Abstract
Objectives:
Crataegus species have been used as food and also in folk medicine for the treatment of various diseases. The present study aimed to make investigations on the biologic properties of different extracts prepared from Crataegus microphylla C. Koch, which was collected from Turkey.
Materials and Methods:
Dried leaf, stem bark, and fresh fruit samples of C. microphylla were separated and ethanol extract, acidified (0.5% HCl, pH: 2.5) ethanol extract, ethanol:water (1:1) extract, methanol extract, acidified (0.5% HCl, pH: 2.5) methanol extract, methanol:water (1:1) extract, water extract, and acidified (0.5% HCl, pH: 2.5) water extract were prepared for each. Various biologic effects such as the prevention of oxidative DNA damage, acetylcholinesterase, tyrosinase, α-glucosidase inhibition, and antioxidant effects with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, PRAP, and FRAP assays of these extracts at different concentrations were studied.
Results:
Acidified methanol extract of stem barks exhibited the highest acetylcholinesterase and tyrosinase inhibitions among the other extracts with IC50 values of 204.02±0.95 μg/mL and 37.30±0.27 μg/mL, respectively. Acidified ethanol extract of leaves was the most efficient extract against α-glucosidase, giving an IC50 of 15.78±0.14 μg/mL. The IC50 value of the acidified ethanol extract for DPPH was 9.89±0.09 µg/mL. Methanol extracts of leaves and stem barks at the dose of 125 µg/mL exhibited significant protective activity against DNA strand scission by hydroxyl radicals (.OH) on supercoiled pBR322 DNA.
Conclusion:
Acidified methanol or ethanol extracts prepared with stem bark and leaf from C. microphylla have potential antioxidant, hypoglycemic, and neuroprotective effects.
Keywords: DPPH, FRAP, hawthorn, PRAP, Rosaceae
INTRODUCTION
The Crataegus genus (Rosaceae) has approximately 200 species worldwide and 24 species in Turkey.1,2 All plant species in this genus have the common name “Hawthorn”.3Crataegus microphylla C. Koch is one of the wild edible fruits in Turkey.4Crataegus species have been used as food and also in folk medicine for the treatment of different heart diseases and diabetes for hundreds of years.3,5,6 Fruits of the Crataegus species are used for stimulating digestion, improving blood circulation, and for the treatment of diarrhea, abdominal pain, amenorrhea, hypertension, and hyperlipidemia in Chinese traditional medicine.3 In addition, products that include the extracts of some Crataegus species are consumed as natural health products in Europe, Asia, and North America.7,8 Epidemiologic studies and associated meta-analyses showed that long-term consumption of plant polyphenols in diet protected against the development of cancers, cardiovascular diseases, diabetes, osteoporosis, and neurodegenerative diseases.9,10,11,12,13
In addition to its ethnopharmacologic use, the preventive effect of C. microphylla fruit extract against genotoxicity induced by methyl methanesulfonate has been investigated in human cultured blood lymphocytes and found to reduce the oxidative stress and genotoxicity induced by toxic compounds. This activity is attributed to its phenolic content and antioxidant potential.14
By the results of many pharmacologic studies performed with extracts and isolated constituents of Crataegus species, flavonoids and proantocyanidins were found to be responsible for the cardiovascular protective activity of the plant.8 With phytochemical studies, D-sorbitol, apigenin, naringenin, eriodictoyl, vitexin, vitexin-4’-O-rhamnoside, hesperetin, luteolin, luteolin 7-O-glucoside, quercetin, and hyperoside have been isolated from C. microphylla.15,16,17,18 Hyperoside was found to be the major compound in leaves and flowers of C. microphylla.17
Oxidative stress is involved in several neurodegenerative disease and degenerative disorders such as cancer, arteriosclerosis, and diabetes.19 As the accepted consent, the phenolic content determines the antioxidative properties of plant species, and polyphenols play a role in the prevention of chronic human diseases.9 The prevention of DNA damage, antioxidant activity, and total phenolic and flavonoid contents of extracts of new sources are very important in explaining their biochemical properties and behavior. In particular, studies of inhibition of these enzymes and prevention of DNA oxidative damage will also enlighten researchers to perform further studies in terms of neurodegenerative enzyme inhibition, anti-diabetic activity, and preventing the conversion to mutagenic forms with various extracts from C. microphylla.
In this study, prevention of oxidative DNA damage, acetylcholinesterase (AChE), tyrosinase, a-glucosidase inhibition behaviours and antioxidant effects: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging effect, phosphomolybdenum-reducing antioxidant power (PRAP), ferric-reducing antioxidant power (FRAP) with total phenolic and total flavonoid contents of the C. microphylla leaves, stem barks and fruits that were extracted with ethanol, methanol, and water were investigated. The biologic evaluation of the aerial part extracts of C. microphylla was investigated for the first time in this work.
EXPERIMENTAL
Plant material and sample preparation
Leaf, stem bark (B), and fruit of C. microphylla were collected from Kale, Gümüşhane-Turkey, in September 2015. A voucher specimen was deposited at the Hacettepe University, Faculty of Pharmacy, Herbarium (Voucher No: HUEF 15021).
Dried leaf (L), B and fresh fruit (F) samples of C. microphylla were separated and 50 g of L, B, and F was extracted with 250 mL of various solvents to obtain ethanol extract (1), acidified (0.5% HCl, pH: 2.5) ethanol extract (2), ethanol:water (1:1) extract (3), methanol extract (4), acidified (0.5% HCl, pH: 2.5) methanol extract (5), methanol:water (1:1) extract (6), water extract (7), and acidified (0.5% HCl, pH: 2.5) water extract (8) for each, respectively. Extractions were performed in a shaker for 4 h x 3 times, for each sample. Extracts were filtered and evaporated under reduced pressure using a rotary evaporator. Crude extracts were kept in a refrigerator at +4°C until used. All of the extracts in Table 1 were tested in all assays.
Table 1. The codes and yields (w/w) of the extracts prepared with various solvents of leaf, bark and fruit from C. microphylla.

Enzyme inhibitions
Acetylcholinesterase inhibition
AChE inhibition was examined using the method described by Ellmanet al.20 and Ingkaninan et al.21 Galantamine was used as the positive control. All extracts (L1-8, B1-8 and F1-8) at various concentrations were separately added to a 96-well microplate and incubated for 15 min at 25°C. Absorbance was measured at 412 nm using a 96-well microplate reader. Inhibition of AChE was calculated using Formula 1, in which Acontrol is the activity of enzyme without extract (solvent in buffer pH=8) and Asample is the activity of enzyme with extract at various concentrations. The inhibitory concentrations of 50% of AChE (IC50) values were calculated from the graph of the percentage inhibition against extract concentrations.
Tyrosinase inhibition
Tyrosinase inhibition was examined using the method described by Masuda et al.22 Kojic acid was used as the positive control. The tyrosinase inhibition percentage of all extracts (L1-8, B1-8 and F1-8) (20 µL) at various concentrations was calculated using Formula 1. The inhibitory concentration of 50% of tyrosinase (IC50) values was calculated from the graph of the percentage inhibition against extract concentrations.
α-glucosidase inhibition
α-glucosidase inhibition was examined using the method described by da Silva Pinto et al.23 Acarbose was used as the reference drug. The α-glucosidase inhibition percentage of all extracts (L1-8, B1-8 and F1-8) at various concentrations was calculated using Formula 1. The inhibitory concentration of 50% of a-glucosidase (IC50) values was calculated from the graph of the percentage inhibition against extract concentrations.
Antioxidant activities
Determination of total phenolic contents
The Folin–Ciocalteu reagent was used to determine the total phenolic content according to the method described by Kähkönen et al.24 Gallic acid was also used as standard compound. The total phenolic contents of all extracts (L1-8, B1-8 and F1-8) were expressed as mg gallic acid equivalents (GAE) per g of dry weight sample.
Determination of total flavonoid contents
The total flavonoid content was measured by using the aluminum nitrate assay (Chang et al.25 2002). Quercetin was used as the standard compound. The total flavonoid contents of all extracts (L1-8, B1-8 and F1-8) were expressed as mg quercetin equivalents (QE) per g of dry weight sample.
DPPH radical scavenging assay
The DPPH radical scavenging activities of all extracts (L1-8, B1-8 and F1-8) were examined using the method described by Blois compared with gallic acid and ascorbic acid as the reference compounds.26 The absorbance of the sample (Asample) was measured at 517 nm. An assay mixture without samples was used as a control (Acontrol). The inhibition percentage was calculated using Formula 2. The scavenging concentrations of 50% of DPPH (SC50) values were calculated from the graph of the percentage inhibition against extract concentrations.
Formula 2.
PRAP assay
PRAP of all L1-8, B1-8 and F1-8 extracts were examined using phosphomolybdic acid.27 The PRAP of extracts was expressed as mg QE per g of dry weight sample.
FRAP assay
FRAP of all L1-8, B1-8 and F1-8 extracts was examined using the method described by Oyaizu.28 The ferric-reducing power of extracts was expressed as butylated hdroxyanisole equivalents (BHAE) per g of dry weight sample.
Prevention of DNA oxidative damage
The protective effects of all L1-8, B1-8 and F1-8 extracts of C. microphylla against DNA oxidative damage induced by hydroxyl radical were monitored by the conversion of pBR322 to open circular form according to Yeung et al.29 Total volume of reaction mixture (10 µL) contained Tris-HCl buffer (pH 7.0), supercoiled plasmid pBR322 DNA (250 ng), 1 mM FeSO4, 2% H2O2 and 125 µg/mL of extracts. The mixtures were incubated at 37°C for 1 h. The reaction was stopped by adding 5 µL of loading buffer (0.2% bromophenol blue, 4.5% sodium dodecyl sulfate, 0.2% xylene cyanol, 30% glycerol). The mixtures were then loaded on 0.8% agarose gel containing EB 1 mg/mL in TAE (Tris-acetate-EDTA). Electrophoresis was carried out at 100 V for 90 min. and the resulting image was visualized with BioRad Gel Doc XR system.
Statistical analysis
The experiments were performed in triplicate and the results are expressed as the mean ± standard deviation. The statistical analysis was performed with SPSS 15.0 for Windows and Microsoft Excel for Windows 10. The differences between the extracts were evaluated using one-way analysis of variance flowed by Duncan’s multiple range tests. P<0.05 was considered statistically significant.
RESULTS
Enzyme inhibition
AChE inhibition results of extracts of leaf, stem bark and fruit from C. microphylla are presented in Table 2. All of the extracts had low AChE inhibition values when compared with galanthamine with IC50 values of 7.34±0.09 µg/mL. However, among the tested extracts, B5 and B2 exhibited the highest AChE inhibitions with IC50 values of 204.02±0.95 µg/mL and 230.58±3.18 µg/mL, respectively. Some of the extracts (L8, B3, B7, F1, F3, F4, F6, F7 and F8) were inactive against AChE enzyme.
Table 2. IC50 (μg/mL) of aceytlcholinesterase, tyrosinase and α-glucosidase inhibitory activities of leaf, bark, and fruit extracts from C. microphylla.

The results of the tyrosinase enzyme inhibitory effect of the extracts are given in Table 2. The lowest IC50 values of the extracts indicate a higher inhibition effectiveness. All of the extracts from C. microphylla exhibited promising activity against tyrosinase compared with kojic acid. Methanol and ethanol extracts of stem bark of C. microphylla displayed remarkable tyrosinase inhibitory activities with IC50 values of lower than 50 µg/mL. The B2 extract exhibited the highest tyrosinase inhibition with IC50 values of 37.30±0.27 µg/mL (p<0.05), and B5 inhibited tyrosinase with IC50 values of 37.41±0.17 µg/mL.
In this work, IC50 values of α-glucosidase inhibition of C. microphylla extracts are presented in Table 2. A lower IC50 value indicates strong inhibitory activity. L2, L5, B2, B5 and B8 extracts exhibited significant (p<0.05) α-glucosidase inhibition as shown in Table 2. IC50 values of L2, L5, B2, B5 and B8 extracts were found to 15.78±0.14, 29.92±0.26, 38.25±0.51, 39.63±0.62 and 46.02±0.52 µg/mL, respectively. On the other hand, F1, F3, F6, and F7 extracts had no α-glucosidase inhibition effects. All of the data of α-glucosidase inhibition indicated that L2, L5, B2, B5, and B8 extracts of C. microphylla could be effective hypoglcemic agents.
Antioxidant activities
The total phenolic contents of various extracts of C. microphylla leaves, stem barks, and fruits were determined from the gallic acid standard curve (y=1.9251x + 0.3125, R2=0.9967) and expressed as mg GAE/g dry weight. The total phenolic contents of C. microphylla stem barks and leaves were in the range of 13.22±0.38 to 132.26±1.83 mg GAE/g dry weight and 30.93±0.64 to 85.26±1.60 mg GAE/g dry weight, whereas extracts of fruits exhibited 5.00±0.18 to 57.28±1.35 mg GAE/g dry weight as shown in Figure 1. B1 (123.11±2.38), B2 (132.26±1.83), B4 (111.84±2.19), B5 (120.40±2.89), and B6 (112.46±2.13) extracts contained more than 100 mg GAE/g dry weight. On the other hand, B7 and F8 extracts exhibited the lowest total phenolic contents (13.22±0.38, 5.00±0.18 and 14.89±0.73 mg GAE/g dry weight).
Figure 1.
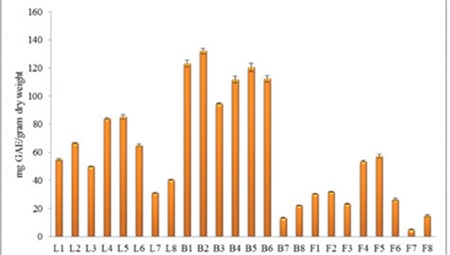
Total phenolic contents of the extracts
*L: Leaf, B: Bark, F: Fruit
Total flavonoid contents of leaf, stem bark, and fruit extracts from C. microphylla were determined from the quercetin standard curve (y=12.632x ± 0.509, R2=0.9981) as shown in Figure 2. The total flavonoid contents expressed as mg QE/g dry weight found in our extracts ranged from 0.97±0.09 to 63.34±0.92 mg QE/g dry weight. Total flavonoid contents of leaf extract from C. microphylla appeared higher than other extracts. The highest total flavonoid content was found in the L1 (63.34±0.92 mg QE/g dry weight) extract, followed by the L2 (56.25±0.73 mg QE/g dry weight), L4 (52.89±0.47 mg QE/g dry weight), L5 (49.39±1.03 mg QE/g dry weight), and L6 (50.53±0.92 mg QE/g dry weight) extracts. Stem bark extracts of C. microphylla were in the range of 0.97±0.09 to 4.78±0.24 mg QE/g dry weight.
Figure 2.

Total flavonoid contents of the extracts
*L: Leaf, B: Bark, F: Fruit
Among the tested extracts, B2 (9.89±0.09 µg/mL), B5 (10.47±0.29 µg/mL), B1 (11.94±0.07 µg/mL) and L2 (12.29±0.07 µg/mL) (p<0.05) extracts showed the highest scavenging activity in this assay as shown in Table 3. The IC50 values of ethanol, acidified ethanol, methanol, and acidified methanol extracts of leaf and stem bark of C. microphylla were found lower than 70 µg/mL. In the leaf, stem bark, and fruit extracts of C. microphylla, F7 extract showed the lowest DPPH radical scavenging activities. F5 extract exhibited the highest scavenging activities among the leaf extracts with 123.50±1.31 µg/mL.
Table 3. DPPH radical scavenging, phosphomolybdenum-reducing antioxidant power (PRAP) and ferric-reducing antioxidant power (FRAP) assay values of leaf, bark, and fruit extracts from C. microphylla.

PRAP of leaf, stem bark, and fruit extracts from C. microphylla were determined from the quercetin standard curve (y=0.0066x ± 0.5295, R2=0.9986) as shown in Table 3. B2, B5, and B4 extracts displayed the highest reducing activities with 368.37±2.41, 324.69±3.69 and 247.75±2.73 mg QE/g dry weight, respectively; F7 extract indicated the lowest activity 25.68±0.82 mg QE/g dry weight dry weight.
The results of the ability to reduce Fe3+ to Fe2+ are presented in Table 3. Stem bark and leaf extracts have a strong ferric reducing power. B2 and B5 extracts demonstrated the highest ferric reducing activity with 240.62±1.03 mg BHAE/g dry weight and 232.26±1.83 mg BHAE/g dry weight, respectively; F7 extract exhibited the lowest activity 25.00±2.38 mg BHAE/g dry weight.
Prevention of DNA oxidative damage
It is known that when circular plasmid DNA is subjected to electrophoresis, the fastest to migrate is the supercoiled Form I, the slowest moving is the open circular Form II, and the linear Form III runs in between the other two forms.30 Prevention of DNA oxidative damage by C. microphylla is shown in Figure 3. The assay revealed that there was a formation of Form II and Form III because of hydroxyl radicals, as shown in Lane 2 on Figure 3.
Figure 3.
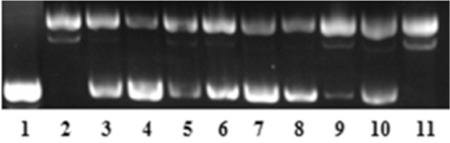
Protective effect of ethanol, methanol and water extracts of leaf, stem bark and fruit from C. microphylla in DNA oxidative damage assay. Lane 1: DNA control; Lane 2: DNA + 2% H2O2 + 1 mM FeSO4; Lane 3: DNA + 2% H2O2 + 1 mM FeSO4 + L1; Lane 4: DNA + 2% H2O2 + 1 mM FeSO4 + L4; Lane 5: DNA + 2% H2O2 + 1 mM FeSO4 + L7; Lane 6: DNA + 2% H2O2 + 1 mM FeSO4+ B1; Lane 7: DNA + 2% H2O2 + 1 mM FeSO4 + B4; Lane 8: DNA + 2% H2O2 + 1 mM FeSO4+ B7; Lane 9: DNA + 2% H2O2 + 1 mM FeSO4+ F1; Lane 10: DNA + 2% H2O2 + 1 mM FeSO4+ F4; Lane 11: DNA + 2% H2O2 + 1 mM FeSO4+ F7.
*L: Leaf, B: Bark, F: Fruit
However, with the addition of extracts, the conversion of supercoiled pBR322 DNA to open circular and linear forms decreased except with F8 extract at 125 µg/mL. L4 and B4 extracts exhibited the highest preventative effect of DNA oxidative damage at 125 µg/mL. The results proved that the prevention of DNA oxidative damage results were compatible with the radical scavenging assay.
DISCUSSION
Alzheimer’s disease (AD) is one of the most frequent forms of dementia among older people.31 Although AChE inhibitors such as tacrine, donepezil, galantamine, and rivestigmine are important in the treatment for AD, they have adverse effects including gastrointestinal problems.32,33 Considering all the extracts, stem bark extracts, which had promising results at AChE inhibition, presented higher phenolic content than the other extracts (Figure 1). Recent studies have shown that antioxidants could scavenge oxygen radicals and could also attenuate inflammation pathways, and also pointed toward an association between AD and inflammatory processes as well as antioxidant activity.34 From this point of view, it is stated that the use of antioxidants could be considered in the treatment of AD.35
Parkinson’s disease (PD) is one of the neurodegenerative diseases caused by dopaminergic neuron deficiency in the brain.36 Methanol and ethanol extracts from C. microphylla had higher inhibition activity than water extracts of C. microphylla due to total phenolic contents. There is a positive correlation between phenolic content and tyrosinase inhibition.37 These results showed that, extracts of C. microphylla, especially B5 extract, had promising neuroprotective potential due to AChE and tyrosinase inhibition.
α-Glucosidase is a key enzyme in the hydrolysis of oligosaccharide and contributes to the formation of glucose.38 It is important to find a new α-glucosidase inhibitor for DM, such as natural products with low toxicity and adverse effects.
Organic solvents such as methanol and ethanol are known to be efficient for the extraction of phenolics. Besides, water is a good choice because it is used to make infusions and decoctions in herbal medicine. Also, acidified extraction systems were shown to be more efficient, especially for the hydrolysis of bound phenolic compounds.39,40 Due to the fact that many solvents may extract different compounds from plant tissues, we wanted to compare the results. The hydrolysation process was done with acidification and aglycones were obtained with acidified extracts (L2, 5, 8; B2, 5, 8; F2, 5, 8) (Table 1).
When we compared the extracts that were prepared with the same solvents, total phenolic contents of the acidified ones were found to be higher than the non-acidified ones (Figure 1). The total phenolic content of L2 was found to be higher than L1, L5 was higher than L4, and L8 was higher than L7. The same results were also obtained with B and F series (Figure 1).
Similar to our findings, it was reported that methanol extract of C. microphylla leaves indicated scavenging activity to 92.82±0.79% at 500 µg/mL.41 According to Sharifi et al.42, IC50 values of methanol extract of C. microphylla were found as 13.01±0.2 µg/mL.
The efficiency of an antioxidant extract was reported to be dependent on the pH of the solvents, as well as the solubility of antioxidant compounds by the solvents used for the extraction.43 Besides, methanol, ethanol, and water, which are commonly used solvents for extraction, and acidified alcohols are also widely used for extraction to release aglycone by chemical hydrolysis under acidic conditions.44 These results confirm that higher contents of total phenolic displayed higher DPPH free radical scavenging activities. All data showed that there was a relationship between the total phenolic and radical scavenging activities.
The results showed that methanol and ethanol extracts of leaf and bark from C. microphylla had more effective phosphomolybdenum-reducing power than its water extractst. The B2 and B5 extracts with higher reducing power showed a positive correlation with phosphomolybdenum-reducing power assay.
Prevention of DNA oxidative damage was based on the ability of extracts (L1-8, B1-8 and F1-8) from C. microphylla to protect the supercoiled pBR322 DNA against damage caused by hydroxyl radicals (.OH). The antioxidant activity of 50% aqueous methanolic extract of whole plant of C. microphylla was studied before with an in vitro study and found to have moderate antioxidant activity.45 However, there are no previous works on the AChE, tyrosinase, α-glucosidase inhibitory effects and oxidative DNA damage protective effects of various extracts of C. microphylla. In this context, it was aimed to compare the extractability of compounds that serve a function in the activity by various solvents.
CONCLUSION
This study presented the potential AChE, tyrosinase, α-glucosidase inhibitory effects, total phenolic, total flavonoid contents, the antioxidant effects, and prevention of oxidative DNA damage of leaf, stem bark and fruit of various extracts (L1-8, B1-8 and F1-8) from C. microphylla. Concurrently, the correlation between the antioxidant activity and the DNA damage protective effects of the extracts (L1-8, B1-8 and F1-8) was described. Our results can be evaluated as a preliminary work for the use of C. microphylla extracts in herbal products.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Dönmez AA. The Genus Crataegus L. (Rosaceae) with special reference to hybridisation and biodiversity in Turkey. Turk J Bot. 2004;28:29–37. [Google Scholar]
- 2.Dönmez AA. A new species of Crataegus (Rosaceae) from Turkey. Bot J Linn Soc. 2005;148:245–249. [Google Scholar]
- 3.Chang Q, Zhong Z, Harrison F, Chow MS. Hawthorn. J Clin Pharmacol. 2002;42:605–612. doi: 10.1177/00970002042006003. [DOI] [PubMed] [Google Scholar]
- 4.Browicz K, Crataegus L. In: PH Davis, ed. Flora of Turkey and the East Aegean Islands, Edinburgh University Press. Edinburgh. 1972;4:133–147. [Google Scholar]
- 5.Kumar D, Arya V, Bhat ZA, Khan NA, Prasad DN. The genus Crataegus: chemical and pharmacological perspectives. Rev Bras Farmacogn. 2012;22:1187–1200. [Google Scholar]
- 6.Rastogi S, Pandey MM, Rawat AK. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine. 2016;23:1082–1089. doi: 10.1016/j.phymed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JE, Brown PN, Talent N, Dickinson TA, Shipley PR. A review of the chemistry of the genus Crataegus. Phytochemistry. 2012;79:5–26. doi: 10.1016/j.phytochem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.No authors listed. Assessment report on Crataegus spp., folium cum flöre, European Medicines Agency Committee on Herbal Medicinal Products (HMPC) EMA/HMPC/159076/2014, Accessed on: 13 October. 2014. [Google Scholar]
- 9.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxi Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abidi E, Habib J, Yassine A, Chahine N, Mahjoub T, Ellak A. Effects of methanol extracts from roots, leaves, and fruits of the Lebanese strawberry tree (Arbutus andrachne) on cardiac function together with their antioxidant activity. Pharm Biol. 2016;54:1035–1041. doi: 10.3109/13880209.2015.1100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8:281–290. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 12.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317–325. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 13.Palma-Duran SA, Vlassopoulos A, Lean M, Govan L, Combet E. Nutritional intervention and impact of polyphenol on glycohemoglobin (HbA1c) in non-diabetic and type 2 diabetic subjects: Systematic review and nmetaanalysis. Crit Rev Food Sci Nutr. 2017;57:975–986. doi: 10.1080/10408398.2014.973932. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinimehr SJ, Azadbakht M, Tanha M, Mahmodzadeh A, Mohammadifar S. Protective effect of hawthorn extract against genotoxicity induced by methyl methanesulfonate in human lymphocytes. Toxicol Ind Health. 2011;27:363–369. doi: 10.1177/0748233710387010. [DOI] [PubMed] [Google Scholar]
- 15.Strain HH. Sources of d-sorbitol. J Am Chem Soc. 1937;59:2264–2266. [Google Scholar]
- 16.Meriçli AH, Melikoğlu G. Investigations on Turkish Crataegus species. Acta Pharm Turcica. 2002;44:169–173. [Google Scholar]
- 17.Melikoğlu G, Bitiş L, Meriçli AH. Flavonoids of Crataegus microphylla. Nat Prod Res. 2004;18:211–213. doi: 10.1080/14786410310001620673. [DOI] [PubMed] [Google Scholar]
- 18.Tajali AA, Khazaeipool M. Effects of height and organs on flavonoids of Crataegus microphylla C. Koch in Iran Int J Biosci. 2012;2:54–58. [Google Scholar]
- 19.Moyo MS, Amoo O, Ncube B, Ndhlala AR, Finnie JF, Van Staden J. Phytochemical and antioxidant properties of unconventional leafy vegetables consumed in Southern Africa. S Afr J Bot. 2013;84:65–71. [Google Scholar]
- 20.Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM. A new and rapid colorimetric determination acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 21.Ingkaninan K, de Best CM, van der Heijden R, Hofte AJ, Karabatak B, Irth H, Tjaden UR, van der Greef J, Verpoorte R. High-performance liquid chromatography with on-line coupled UV, mass spectrometric and biochemical detection for identification of acetylcholinesterase inhibitors from natural products. J Chromatogr A. 2000;872:61–73. doi: 10.1016/s0021-9673(99)01292-3. [DOI] [PubMed] [Google Scholar]
- 22.Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotech Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 23.da Silva Pinto M, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Functionality of bioactive compounds in Brazilian strawberry (Fragaria x Ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J Agric Food Chem. 2008;56:4386–4392. doi: 10.1021/jf0732758. [DOI] [PubMed] [Google Scholar]
- 24.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J Agr Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 25.Chang CC, Yang MH, Wen MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 26.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 27.Falcioni G, Fedeli D, Tiano L, Calzuola I, Mancinelli L, Marsili V, Gianfranceshi G. Antioxidant activity of wheat sprouts extract in vitro: inhibition of DNA oxidative damage. J Food Sci. 2002;67:2918–2922. [Google Scholar]
- 28.Oyaizu M. Studies on products of browning reactions-antioxidative activities of browning reaction prepared from glucosamine. Jpn J Nut. 1986;44:307–315. [Google Scholar]
- 29.Yeung SY, Lan WH, Huang CS, Lin CP, Chan CP, Chang MC, Jeng JH. Scavenging property of three cresol isomers against H2O2, hypochlorite, superoxide and hydroxyl radicals. Food Chem Toxicol. 2002;40:1403–1413. doi: 10.1016/s0278-6915(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 30.Özel A, Barut B, Demirbaş Ü, Biyiklioglu Z. Investigation of DNA binding, DNA photocleavage, topoisomerase I inhibition and antioxidant activities of water soluble titanium(IV) phthalocyanine compounds. J Photochem Photobiol B. 2016;157:32–38. doi: 10.1016/j.jphotobiol.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Lewis WG, Green LG, Grynszpan F, Radic Z, Carlier PR, Taylor P, Finn MG, Sharpless KB. Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew Chem Int Engl. 2002;41:1053–1057. doi: 10.1002/1521-3773(20020315)41:6<1053::aid-anie1053>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Tian T, Weng L, Wang S, Weng X, Zhang L, Zhou X. Cationic tetrapyrrolic macromolecules as new acetylcholinesterase inhibitors. J Porphyr Phthalocyanines. 2009;13:893–902. [Google Scholar]
- 33.Zengin G, Nithiyanantham S, Locatelli M, Ceylan R, Uysal S, Aktumsek A, Kalai Selvi P, Maskovic P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur J Integr Med. 2016;8:286–292. [Google Scholar]
- 34.Ferreira A, Proença C, Serralheiro MLM, Araujo ME. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 2016;108:31–37. doi: 10.1016/j.jep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Gibson GE, Huang HM. Oxidative stress in Alzheimer’s disease. Neurobiol Aging. 2005;26:575–578. doi: 10.1016/j.neurobiolaging.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Bao K, Dai Y, Zhu ZB, Tu FJ, Zhang WG, Yao XS. Design synthesis biphenyl derivatives mushroom tyrosinase inhibitors. Bioorg Med Chem. 2010;18:6708–6714. doi: 10.1016/j.bmc.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 37.Choi HK, Lim YS, Kim YS, Park YS, Lee CH, Hwang KW, Kwon DY. Free-radical- scavenging and tyrosinase-inhibition activities of Cheonggukjang samples fermented for various time. Food Chem. 2008;106:564–568. [Google Scholar]
- 38.Majouli K, Hlila MB, Hamdi A, Flamini G, Jannet HB, Kenani A. Antioxidant activity and α-glucosidase inhibition by essential oils from Hertia cheirifolia (L) Ind Crop Prod. 2016;82:23–28. [Google Scholar]
- 39.Acosta-Estrada BA, Gutierrez-Uribe JA, Serna-Saldivar SO. Bound phenolics in foods, a review. Food Chem. 2014;152:46–55. doi: 10.1016/j.foodchem.2013.11.093. [DOI] [PubMed] [Google Scholar]
- 40.Mushtaq M, Sultana B, Anwar F, Batool S. Antimutagenic and antioxidant potential of aqueous and acidified methanol extracts from citrus limonum fruit residues. J Chil Chem Soc. 2015;60:2979–2983. [Google Scholar]
- 41.Artun FT, Karagöz A, Özcan G, Melikoğlu G, Anıl S, Kultur S, Sutlupinar N. In vitro evaluation of antioxidant activity of some plant methanol extracts. J BUON. 2016;21:720–725. [PubMed] [Google Scholar]
- 42.Sharifi N, Souri E, Ziai SA, Amin G, Amanlou M. Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. DARU. 2013;21:74. doi: 10.1186/2008-2231-21-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutlu T, Durmaz G, Ateş B, Yilmaz İ, Çetin MŞ. Antioxidant properties of different extracts of black mulberry (Morus nigra L.) Turk J Biol. 2011;35:103–110. [Google Scholar]
- 44.Nollet LML, Toldra F. Handbook of Analysis of Active Compounds in Functional Foods, CRC Press. Boca Raton. 2012. [Google Scholar]
- 45.Serteser A, Kargioğlu M, Gök V, Bağci Y, Ozcan MM, Arslan D. Determination of antioxidant effects of some plant species wild growing in Turkey. Int J Food Sci Nutr. 2008;59:643–651. doi: 10.1080/09637480701602530. [DOI] [PubMed] [Google Scholar]


