrer for an hour. The same protocol was applied to obtain insulin-loaded LCS-NPs. The initial insulin amount was determined according to the preliminary drug loading studies.
Preparation of liposomes (LPs)
Blank liposomes were prepared using dry film hydration according to Bangham et al.11 DSPC and CHOL were added to the round-bottomed flask in a 6:4 molar ratio and dissolved in chloroform. The organic solvent was then evaporated on a rotary evaporator (Heidolph) at ~45°C. The resulting thin film was then hydrated with 10 mL water for 30 min and vortexed.
Preparation of the complexes
Blank and insulin-loaded LCS-NP complexes were formed by the combination of liposomes/nanoparticles at a 2:1 ratio (w/w) followed by lyophilization (Labconco) in the presence of 5.0% mannitol for 24 h.12,13 Finally, the lyophilized powders were reconstituted with distilled water for their physicochemical characterization and encapsulation efficiency (EE) determination.
Characterization of the LCS-NP complexes
The surface morphologies of the blank and insulin-loaded complexes were determined using an inverted microscope (Zeiss Primo Star, Germany). Particle size and zeta potential were determined using a Malvern Zeta Sizer. The EE of insulin was determined after centrifugation at 14.000 rpm, for 25 min of the LCS-NP complex dispersions. The amount of free insulin in the supernatant was measured using the Lowry method at 756 nm.
In vitro release studies
LCS-NP complex were placed in a tube and 2 mL of pH 7.4 phosphate buffer was added as release medium. The tubes were immersed in a water bath (37±0.5°C) with a horizontal shaker. At various time intervals, samples of 1.5 mL were withdrawn and replaced with equal volumes of fresh medium. The samples were centrifuged at 14.000 rpm, for 25 min. Insulin in the supernatant was measured using the Lowry method as described above.14
Cell culture studies
β Tc cells were grown in a medium composed of DMEM containing 25 mM glucose, 5 mM glutamine supplemented with 15% fetal bovine serum and 1% penicilin-streptomycin in an incubator at 37°C under 5% CO2 atmosphere. The medium was replaced with fresh DMEM every 48 h. The presence of a confluent monolayer was checked using a microscope.
Cytotoxicity assay
The effect of the insulin solution and formulation components on cell viability was investigated using the MTT [3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide] test.15 β Tc cells were seeded (20.000 cells/well) in 96-well culture plates and kept at 37°C for 24 h for cell adherence.16 Then, the cells were treated with various insulin concentrations (100-6.25 µg/mL) for 24 hours. The color intensity corresponding to cellular activity was measured at 570 nm using a multi-well ELISA reader.
Transport experiments
β Tc cells were seeded at 4x104 cells/well on polycarbonate membranes (Corning® 6 Well Transwell® Inserts) with a pore size of 0.4 µm.17 Insulin, blank, and insulin-loaded complexes were dissolved in medium for the donor compartment. 95% O2 and 5% CO2 were delivered to the system at 37°C to maintain cell viability. Samples were collected from the receptor compartment after 30, 60, 120, 180, 240, 360, 480, 720, and 1440 min. The insulin content of the samples was analyzed using the Lowry method and an ELISA kit and the apparent permeability (Papp) value was calculated using the following equation:18
Papp = (dQ / dt) x (1 / A x C0)
dQ/dt refers to the permeability rate, A (cm2) is the membrane diffusion area, and C0 (mg/mL) is the initial concentration of insulin in the donor compartment.
RESULTS
In vitro studies
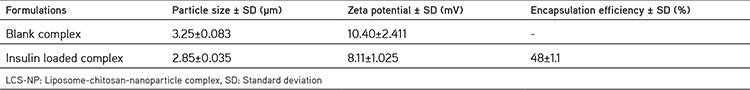
The surface morphologies of the LCS-NP complexes are shown in Figure 1. The mean particle size and zeta potential of the LCS-NP complexes are shown in Table 1.
Figure 1.
The surface morphologies of the LCS-NP complexes: Blank LCS-NP complex X40 (a), Insulin loaded LCS-NP complex X40 (b)
Table 1. Characterization parameters of the LCS-NP complexes (n=3).
The extent of the in vitro insulin release from LCS-NP complexes for 24 h was found as 80.9%. Insulin release was found to be compatible RRSBW kinetics and the correlation coefficient was found as 0.8995, as shown in Figure 2.
Figure 2.
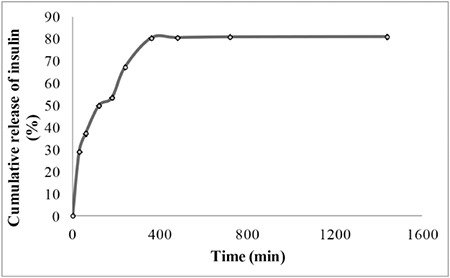
In vitro release profiles of insulin from LCS-NP complexes at pH 7.4 phosphate buffer (error bars represent standard deviations, n=3)
Cell culture studies
The MTT assay was used to determine the toxic effects on β Tc cells. The effect of insulin solution, blank and insulin-loaded LCS-NP complexes on cell viability were investigated for 24 h. The wells containing only the medium were accepted as a positive control with a cell viability of 100%. The viability of cells as percentages is given on Figure 3.
Figure 3.
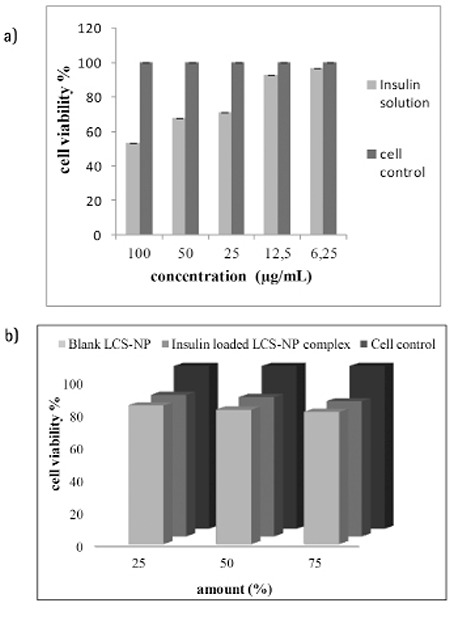
Cytotoxicity of various concentrations of insulin solutions (a) blank and insulin loaded LCS-NP complexes (b)
Transport experiments of insulin from the solution and LCS-NP complex through β Tc cells were evaluated. The cumulative amounts of transported insulin from solution and LCS-NP complex at the end of the 24 hours were found as 37.46% and 30.50%, respectively. The results are presented in Figure 4. Papp (log k) values were calculated for insulin solution and LCS-NP complex (Table 2).
Figure 4.
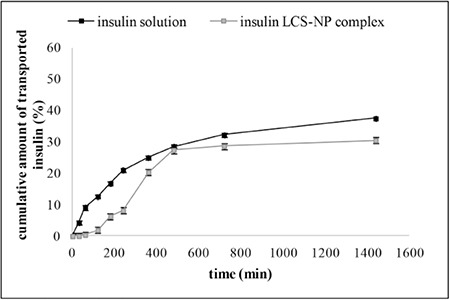
Cumulative amount of insulin from solution and LCS-NP complex transported through β Tc cells (error bars represent standard deviations, n=3)
LCS-NP: Liposome-chitosan-nanoparticle complex
Table 2. Papp (log k) values was calculated from β Tc cell transport study results (n=3).

DISCUSSION
Diabetes is a disease with an increasing prevalence in the world, which occurs as hyperglycemia due to a relative deficiency of the production of insulin by the pancreatic beta-cells.19 Insulin is a hydrophilic molecule, used in the treatment of diabetes mellitus that is commonly administered as multiple daily subcutaneous injections. Alternative routes for administration to improve patient compliance for insulin therapy have been investigated by designing drug delivery systems. Furthermore, various studies have been made on insulin-loaded drug delivery systems such as liposomes and polymeric micro/nanoparticles to improve bioavailability and provide long-term stability.20,21
The aim of the present study was to combine the CS-NPs with the similarity of the liposomes to the biologic membranes to obtain a good alternative to insulin treatment. We developed and characterized insulin-encapsulated LCS-NP complexes to improve drug entrapment efficiency and evaluate their sustained efficiencies on pancreatic β Tc cell line.
In this study, blank liposomes and insulin-loaded CS-NPs were prepared using dry film hydration11 and an ionotropic gelation process as described by Aktaş et al.,10 respectively. For the preparation of the LCS-NP complex, liposomes/nanoparticles at a 2:1 ratio (w/w) were used according to the method described by Carvalho et al.21 In the characterization studies, the mean particle size and zeta potential of blank and insulin-loaded LCS-NP complexes were found as 3.25±0.083 µm, 10.395±2.411 mV, 2.85±0.035 µm, and 8.115±1.025 mV, respectively. The encapsulating efficiency of the insulin-loaded LCS-NP complex was found as 48±0.1%, which is reported to be quite high.22 In the literature, Diebold et al.23 developed and studied LCS-NPs, a class of colloidal system that combines liposomes and CS-NPs as a potential ocular drug delivery system, which had a mean size of from 407.8±9.6 to 755.3±30.0 nm and zeta potential of from +14.7±0.4 to +5.8±1.3 mV.
In another study, microspheres containing lipid/CS nanoparticle complexes were prepared for pulmonary delivery of therapeutic proteins. Lipid and CS-NPs complexes had a particle size of approximately 2 µm and zeta potential of 2 mV.24 Cui et al.4 prepared nanoparticles with biodegradable polymers such as poly(lactic acid), and poly(D,L-lactide-co-glycolide acid), loaded with an insulin-phospholipid complex for oral delivery. Spherical particles with a 200-nm mean diameter and a narrow size distribution were obtained.4 In another study, bioadhesive polysaccharide CS nanoparticles containing insulin were prepared by ionotropic gelation and particle size distributions and zeta potentials were determined. The ability of CS-NPs to enhance intestinal absorption of insulin and increase the relative pharmacologic bioavailability of insulin was investigated. The authors concluded that CS-NPs had shown an excellent capacity for the association of insulin. CS-NPs loading insulin showed a positive charge and rapid release kinetics in vitro. Also, obtaining a positive zeta potential is important for interaction with the negatively charged cell membrane. Therefore, our characterization findings appeared to be sufficient.8,25
The in vitro release experiment was performed in pH 7.4 phosphate buffer. The medium for LCS-NP complex was selected as pH 7.4 phosphate buffer because the pH of DMEM (cell culture medium) was measured as 7.37 pH. The kinetic releases of insulin were found to be with RRSBW (r2=0.8995) for insulin solution and LCS-NP complex. In this RRSBW kinetic, a steeper initial slope followed by a flattened tail in the final part was obtained.26
For cytotoxicity studies, we used the MTT test, which is the most commonly used. The effects of insulin solution and formulation components on β Tc cell viability were investigated for 24 h. According to the MTT test results, insulin caused no cellular toxicity with the used dose of 33 µg/mL, which was decided as the encapsulated concentration in preparing CS-NPs. Additionally, blank and insulin-loaded LCS-NP complexes were also not found to be toxic to cells even at the highest concentrations (75%). In the literature, nanosystems have been designed consisting of CS nanoparticles and liposomes for ocular delivery. Diebold et al.23 hypothesized that a combination of CS nanoparticles and liposomes would protect the peptide fluorescein isothiocyanate (FITC)-conjugated bovine serum albumin (BSA) from harsh environmental conditions while providing its sustained release. The authors showed that these complexes interacted with the mucus layer and transported the conjunctival cells. Furthermore, toxicity of the LCS-NP complexes in conjunctival cells was found very low.23 Carvalho et al.21 developed nanosystems using the same method. They encapsulated insulin and (FITC)-conjugated BSA in LCS-NP complexes then evaluated these activities in conjunctival cells in in vitro cell culture studies. They also investigated activities of these complexes via the oral route and found that the complexes were well suited for controlled release with great stability in biologic fluids and provided a significant reduction in plasma glucose levels. They also found that the toxicity of LCS-NP complexes in conjunctival cells was found very low.21
The transport studies were performed for insulin solution and insulin-loaded LCS-NP complexes through pancreatic β Tc cells and permeability coefficients were calculated (Table 2).
Permeability coefficients (log k) were also calculated from solution and the LCS-NP complex and the lower value was -1.280 cm/h for insulin solution, the penetration value found for the insulin--loaded LCS-NP complex was -1.020 cm/h.
CONCLUSION
Although further experiments are warranted, these data indicate that LCS-NPs are potentially useful candidates for insulin delivery. Due to the similarities of liposomes in the cell membrane structures, LCS-NP complexes are able to penetrate more into the cells and are endocytosed more easily by the cells.
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Swenne I. Pancreatic Beta-cell growth and diabetes mellitus. Diabetologia. 1992;35:193–201. doi: 10.1007/BF00400917. [DOI] [PubMed] [Google Scholar]
- 3.Elsayed AM. Oral Delivery of Insulin. In: Sezer AD, ed. Recent Advances in Novel Drug Carrier Systems 1th ed. Crotia; InTech. 2012:281–314. [Google Scholar]
- 4.Cui F, Shi K, Zhang L, Tao A, Kawashima Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114:242–250. doi: 10.1016/j.jconrel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay P, Mishra R, Rana D, Kundu PP. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Prog Polym Sci. 2012;37:1457–1475. [Google Scholar]
- 6.Barenholz Y. Liposome application: problems and prospects. Curr Opin. Colloid Interface Sci. 2001;6:66–77. [Google Scholar]
- 7.Ding X, Alani AWG, Robinson JR. Extended release and Targeted Drug Delivery Systems. In: Troy DB, Beringer P, eds. Remington: The Science and Practice of Pharmacy 21st ed. USA; Lippincott Williams & Wilkins. 2006:939–964. [Google Scholar]
- 8.Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, Hao JS, Cui FD. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249:139–147. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 9.Skelin M, Rupnik M, Cencic A. Pancreatic Beta Cell Lines and their Applications in Diabetes Mellitus Research. ALTEX. 2010;27:105–113. doi: 10.14573/altex.2010.2.105. [DOI] [PubMed] [Google Scholar]
- 10.Aktaş Y, Andrieux K, Alonso MJ, Calvo P, Gürsoy RN, Couvreur P, Capan Y. Preparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitor. Int J Pharm. 2005;298:378–383. doi: 10.1016/j.ijpharm.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Bio. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 12.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 13.Diop M, Auberval N, Viciglio A, Langlois A, Bietiger W, Mura C, Peronet C, Bekel A, Julien David D, Zhao M, Pinget M, Jeandidier N, Vauthier C, Marchioni E, Frere Y, Sigrist S. Design, characterisation, and bioefficiency of insulin-chitosan nanoparticles after stabilisation by freeze-drying or cross- linking. Int J Pharm. 2015;491:402–408. doi: 10.1016/j.ijpharm.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 14.Sajeesh S, Sharma CP. Cyclodextrin-insulin complex encapsulated polymethacrylic acid based nanoparticles for oral insulin delivery. Int J Pharm. 2006;325:147–154. doi: 10.1016/j.ijpharm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Lapidot T, Walker MD, Kanner J. Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J Agric. Food Chem. 2002;50:7220–7225. doi: 10.1021/jf020615a. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki R, Okada N, Miyamoto H, Yoshioka T, Sakamoto K, Oka H, Tsutsumi Y, Nakagawa S, Miyazaki J, Mayumi T. Cyotomedical therapy for insulinopenic diabetes using microencapsulated pancreatic β cell lines. Life Sci. 2002;71:1717–1729. doi: 10.1016/s0024-3205(02)01724-1. [DOI] [PubMed] [Google Scholar]
- 18.Yücel C, Değim Z, Yilmaz Ş. Nanoparticle and liposome formulations of doxycycline: Transport properties through Caco-2 cell line and effects on matrix metalloproteinase secretion. Biomed Pharmacother. 2013;67:459–467. doi: 10.1016/j.biopha.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased betacell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 20.Vila A, Sanchez A, Tabio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002;78:15–24. doi: 10.1016/s0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho EL, Grenha A, Remuñán-López C, Alonso MJ, Seijo B. Mucosal delivery of liposome-chitosan nanoparticle complexes. In: Düzgüneş N, ed. Methods in Enzymology. USA; Burlington: Academic Press; 2009:289–312. doi: 10.1016/S0076-6879(09)65015-1. [DOI] [PubMed] [Google Scholar]
- 22.Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16:307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 23.Diebold Y, Jarrín M, Sáez V, Carvalho EL, Orea M, Calonge M, Seijo B, Alonso MJ. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP) Biomaterials. 2007;28:1553–1564. doi: 10.1016/j.biomaterials.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Grenha A, Remunan-Lopez C, Carvalho EL, Seijo B. Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins. Eur J Pharm Biopharm. 2008;69:83–93. doi: 10.1016/j.ejpb.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y. Enteral absorption of insulin in rats from mocoadhesive chitosan-coated liposomes. Pharm Res. 1996;13:896–901. doi: 10.1023/a:1016009313548. [DOI] [PubMed] [Google Scholar]
- 26.Özkan Y, Savaşer A, Özalp Y, Işımer A. Dissolution properties of different designed and formulated salbutamol tablet dosage forms. Acta Pol Pharm. 2000;57:271–276. [PubMed] [Google Scholar]


