Abstract
Objectives:
In the present study, the antioxidant potency of ethyl acetate (AcOEt) and methanol (MeOH) extracts from the aerial parts of Seseli L. species was investigated for the first time.
Materials and Methods:
Seseli species L. such as Seseli andronakii Woronow ex Schischk., S. campestre Besser, S. corymbosum Boiss. & Heldr., S. gummiferum subsp. gummiferum Pall. ex Sm., S. hartvigii Parolly & Nordt, S. libanotis (L.) W.Koch, S. petraeum M.Bieb., S. peucedanoides (M.Bieb.) Koso-Pol., S. resinosum Freyn & Sint., and S. tortuosum L. growing in Turkey were collected and evaluated for their antioxidant capacity by using 1.1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and lipid peroxidation (LPO) inhibition methods.
Results:
The highest activities as a scavenger of DPPH radicals were found in the AcOEt extracts of S. peucedanoides (M.Bieb.) Koso-Pol (IC50=0.49 mg/mL), and S. libanotis (IC50=0.75 mg/mL); α-tocopherol was used as a positive control. On the other hand, in the LPO assay, the highest activities were determined in AcOEt and MeOH extracts (at 5 mg/mL) of S. tortuosum and S. libanotis (84-94%).
Conclusion:
This report gives important information about the antioxidant capacity of Seseli L. species. This research on antioxidant capacity proves that the use of some species used in Eastern Anatolia (in salads) is correct. With this screening study performed in Seseli L. species growing in Turkey, in the future, it is planned to isolate antioxidant compounds from the most active strains of Seseli L.
Keywords: Antioxidant, Apiaceae, DPPH, LPO, Seseli
INTRODUCTION
The Apiaceae (previously Umbelliferae) is a well-known family in the plant kingdom with aromatic plants and economically important species.1 Some members of the family are used as foods, spices, condiments, and ornaments.2,3,4 The genus Seseli L. belongs to the family Apiaceae and is distributed in Asia and Europe, comprising more than 12 taxa in Turkey, of which 4 are native to the region.5,6,7,8 In addition, new species have recently been discovered.9,10,11,12 Moreover, the latest taxonomy of the type section of the genus Seseli has been given based on the molecular data with recently updated names.13 Seseli is an ancient Greek name given to some individual members of the family Apiaceae by Hippocrates.14 Seseli species are mainly rich in coumarins as well as terpenoids, essential oils, etc.15,16 and have many important pharmacological activities with healing effects such as in inflammation, swelling, rheumatism, pain, and the common cold.17 On the other hand, the fruit of S. indicum has been reported to have anthelmintic, carminative, stomachic, and stimulant properties.18 S. sibiricum is used for blending beverages and as a medicine for livestock in Kashmir.19 In addition, the fruit of S. libanotis is a local remedy for blood pressure control in Pakistan, and its essential oil from the fruit has potent antimicrobial activity.20 While S. indicum exhibited strong insect repellent activity21 and fungitoxicity,22 the fruit of S. tortuosum is recorded to have emmenagogic and antiflatulent effects.23 Moreover, the leaves of S. libanotis (Kelemkeşir or Kelemenkeşir in Turkish) are consumed as a vegetable in salads in Eastern Turkey.24
In Turkey, there are limited studies on Seseli species based on coumarins25,26,27,28,29 and essential oils.30,31,32,33,34 Previously, antimicrobial,35 anti-inflammatory, and antinociceptive36,37,38 effects have been examined in Turkish Seseli species.
The plant kingdom presents secondary plant metabolites (especially polyphenols) as a wide range of natural antioxidants.39,40,41,42 The natural antioxidants in plants are of great interest in natural product science and many herbs have significant antioxidant potency.43 Antioxidants decrease oxidative stress in cells and are therefore very useful in the treatment of major degenerative diseases.44 The physiological role of antioxidant agents is to scavenge for free radicals45,46 in the case of overproduction of these reactive species.47
Therefore, in the present study, we aimed to investigate the antioxidant potential of the aerial parts of Turkish Seseli species. The species were screened using in vitro 1.1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and lipid peroxidation (LPO) inhibition assays.
MATERIALS AND METHODS
Plant material
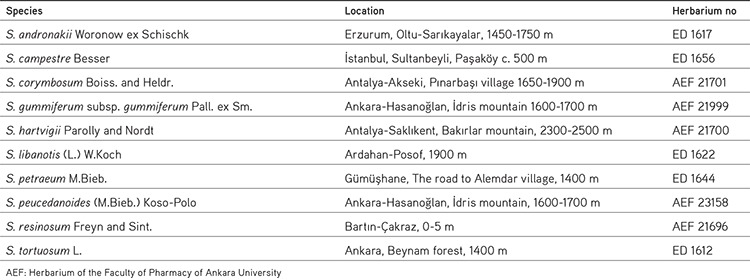
Plant materials were collected from different localities in Turkey. All of the Seseli L. species were identified by Prof. H. Duman from the Department of Biology, Faculty of Science and Arts, Gazi University, Ankara, Turkey. Voucher specimens were deposited at the Herbarium of the Faculty of Pharmacy of Ankara University and the Herbarium of Gazi University, Ankara, Turkey. The species are listed in Table 1 (ethical committee approval and patient consent were not required).
Table 1. Plant names and collection sites of Turkish Seseli L. species.
Extraction of the plants
The extraction method in Fenglin et al.48 and Báthori et al.49 was used with some modifications. The aerial parts of each plant material, which were dried and powdered, were prepared according to the procedures described below:
-The ethyl acetate (AcOEt) extract: The plant material (10 g) was extracted with AcOEt at room temperature by a magnetic stirrer (x200 mL) for 24 hour. The extract was evaporated to dryness in a vacuum to give a crude AcOEt extract.
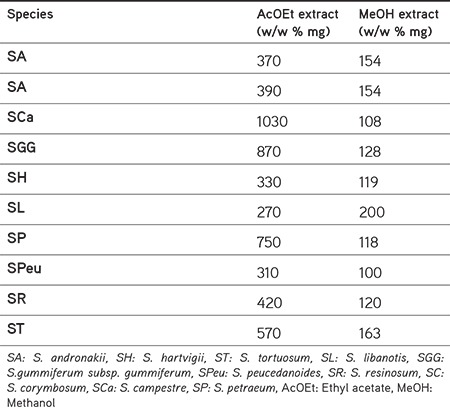
-The methanol (MeOH) extract: After the AcOEt extraction, the plant material (10 g) was extracted with MeOH (80%) at room temperature by a magnetic stirrer (x200 mL) for 24 hour. The extract was evaporated to dryness in vacuo to give a crude methanolic extract. The yields of all extracts are given in Table 2.
Table 2. The yield of extracts from Turkish Seseli L. species.
Chemicals
Ascorbic acid, thiobarbituric acid (TBA), DPPH, and α-tocopherol were purchased from Sigma Chemical Co (St. Louis, MO, USA).
Antioxidant capacity of the extracts
Radical scavenging capacity (DPPH)
The model of scavenging stable DPPH radicals is a widely used method to evaluate antioxidant activities in a relatively short time compared with other methods. The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability.50 The reaction mixture contained 100 µM DPPH in MeOH and different concentrations of the crude extract. Absorbance at 517 nm was measured on a Shimadzu UV-1601 UV-VIS spectrometer at various concentrations (30 min after starting the reaction) at room temperature and the scavenging activity was calculated as the percentage of radical reduction. In our study, samples were dissolved in MeOH (80%) and AcOEt to 10 mg/mL and diluted to various concentrations. The scavenging activity was calculated as the percentage of radical reduction. The values of IC50 were determined from a calibration curve for each plant extract. Each experiment was performed in triplicate. IC50 values were determined from a calibration curve for each plant extract and α-tocopherol was used as the reference compound.
Assay of lipid peroxidation (LPO)
LPO was determined by a modified version of the method described by Mihara et al.51 It was measured spectrophotometrically by estimation of the TBA reactant substances (TBARS). Amounts of TBARS were expressed in nmoL malondialdehyde/g tissue. A typical optimized assay mixture containing 0.5 mL of liver homogenate, 0.1 mL of Tris-HCl buffer (pH 7.2), 0.05 mL of 0.1 mM ascorbic acid, and 0.05 mL of 4 mM FeCl2 and 0.05 mL of various concentrations of crude extract or α-tocopherol were incubated for 1 h at 37°C. After incubation, 3.0 mL of H3PO4 and 1 mL of 0.6% TBA were added and the resulting mixture was shaken vigorously. The mixture was boiled for 30 minute. After cooling, n-butanol was added and the mixture was shaken vigorously. Then the n-butanol phase was separated by centrifugation at 3000 rpm for 10 minute. The absorbance of the supernatant was measured at 532 nm against a blank, which contained all reagents except the liver homogenate.
Statistical analysis
Values of experimental results were considered as the mean of at least three determinations (± standard deviation).
RESULTS AND DISCUSSION
The present study deals with the radical scavenging activity (Table 3) and LPO (Table 4) of the AcOEt and MeOH extracts obtained from Seseli L. species growing in Turkey such as Seseli andronakii, S. campestre, S. corymbosum, S. gummiferum subsp. gummiferum, S. hartvigii, S. libanotis, S. petraeum, S. peucedanoides (M.Bieb.) Koso-Pol, S. resinosum, and S. tortuosum. The antioxidant activities of AcOEt and MeOH extracts obtained from the Seseli species were investigated by the DPPH scavenging and nonenzymatic rat hepatic microsomal LPO methods. In addition, their antioxidant activities were compared with those of the standard antioxidant α-tocopherol. The DPPH free radical scavenger assay is a simple and basic screening method for the discovery of bioactive substances. Free radicals are species that damage all the components of the body (lipids, proteins, DNA, etc.) and take part in mutations. In this case, antioxidants are important for body protection, helping reduce oxidative damage in the human body, and prevent LPO in foods.52,53
Table 3. Inhibitory effects of Seseli extracts on DPPH stable radicals.
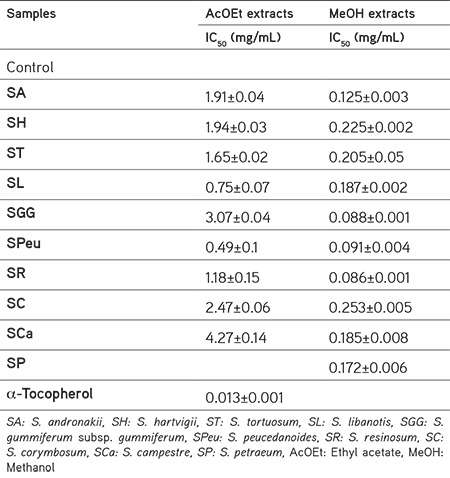
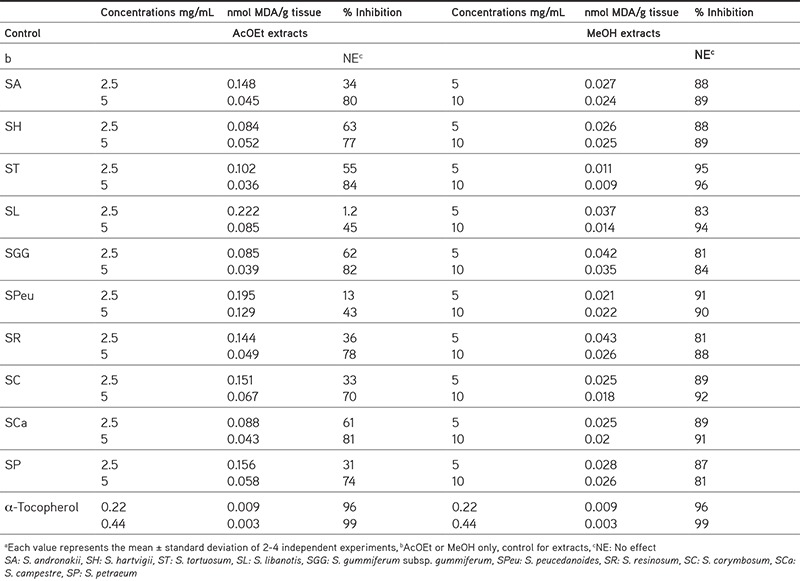
Table 4. Antilipid peroxidation effects of Seseli extractsa.
In our experiments, the results indicated that the extracts of some Turkish Seseli species have considerable effects on scavenging DPPH radicals (Figure 1). The AcOEt extract of S. peucedanoides (IC50=0.49 mg/mL) and S. libanotis (IC50=0.75 mg/mL) showed the most potent radical scavenging capacity (Table 3). These extracts were followed by S. resinosum (IC50=1.18 mg/mL), S. tortuosum (IC50=1.65 mg/mL), S. andronakii (IC50=1.91 mg/mL), S. hartvigii (IC50=1.94 mg/mL), S. corymbosum (IC50=2.47 mg/mL), S. gummiferum subsp. gummiferum (IC50=3.07 mg/mL), and S. campestre (IC50=4.27 mg/mL) extracts.
Figure 1.
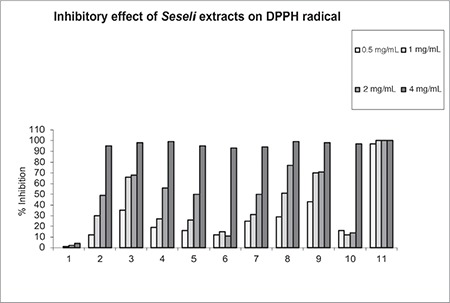
Ethyl acetate extracts of Seseli species (1-10) and (11) α-tocopherol at various concentrations
(1) S. andronakii, (2) S. hartvigii, (3) S. tortuosum, (4) S. libanotis,
(5) S. gummiferum subsp. gummiferum, (6) S. peucedanoides, (7) S. resinosum, (8) S. corymbosum, (9) S. campestre, (10) S. petraeum
The MeOH extracts of Seseli species have a higher DPPH radical scavenging effect than AcOEt extracts. The results showed that MeOH extracts of S. resinosum, S. gummifeum subsp. gummiferum, and S. peucedanoides have the highest scavenging capacity (IC50=0.086, IC50=0.088, and IC50=0.091, respectively).
The TBA test results showed that MeOH extracts of Seseli spp. exhibited potent antioxidant effects (81-96% inhibition at 5 and 10 mg/mL concentrations) when compared to α-tocopherol. The AcOEt and MeOH extracts of S. tortuosum have the strongest anti-LPO activity (84-96% inhibition at a dose of 10 mg). The AcOEt and MeOH extracts of S. campestre, S. andronakii, and S. gummiferum subsp. gummiferum also exhibited a high anti-LPOeffect in the LPO assay (Table 4).
In previous studies, the antioxidant potency of MeOH extract of S. pallasii, S. libanotis subsp. libanotis, and S. libanotis subsp. intermedium (aerial parts and fruits) was determined. S. libanotis subsp. libanotis showed the strongest antioxidant activity in the DPPH assay.54 Various extracts in different polarities from the roots, leaves, flowers, and fruit of S. rigidum were also studied, and the hexane extract of the root had the best effect among the other plant parts in the DPPH assay.55,56 In another study, the antioxidant activity of Seseli rigidum was evaluated in five extracts in different polarities (water, MeOH, acetone, ethyl acetate, and petroleum ether). The antioxidant effect of the aerial parts of the species was determined in vitro using DPPH reagent, and the highest antioxidant activity was expressed in water extract (46.15 µg/mL).57 Moreover, some of the compounds isolated from the methanolic extracts (80%) of Seseli diffusum have been found to have a strong antioxidant effect.58
It is known that Seseli species contain phenolic compounds consisting mainly of coumarins,16 which have notable antioxidant potency.59,60,61 In addition, mostly oxygenated coumarins are accumulated in the AcOEt fractions, and the glycosides are present in the MeOH extract. The MeOH extract exhibits higher antioxidant activity, which may be explained by the presence of coumarin glucosides as highly polar compounds in the extract. The results show that there seemed to be a good match between the content of the extracts and the antioxidant capacity. Finally, the activity might be due to the polar coumarins of the active Seseli species.52,62
CONCLUSION
Natural products are generally known to be a good source of active compounds that have potential for the development of new therapeutic agents. The antioxidant properties of the AcOEt and MeOH extracts of Seseli species expressed as α-tocopherol equivalent antioxidant capacity were studied using DPPH and LPO assays. These results indicate that plant extracts prevent oxidative damage in normal cells due to their antioxidant properties. The best part of our research was that Seseli species growing in Turkey were screened for the first time for their antioxidant capacity. In addition, this research provides a scientific basis for the medicinal use of these plant materials. Therefore, we can conclude from the results of the present study that Seseli species may be a potential source of natural antioxidant compounds for the treatment of oxidative degeneration.
Footnotes
Conflicts of interest: No conflict of interest was declared by the authors.
References
- 1.Sayed-Ahmad B, Talou T, Saad Z, Hijazi A, Merah O. The Apiaceae: ethnomedicinal family as a source for industrial uses. Ind Crops Prod. 2017;109:661–671. [Google Scholar]
- 2.Crowden RK, Harborne JB, Heywood VH. Chemosystematics of the Umbelliferae - a general survey. Phytochemistry. 1969;8:1963–1984. [Google Scholar]
- 3.Lawrence GHM. Taxonomy of Vascular Plants. New York; Macmillan. 1969:642–646. [Google Scholar]
- 4.Pimenov MG, Leonov MV. The Genera of the Umbelliferae. London; Royal Botanic Gardens. 1993. [Google Scholar]
- 5.Hedge IC, Lamond JM, Seseli L. In Davis PH, ed. Flora of Turkey and the East Aegean Islands, vol. 4. Edinburgh; Edinburgh University Press. 1972. [Google Scholar]
- 6.Davis PH, Mill RR, Tan K. Flora of Turkey and the East Aegean Islands, vol. 10. Edinburgh; Edinburgh University Press. 1988. [Google Scholar]
- 7.Duman H, Seseli L. In Güner A, Özhatay N, Ekim T, Başer KHC, eds. Flora of Turkey and the East Aegean Islands, vol. 11. Edinburgh; Edinburgh University Press. 2000. [Google Scholar]
- 8.Parolly G, Nordt B. Seseli hartvigii (Apiaceae), a new name for S. ramosissimum Hartvig & Strid, with carpological and ecological notes on this species. Willdonowia. 2001;31:87–93. [Google Scholar]
- 9.Pimenov MG, Kljuykov EV. Inclusion of eriocycla into Seseli (Umbelliferae) and description of some new sections and subsections within the genus Seseli. Bot Zhurn. 2000;85:96–109. [Google Scholar]
- 10.Pimenov MG, Kljuykov EV. Two new species of Seseli (Umbelliferae) from Turkey. Fl Medit. 2010;20:19–27. [Google Scholar]
- 11.Güner E, Duman H. The revision of genus Seseli (Umbelliferae) in Turkey. Turk J Bot. 2013;37:1018–1037. [Google Scholar]
- 12.Çetin Ö, Şeker MÖ, Duran A. A new subspecies of Seseli gummiferum (Apiaceae) from the Ilgaz Mountain National Park, Northern Turkey. Phyto Keys. 2015;56:99–110. doi: 10.3897/phytokeys.56.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyskov D, Doğan Güner E, Samigullin T, Duman H, Pimenov M. Molecular data to elucidate the taxonomy of Seseli sect. Seseli (Apiaceae) in east Mediterranean and southern Europe. Nordic J Bot. 2018;2018:e01857. [Google Scholar]
- 14.Hamlyn P. The Marshall Cavendish Encyclopedia of Gardening. vol. 19. London; Garrod and Lofthouse International. 1969. [Google Scholar]
- 15.Barrero AF, Herrador MM, Arteaga P. Cumarinas en especies del genero Seseli. (Fam. Umbelliferae) Ars Pharm. 1990;31:241–256. [Google Scholar]
- 16.Tosun A, Özkal N, Seseli L. (Umbelliferae) türlerinin kimyasal bileşimi ve biyolojik aktiviteleri/Chemical constituents and biological activities of Seseli L. (Umbelliferae) species. J Fac Pharm Ankara. 2003;32:269–284. [Google Scholar]
- 17.Hu CQ, Chang JJ, Lee KH. Antitumor agents, 115. Seselidiol, a new cytotoxic polyacetylene from Seseli mairei. J Nat Prod. 1990;53:932–935. doi: 10.1021/np50070a022. [DOI] [PubMed] [Google Scholar]
- 18.Tandan SK, Chandra S, Tripathi HC, Lal J. Pharmacological actions of seselin, a coumarin from Seseli indicum seeds. Fitoterapia. 1990;61:360–363. [Google Scholar]
- 19.Austin PW, Seshadri TR, Sood MS. Vishwapaul. Components of Seseli sibiricum: constitution and synthesis of sibiricin, a new coumarin. Tetrahedron. 1968;24:3247–3253. [Google Scholar]
- 20.Syed M, Chaudhary FM, Bhatty MK. Antimicrobial activity of the essential oils of the Umbelliferae family. Part VIII. Seseli libanotis, Ligusticum stewartii, and Pycnocycla aucheriana oils. Pak J Sci Ind Res. 1989;32:316–319. [Google Scholar]
- 21.Dixit V, Chaturvedi RV, Tripathi SC. Evaluation of some essential oils against Pulse Bettle (Callosobruchus chinensis) Natl Acad Sci Lett. 1992;15:255–257. [Google Scholar]
- 22.Chaturvedi RV, Tripathi SC. Fungutoxic, physicochemical and phytotoxic properties of essential oil of Seseli indicum W and A. J Phytopathol. 1989;124:316–322. [Google Scholar]
- 23.Baytop T. Türkiye’de Bitkilerle Tedavi. Therapy with Plants in Turkey (Past and Present) (2nd ed) İstanbul; Nobel Medical House. 1999. [Google Scholar]
- 24.Baytop T. Türkçe Bitki Adları Sözlüğü, Atatürk Kültür, Dil ve Tarih Yüksek Kurumu, TDKY 3578. Ankara; TTK Basımevi. 1994. [Google Scholar]
- 25.Tosun A, Özkal N, Baba M, Okuyama T. Pyranocoumarins from Seseli gummiferum subsp. corymbosum growing in Turkey. Turk J Chem. 2005;29:327–334. [Google Scholar]
- 26.Tosun A. Occurrence of coumarins in Seseli hartvigii growing in Turkey. Chem Nat Compd. 2006;42:608–609. [Google Scholar]
- 27.Tosun A, Baba M, Bahadır Ö, Okuyama T. Coumarins isolated from the roots of Seseli resinosum in Turkey. Pharm Biol. 2006;44:528–533. [Google Scholar]
- 28.Zhang L, Tosun A, Baba M, Okada Y, Wu L, Okuyama T. Coumarins from Seseli hartvigii. Nat Prod Commun. 2010;5:1067–1070. [PubMed] [Google Scholar]
- 29.Shehzad O, Khan S, Ha IJ, Park Y, Tosun A, Kim YS. Application of stepwise gradients in counter-current chromatography: a rapid and economic strategy for the one-step separation of eight coumarins from Seseli resinosum. J Chromatogr A. 2013;1310:66–73. doi: 10.1016/j.chroma.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Başer KHC, Özek T, Kürkçüoglu M, Aytaç Z. Essential oil of Seseli campestre Besser. J Essent Oil Res. 2000;12:105–107. [Google Scholar]
- 31.Kaya A, Demirci B, Baser KHC. The essential oil of Seseli tortuosum growing in Turkey. Flavour Fragrance J. 2003;18:159–161. [Google Scholar]
- 32.Tosun A, Baba M, Kodama T, Nakanishi H, Okuyama T. The composition of essential oils of Seseli species growing in Turkey. Nat Med. 2005;59:85–90. [Google Scholar]
- 33.Tosun A, Doğan E, Duman H, Kürkçüoğlu M, Başer KHC. Essential oil composition of the fruits of Seseli resinosum Freyn et Sint. and Seseli tortuosum L. growing in Turkey. J Essent Oil Res. 2006;18:57–59. [Google Scholar]
- 34.Tosun A, Kürkçüoğlu M, Doğan E, Duman H, Başer KHC. Essential oil composition of Seseli petraeum M. Bieb. and Seseli andronakii Woron. growing in Turkey. Flavour Fragrance J. 2006;21:257–259. [Google Scholar]
- 35.Tosun A, Özkal N, Yıldız S. Antimicrobial activity screening of some Seseli L. species growing in Turkey. Ankara Ecz Fak Derg. 2004;33:151–155. [Google Scholar]
- 36.Küpeli E, Tosun A, Yeşilada E. Anti-inflammatory and antinociceptive activities of Seseli L. species (Umbelliferae) growing in Turkey. J Ethnopharmacol. 2006;104:310–314. doi: 10.1016/j.jep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Tosun A, Akkol EK, Yeşilada E. Anti-inflammatory and antinociceptive activities of Seseli L. species (Apiaceae) growing in Turkey. Z Natur Forsch C. 2009;64:56–62. doi: 10.1016/j.jep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Chun J, Tosun A, Kim YS. Anti-inflammatory effect of corymbocoumarin from Seseli gummiferum subsp. corymbosum through suppression of NF-κB signaling pathway and induction of HO-1 expression in LPS-stimulated RAW 264.7 cells. Inter Immunopharmacol. 2016;31:207–215. doi: 10.1016/j.intimp.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Lee SE, Hwang HJ, Ha J-S, Jeong H-S, Kim JH. Screening medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–179. doi: 10.1016/s0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 40.Kim M-B, Park J-S, Lim S-B. Antioxidant activity and cell toxicity of pressurized liquid extracts from 20 selected plant species in Jeju, Korea. Food Chem. 2010;122:546–552. [Google Scholar]
- 41.Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int J Mol Sci. 2018;25:19:1573. doi: 10.3390/ijms19061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olas B. Berry phenolic antioxidants - implications for human health? Front Pharmacol. 2018;9:78. doi: 10.3389/fphar.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–723. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 44.Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–233. [Google Scholar]
- 45.Muraina IA, Suliman MM, Eloff JN. Can MTT be used to quantify the antioxidant activity of plant extracts? Phytomedicine. 2009;16:665–668. doi: 10.1016/j.phymed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford; Clarendon Press. 1989. [Google Scholar]
- 47.Wong SP, Leong LP, Koh JHW. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;9:775–783. [Google Scholar]
- 48.Fenglin H, Ruili L, Bao H, Liang M. Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia. 2004;75:14–23. doi: 10.1016/j.fitote.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Báthori M, Zupkó I, Hunyadi A, Gácsné-Baitz E, Dinya Z, Forgó P. Monitoring the antioxidant activity of extracts originated from various Serratula species and isolation of flavonoids from Serratula coronata. Fitoterapia. 2004;75:162–167. doi: 10.1016/j.fitote.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 51.Mihara M, Uchiyama M, Fukuzawa K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem Med. 1980;23:302–311. doi: 10.1016/0006-2944(80)90040-x. [DOI] [PubMed] [Google Scholar]
- 52.Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000;66:725–735. doi: 10.1016/s0024-3205(99)00643-8. [DOI] [PubMed] [Google Scholar]
- 53.Ellnain-Wojtaszek M, Kruczynski Z, Kasprzak J. Investigation of the free radical scavenging activity of Ginkgo biloba L. leaves. Fitoterapia. 2003;74:1–6. doi: 10.1016/s0367-326x(02)00306-4. [DOI] [PubMed] [Google Scholar]
- 54.Matejić JS, Džamić AM, Mihajilov-Krstev T, Ranđelović VN, Krivošej ZD, Marin PD. Total phenolic content, flavonoid concentration, the antioxidant and antimicrobial activity of methanol extracts from three Seseli L. taxa. Cent Eur J Biol. 2012;7:1116–1122. [Google Scholar]
- 55.Stojkovic S, Petrovic S, Kukic J, Dzamic A, Ristic M, Milenkovic M, Glamoclija J, Sokovic M, Stojkovic D. Chemical composition and antimicrobial and antioxidant activity of Seseli rigidum flower essential oil. Chem Nat Compd. 2009;45:253–256. [Google Scholar]
- 56.Stankov-Jovanović VP, Ilić MD, Mitić VD, Mihajilov-Krstev TM, Simonović SR, Nikolić Mandić SD, Tabet JC, Cole RB. Secondary metabolites of Seseli rigidum: chemical composition plus antioxidant, antimicrobial and cholinesterase inhibition activity. J Pharm Biomed Anal. 2015;111:78–90. doi: 10.1016/j.jpba.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Jakovljević D, Vasić S, Stanković M, Čomić L, Topuzović M. In vitro biological activity of secondary metabolites from Seseli rigidum Waldst. et Kit. (Apiaceae) Acta Biol Hung. 2015;66:395–405. doi: 10.1556/018.66.2015.4.4. [DOI] [PubMed] [Google Scholar]
- 58.Abbaskhan A, Choudhary MI, Ghayur MN, Parween Z, Shaheen F, Gilani AU, Maruyama T, Iqbal K, Tsuda Y. Biological activities of Indian celery, Seseli diffusum (Roxb. ex Sm.) Sant. & Wagh. Phytother Res. 2012;26:783–786. doi: 10.1002/ptr.3600. [DOI] [PubMed] [Google Scholar]
- 59.Torres FC, Brucker N, Andrade SF, Kawano DF, Garcia SC, Poser GL, Eifler-Lima VL. New insights into the chemistry and antioxidant activity of coumarins. Curr Top Med Chem. 2014;14:2600–2623. doi: 10.2174/1568026614666141203144551. [DOI] [PubMed] [Google Scholar]
- 60.Pereira TM, Franco DP, Vitorio F, Kummerle AE. Coumarin compounds in medicinal chemistry: some important examples from the last years. Curr Top Med Chem. 2018;18:124–148. doi: 10.2174/1568026618666180329115523. [DOI] [PubMed] [Google Scholar]
- 61.Zhu JJ, Jiang JG. Pharmacological and nutritional effects of natural coumarins and their structure-activity relationships. Mol Nutr Food Res. 2018;11:e1701073. doi: 10.1002/mnfr.201701073. [DOI] [PubMed] [Google Scholar]
- 62.O’Kennedy R, Thornes RD. Coumarins: Biology, Applications and Mode of Action. Chichester; John Wiley & Sons. 1997. [Google Scholar]


