Abstract
Objectives:
Dried roots of Echium species are used in Turkey for the treatment of wounds, inflammation, and depression. In this study, an reversed-phase-liquid chromatographic method with isocratic elution was developed to determine shikonin derivatives in Echium species.
Materials and Methods:
The chromatographic separation and quantification was performed on a C18 column (ACE, 150 mm × 4.6 mm, 5 μm), with a mobile phase of acetonitrile and 0.1 M acetic acid (70: 30, v/v) at a flow rate of 1 mL/min, and ultraviolet detection at 520 nm.
Results:
Linear behavior was observed over the investigated concentration range (2-500 ppm) for all analytes, with a correlation coefficient of >0.998. The proposed method was found to be specific and precise for the quantification of shikonin derivatives in Echium species.
Conclusion:
The highest content of shikonin derivatives was observed in E. italicum L. compared with the other species studied herein, advocating the use of E. italicum L. roots as an alternate source for shikonin derivatives.
Keywords: Echium sp., shikonin derivatives, HPLC
INTRODUCTION
Species of Echium occur abundantly as a flowering plant native to North Africa, mainland Europe, and the Macaronesian islands, but have also become invasive in southern Africa and Australia. Out of the 60 species of this genus, nine are found in Turkey: E. angustifolium, E. arenarium, E. italicum, E. glomeratum, E. oriantale, E. parviflorum, E. plantagineum, E. russicum, and E. vulgare.
Based on the traditional documents on folk medicine, Echium sp. have been employed as diaphoretics, diuretics, expectorants, febrifuges, sedatives, analgesics, vulneraries, anxiolytics, and in the treatment of upper respiratory tract infections.1,2,3,4 Pharmacologic studies have demonstrated that species of Echium possess antioxidant5,6,7, anti-inflammatory8, antibacterial9,10, antiviral11, antiproliferative12,13,14, analgesic15 and antidepressant16 activities. It is clinically proven as a useful and safe drug in patients with obsessive compulsive disorders.16,17,18 It is also reported that various extracts of E. amoenum have good antianxiety activity on in vivo models.19,20,21,22.23 Echium species contain a variety of phytochemicals, including naphthoquinones, flavonoids, pyrrolizidine alkaloids, steroids, anthocyanins, fatty acids, amino acids, and essential oils. Many shikonin (S) derivates (ShD) have been isolated from the roots of Echium plants. Naphthoquinones are a series of ShD such as S, acetylshikonin (AS), deoxyshikonin (DS), β, β-dimethylacrylshikonin (DMAS), isobutrylshikonin, isovalerylshikonin (IVS), and 2-methyl butrylshikonin (MBS).24
S, R enantiomer of Alkannins, are lipophilic red pigments commonly known as isohexenylnaphthazarins. They are mostly found in more than 150 genus such as Lithospermum, Echium, Onosma, Anchusa, and Cynoglossum of the Boraginaceae family. Historically they were used as dyes by the ancient Greeks and Romans. S contains two parts structurally: the naphthazarine moiety (5, 8-dihydroxy-1, 4 naphthoquinone) and the chiral six-carbon side chain. It is readily sensitive to polymerization following treatment with acids, bases, heat, or light due to the naphthazarine core, and easily to oxidation by exposure to light or air just for the high chemical reactivity. Most of the ShD are present as ester derivatives linked with the hydroxyl group of the side chain, maintaining the naphthazarine moiety.25 ShD have attracted the attention of many researchers due to their several potential pharmacologic activities such as antimicrobial, antitumor, wound healing, and antioxidants. S extracts obtained from Lithospermum erythrorhizon induced apoptosis against human colorectal carcinoma cells, HL60 cells, and HeLa cells by the mechanism of tumor suppression via signaling pathways that possibly involved reactive oxygen species, p53, p27, Bcl-2, caspase, and inhibition of DNA topoisomerase I/II and telomerase.26,27,28 A clinical study showed that ShD were efficacious in the treatment of patients with late-stage lung cancer who were inappropriate for other treatment.29 Furthermore, the cream Helixderm®, made from S and derivatives, has been evaluated for wound healing activity in clinical trials undertaken at the Freie University and showed good results with granulation and epithelization.30
In terms of the toxicologic effect of naphthoquinone compounds, it was reported that ShD did not induce any hematologic toxicity in animal models, which indicates that ShD may be safe for use in vivo31, whereas another report demonstrated toxicity in mice by intraperitoneal administration at a dose of 20 mg/kg for S, 41.0/22.75 mg/kg for AS, and 48 mg/kg for 3,3-β, β-DMAS. In addition, toxicity has also been observed during in vivo testing for the antitumor effects of ShD.32
Herbals and herbal drugs contain active ingredients present in the naturally occurring plant source in certain quantities, and the proportion between different constituents or active principles is a key quality parameter for the efficacy of the product. It is in this regard that the modern tools and techniques of analysis provide vital support and required evidence. According to our knowledge, there are no reports related to the phytochemical analyses of naphthoquinone derivatives in Echium sp. using high-performance liquid chromatography (HPLC) quantitatively. Thus, the aims of the present study were to quantify biologically active naphthoquinone derivatives in the ethanol extracts of the roots of four selected Echium species growing wild in Turkey.
EXPERIMENTAL
HPLC analysis of shikonin derivatives in different samples
Plant material and chemicals
E. italicum L., E. vulgare L., plant procured from 56 km north of Ankara, and E. angustifolium Miller., E. parviflorum Moench collected from the coast of Antalya province of Turkey, identified and authenticated by Dr. Gülderen Yılmaz, Assist. Professor, Ankara University, Faculty of Pharmacy, Department of Pharmaceutical Botany. A voucher specimen has been deposited in the Herbarium of Gazi University, Faculty of Pharmacy (GUE2991, GUE2992) and in the herbarium of Ankara University, Faculty of Pharmacy (AEF26023, AEF26024). The reference compounds were purchased from Pureone biotechnology Co. Ltd, China. Chemicals and reagents used in the study were of HPLC grade or analytical grade.
Extraction of plant samples
Extraction of crude plant extracts
The extraction procedure was performed as follows: the shade-dried and coarsely powdered roots of four Echium sp. (100 g) were extracted with 96% alcohol (2 × 750 mL) in a water bath with temperature of 40°C for 48 h. After cooling, the material was filtered and the residue was further extracted with ethanol (500 mL) twice. Following this procedure, all the extracts were pooled together, concentrated under vacuum using a rotavapor (Büchi, Switzerland) to afford the alcoholic extract, and then freeze-dried (Figure 1).
Figure 1.
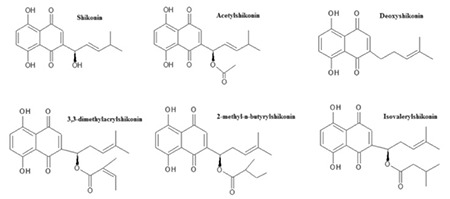
The structures of standard compounds
Identification of shikonin derivatives by thin-layer chromatography
Fifty milligrams of the ethanol extracts made from roots of four Echium species were taken into a set of 10 mL volumetric flasks separately and the volumes were completed to the mark with methanol. A 10-µL aliquot of test samples of different extracts was applied to the TLC plate (aluminum foil-backed TLC plate coated with silica gel 60 F254, Merck, 0.2-mm layer thickness) separately. It was positioned 10 mm from the bottom of the plate, and then the plate was developed to a distance of about 9.5 cm using petroleum ether: ethyl acetate: acetic acid (85:15: 1 /v) as the mobile phase. The plates were then removed, air-dried, and the spots were visualized under an ultraviolet (UV) lamp at 254 nm after spraying 5% sulfuric acid reagent on the plate and heated at 105°C for about 5 min until the bands are clearly visible. The chromatogram obtained with test solution showed a band corresponding to those of ShD (Figure 2).
Figure 2.
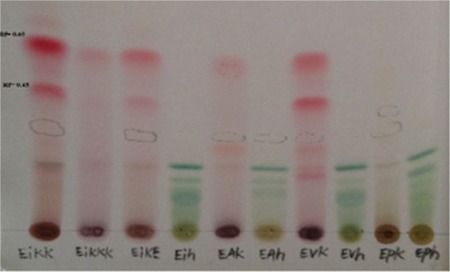
Thin layer chromatogram of four Echium species ethanol extracts from roots and aerial parts
EIKK: Chloroform extract of E. italicum roots, EIKKK: Chloroform extract of E. italicum root barks, EIKE: Ethanol extract of E. italicum roots, EIH: Ethanol extract of E. italicum aerial parts, EAK: Ethanol extract of E. angustifolium roots, EAH: Ethanol extract of E. angustifolium aerial parts, EVK: Ethanol extract of E. vulgare roots, EVH: Ethanol extract of E. vulgare aerial parts, EPK: Ethanol extract of E. parviflorum roots, and EPH: Ethanol extract of E. parviflorum aerial parts
High-performance liquid chromatography
Instrumentation
An Agilent 1200 LC system (equipped with a G1311A pump, G1328B manual injector, G1322A degasser, G1316A column heater, and G1314B variable wavelength UV-detector) was employed (Agilent Technologies-Santa Clara, CA).
Chromatographic conditions
An ACE 5 C18 (150 mm × 4.6 mm, 5 µm) (Scotland) reversed-phase column was used as the stationary phase. An isocratic elution program was applied for chromatographic analysis. The mobile phase was a mixture of acetonitrile: 0.1 M acetic acid contained water=70:30 (pH: 2.82) with isocratic elution, filtered through a 0.45-µm membrane filter (Millipore) and degassed by sonication for 30 min prior to use. The analysis was performed at a flow-rate of 1.0 mL/min. The injection volume was adjusted to 10 µL. The temperature of the column was set to 25°C. The analysis was performed at 520 nm because the maximum UV absorbance for the analytes were obtained at this wavelength.
Preparation of test solution
The dry extracted samples were weighed and finely powdered. Approximately 100 mg of the ground powder was weighed carefully in the vials, then the sample was ultrasonically extracted with 15 mL methanol and 1 mL DMSO for about 30 min. After cooling, the volume was made up to 50 mL with the same solvent. Prior to analysis, the extraction solutions were filtered through a 0.45-µm filter membrane (Merck, Millipore) and 10-µL aliquot was injected into the HPLC system.
Preparation of standard solution
A standard stock solution of S, AS, DS, DMAS, MBS, and IVS was prepared by dissolving 1.0 mg of each compound in 10 mL of acetonitrile in a volumetric flask. For the determination of the limit of detection (LOD) and limit of quantification (LOQ), working standard solutions were prepared by further dilution of this stock solution with aqueous methanol.
Calibration curve
Linear calibration plots were obtained for the related standard compounds at six different concentration levels. After filtering through a 0.45-µm membrane filter, 10 µL of each concentration of the standard solution was injected into the HPLC system for analysis in triplicates. The regression equation and coefficient of correlation is given in Table 1.
Table 1. Validation parameters of the HPLC method for the determination of shikonin derivatives.
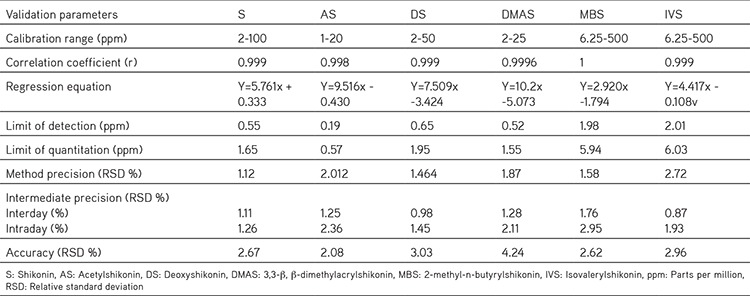
Validation of method
LOD and LOQ were determined experimentally based on the signal-to-noise ratio until the average responses were approximately three (S/N=3) and ten times (S/N=10) that of the blank responses, respectively.
The accuracy of the method was ascertained by spiking the pre-analyzed samples with a known amount of standard solution prepared at three concentration levels (50, 100, and 150 ppm) in triplicate. The average percentage recovery was estimated by applying the values of the peak area to the regression equations of the calibration graph.
Precision test: The mixtures composed of S, DS, AS, DMAS, MBS, and IVS were taken and repeatedly injected six times to measure the peak areas respectively. The results are expressed as relative standard deviation (RSD).
The precision of the method (inter-day and intra-day variations of replicate determinations) was checked by injecting standard solution at different concentrations (12.5, 50, and 100 ppm) in triplicate on the same day and on five consecutive days. The results are reported in terms of RSD.
RESULTS AND DISCUSSION
Identification of active compounds by thin-layer chromatography (TLC)
The structures of the reference compounds analyzed using TLC are shown in Figure 1. The separation of four Echium species root and aerial part ethanol extract with TLC are presented in Figure 2. A comparative chromatographic assessment showed the presence of the ShD in alcoholic extracts of roots of E. italicum L. E. vulgare L. and E. angustifolium Miller. The identification of the bands of ShD (Rf=0.7~0.3) in the sample extract was confirmed by overlaying their absorption with those of the standard compounds at 254 nm and 366 nm.
Optimization of HPLC
The critical aspects of developing a method in liquid chromatography are to provide good resolution between the studied compounds in the shortest analysis time. The current optimized conditions of HPLC with a C18 column and UV detector at 520 nm using isocratic mixture of acetonitrile and water as mobile phase achieved the best-resolved symmetric peaks for ShD. It was observed that the retention time of the reference standards was 4.176~16.58 min (Figure 3). The total run time of the ShD was found to be 20 min and the ShD in each extract could be observed on HPLC-UV chromatogram (Figure 4,5,6,7). This indicates that the present HPLC method is fast, specific, and convenient. The average retention time ± SD for AS, S, DS, DMAS, MBS, and IVS was found to be 4.176±0.003, 8.752±0.005, 11.006±0.004, 15.059±0.003, 15.672±0.004 and 16.584±0.003, respectively, for six replicates.
Figure 3.
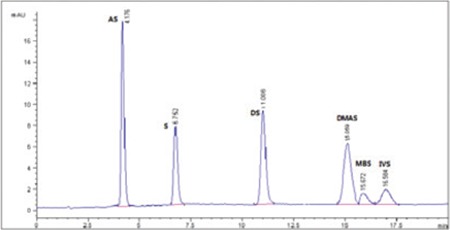
HPLC-UV chromatogram of reference standard mixtures prepared of 50 ppm
HPLC-UV: High-performance liquid chromatography-ultraviolet, AS: Acetylshikonin, S: Shikonin, DS: Deoxyshikonin, DMAS: β, β-dimethylacryshikonin, IVS: Isovalerylshikonin, MBS: 2-methyl butrylshikonin
Figure 4.
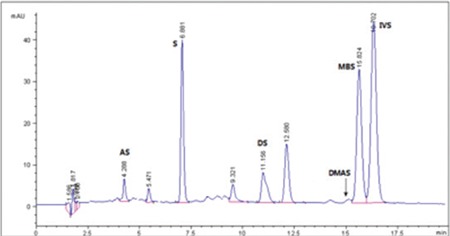
HPLC-UV chromatogram of ethanol extract of E. italicum L. root
HPLC-UV: High-performance liquid chromatography-ultraviolet, AS: Acetylshikonin, S: Shikonin, DS: Deoxyshikonin, DMAS: β, β-dimethylacryshikoninç IVS: Isovalerylshikonin, MBS: 2-methyl butrylshikonin
Figure 5.
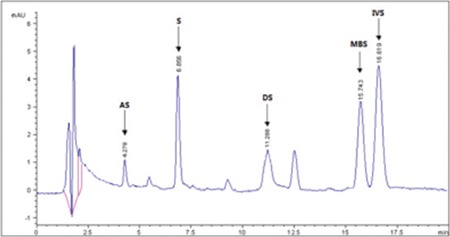
HPLC-UV chromatogram of ethanol extract of E. vulgare L. root
HPLC-UV: High-performance liquid chromatography-ultraviolet, AS: Acetylshikonin, S: Shikonin, DS: Deoxyshikonin, DMAS: β, β-dimethylacryshikonin, IVS: Isovalerylshikonin, MBS: 2-methyl butrylshikonin
Figure 6.
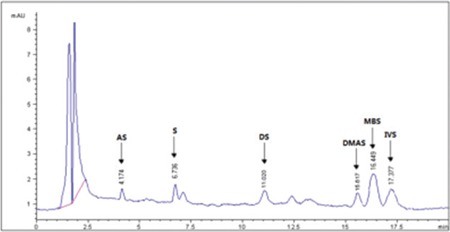
HPLC-UV chromatogram of ethanol extract of E. angustifolium Miller root
HPLC-UV: High-performance liquid chromatography-ultraviolet, AS: Acetylshikonin, S: Shikonin, DS: Deoxyshikonin, DMAS: β, β-dimethylacryshikonin, IVS: Isovalerylshikonin, MBS: 2-methyl butrylshikonin
Figure 7.
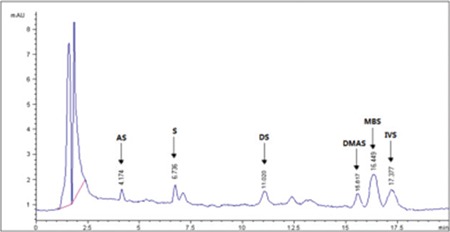
HPLC-UV chromatogram of ethanol extract of E. parviflorum Moench root
HPLC-UV: High-performance liquid chromatography-ultraviolet, AS: Acetylshikonin, S: Shikonin, DS: Deoxyshikonin, DMAS: β, β-dimethylacryshikonin, IVS: Isovalerylshikonin, MBS: 2-methyl butrylshikonin
Validation of method
Calibration graphs were generated by plotting the linear regression of the peak area versus the corresponding analyte concentration; the calibration curve for each analyte was obtained using six concentration in the range of 2-500 ppm. The linear correlation coefficient (r2) for all calibration curves was showing best linearity. The method was validated in terms of precision, accuracy, and other validation method parameters. The repeatability of the HPLC method and intermediate precisions for intra-day and inter-day variations are given in Table 1. The LOD value was found to be 0.19~2.01 ppm, which is the concentration that yields a signal-to-noise (S/N) ratio of 3:1. The LOQ value under the described conditions was 0.57~6.03 ppm with an S/N ratio of 10:1. This confirmed the sensitivity for the quantification of the compounds.
HPLC analysis of shikonin derivatives in different samples
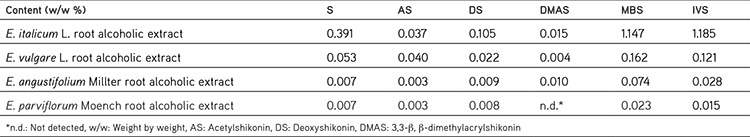
The concentrations of the ShD in ethanol extracts of different Echium species were determined from the peak-area ratios using the equations of linear regression obtained from the calibration curves. Data for the concentration of ShD in ethanol extracts obtained from different Echium species growing wild in Turkey are presented in Table 2. As can be observed, the content of ShD was the highest in E. italicum L. compared with the other species.
Table 2. Quantitative determination of shikonin derivatives in each Echium species.
CONCLUSIONS
S and derivatives are naturally occurring isohexenylnaphthazarine compounds, which are used as a red dye in drugs, cosmetics, and the textile industry in the Far East and Europe33, and comprise the active ingredients of several pharmaceutical preparations because they exert potent biologic activities with wound healing, antimicrobial, cytotoxic, and antioxidant properties. However, these bioactive constituents become expensive due to their physical instability and tedious separation process. Therefore, it is important to develop a simple, fast, and sensitive method to evaluate S compounds in drugs, extracts, and pharmaceutical preparations. The implications of the current study are important because we have standardized the raw materials and preparations of the studied four Echium sp. roots for the first time. To the best of our knowledge, this is the first study to determine the amount of S and ShD in the four Echium sp. We developed a simple, accurate, fast, and reproducible HPLC method to quantify the active principles in Echium species. This method can be used for routine quality control analysis of this species. Although ShD are the main constituents of Echium sp. roots, the results of the current study also indicate that the maximum S derivative content in Echium species can be found in Echium italicum L. extract as compared with other studied extracts. Since most of the biologic activities such as antimicrobial, antiproliferative, antioxidant, analgesic, and antidepressant activities of Echium sp. roots can be attributed to ShD, the quantification of such compounds in the roots of these plants need to be considered as very important for the future use of preparations of these plants in several areas of clinical medicine. By using the optimized separation method described above, ShD can be detected in herbals or formulations prepared from Boraginaceae family plants, which are rich in naphthoquinone derivatives. We have demonstrated that the method is suitable for application in quality control of herbal formulations.
Acknowledgments
The authors thank Osman Üstün, Uğur Tamer and Murat Şüküroğlu for helping with the research. The author would like to thank Ali Çetin for his careful reading of the manuscript and helpful comments and suggestions. This work was supported by the Gazi University Academic Research Grants [grant number: GÜBAP 02-2010/2011].
Footnotes
Conflict of Interest: No conflict of interest was declared by the author.
References
- 1.Ahvazi M, Khalighi-Sigaroodi F, Charkhchiyan MM, Mojab F, Mozaffarian VA, Zakeri H. Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran J Pharm Res. 2012;11:185–194. [PMC free article] [PubMed] [Google Scholar]
- 2.Çakilcioğlu U, Turkoglu I. An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey) J Ethnopharmacol. 2010;132:165–175. doi: 10.1016/j.jep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Chevallier A. The Encyclopedia of Medicinal Plants. Dorling Kindersley Limited, London. 1996. [Google Scholar]
- 4.Grieve M. A Modern Herbal 2. Dover Publications. NewYork. 1982. [Google Scholar]
- 5.Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chemistry. 2009;114:1438–1443. [Google Scholar]
- 6.Niciforovic N, Mihailovic V, Maskovic P, Solujic S, Stojkovic a, Pavlovic Muratspahic D. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem Toxicol. 2010;48:3125–3130. doi: 10.1016/j.fct.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Ranjbar A, Khorami S, Safarabadi M, Shahmoradi A, Malekirad AA, Vakilian K, Mandegary A, Abdollahi M. Antioxidant Activity of Iranian Echium amoenum Fisch & C.A. Mey Flower Decoction in Humans: A cross-sectional Before/After Clinical Trial. vid Based Complement Alternat Med. 2006;3:469–473. doi: 10.1093/ecam/nel031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abed A, Minaiyan M, Ghannadi A, Mahzouni P, Babavalian MR. Effect of Echium amoenum Fisch. et Mey a Traditional Iranian Herbal Remedy in an Experimental Model of Acute Pancreatitis. ISRN Gastroenterol. 2012;2012:141548. doi: 10.5402/2012/141548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abolhassani M. Antibacterial Effect of Borage (Echium amoenum) on Staphylococcus aureus. Braz J Infect Dis. 2004;8:382–385. doi: 10.1590/s1413-86702004000500008. [DOI] [PubMed] [Google Scholar]
- 10.Karakaş FP, Yildirim A, Türker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turk J Biol. 2012;36:641–652. [Google Scholar]
- 11.Farahani M. Antiviral Effect Assay of Aqueous Extract of Echium amoenum-L against HSV-1. Zahedan J Res Med Sci. 2013;15:46–48. [Google Scholar]
- 12.Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Uysal H, Kizilet H, Ayar A, Taheri A. The use of endemic Iranian plant, Echium amoenum, against the ethyl methanesulfonate and the recovery of mutagenic effects. Toxicol Ind Health. 2015;31:44–51. doi: 10.1177/0748233712468019. [DOI] [PubMed] [Google Scholar]
- 14.Wassel G, el-Menshawi B, Saeed A, Mahran G, el-Merzabani M. Screening of selected plants for pyrrolizidine alkaloids and antitumor activity. Pharmazie. 1987;42:709. [PubMed] [Google Scholar]
- 15.Heidari MR, Azad EM, Mehrabani M. Evaluation of the analgesic effect of Echium amoenum Fisch & C.A. Mey. extract in mice: possible mechanism involved. J Ethnopharmacol. 2006;103:345–349. doi: 10.1016/j.jep.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Moallem SA, Hosseinzadeh H, Ghoncheh F. Evaluation of Antidepressant Effects of Aerial Parts of Echium vulgare on Mice. JBMS. 2007;10:189–196. [Google Scholar]
- 17.Sayyah M, Kamalinejad M. A preliminary randomized double blind clinical trial on the efficacy of aqueous extract of Echium amoenum in the treatment of mild to moderate major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:166–169. doi: 10.1016/j.pnpbp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Sayyah M, Boostani H, Pakseresht S, Malaieri A. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1513–1516. doi: 10.1016/j.pnpbp.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Gholamzadeh S, Zare S, Ilkhanipoor M. Evaluation of the anxiolytic effect of Echium amoenum petals extract, during chronic treatment in rat. Research in pharmaceutical science. 2007;2:91–95. [Google Scholar]
- 20.Gholamzadeh S, Zare S, Ilkhanipoor M. Anxiolytic Effect of Echium amoenum During Different Treatment Courses. Research Journal of Biological Sciences. 2008;3:176–178. [Google Scholar]
- 21.Gomes NG, Campos MG, Orfao JM, Ribeiro CA. Plants with neurobiological activity as potential targets for drug discovery. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1372–1389. doi: 10.1016/j.pnpbp.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Rabbani M, Sajjadi SE, Vaseghi G, Jafarian A. Anxiolytic effects of Echium amoenum on the elevated plus-maze model of anxiety in mice. Fitoterapia. 2004;75:457–464. doi: 10.1016/j.fitote.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Shafaghi B, Naderi N, Tahmasb L, Kamalinejad M. Anxiolytic Effect of Echium amoenum L. in Mice. Iranian Journal of Pharmaceutical Research. 2002;1:37–41. [Google Scholar]
- 24.Albreht A, Vovk I, Simonovska B, Srbinoska M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J Chromatogr A. 2009;1216:3156–3162. doi: 10.1016/j.chroma.2009.01.098. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Yin R, Zhou W, Xu DF, Li S. Shikonin and its derivatives: a patent review. Expert Opin Ther Pat. 2012;22:977–997. doi: 10.1517/13543776.2012.709237. [DOI] [PubMed] [Google Scholar]
- 26.Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK, Pan MH. Induction of apoptosis by shikonin through coordi native modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem. 2004;52:6330–6337. doi: 10.1021/jf0495993. [DOI] [PubMed] [Google Scholar]
- 27.Gao D, Hiromura M, Yasui H, Sakurai H. Direct reaction between shikonin and thiols induces apoptosis in HL60 cells. Biol Pharm Bull. 2002;25:827–832. doi: 10.1248/bpb.25.827. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Wu LJ, Li LH, Tashiro S, Onodera S, Ikejima T. Shikonin regulates HeLa cell death via caspase-3 activation and blockage of DNA synthesis. J Asian Nat Prod Res. 2004;6:155–166. doi: 10.1080/1028602032000169622. [DOI] [PubMed] [Google Scholar]
- 29.Guo XP, Zhang XY, Zhang SD. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi. 1991;11:598–599. [PubMed] [Google Scholar]
- 30.Papageorgiou VP, Assimopoulou AN, Ballis AC. Alkannins and Shikonins: A New Class of Wound Healing Agents. Curr Med Chem. 2008;15:3248–3267. doi: 10.2174/092986708786848532. [DOI] [PubMed] [Google Scholar]
- 31.Su L, Liu LH, Wang Y, Yan G, Zhang Y. Long term systemic toxicity of shikonin derivatives in Wistar rats. Pharm Biol. 2014;52:486–490. doi: 10.3109/13880209.2013.846913. [DOI] [PubMed] [Google Scholar]
- 32.Papageorgiou VP, Assimopoulou AN, Couldouros EA, Heapworth D, Nicoloau KC. The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products. Angew Chem Int Ed. 1999;38:270–300. doi: 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Bozan B, Baser KHC, Kara S. Quantitative determination of naphthoquinones of Arnebia densiflora by TLC-densitometry. Fitoterapia. 1999;70:402–406. [Google Scholar]


