Abstract
Objectives:
Hydrogels are macromolecular networks able to absorb and release water/biological fluids in a reverse-phase manner, in response to specific environmental stimuli. Such stimuli-sensitive behavior makes hydrogels interesting for the design of smart devices applicable to a variety of technological fields. They are able to absorb and retain 10-20% and up to 1000 times the water or biological fluids than their dry weight can. The aim of this study was to extend the work on drug delivery in the stomach at pH 2-2.2.
Materials and Methods:
The authors synthesized sodium alginate (SA)/poly(vinyl alcohol) (PVA) hydrogels. These hydrogels were characterized by fourier transform infrared spectroscopy and scanning electron microscopy, and the swelling properties of the hydrogels were examined at different pH values, in different salts, at different temperatures, and in different acids and bases.
Results:
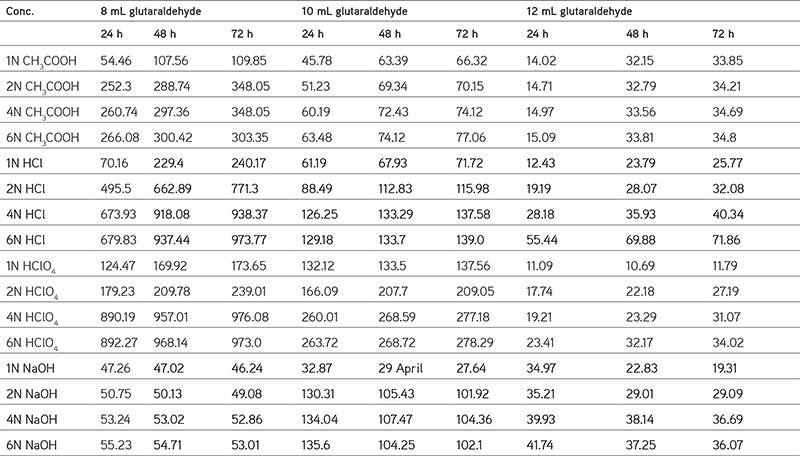
The authors studied and reported the swelling effects or variations such as the effects of salts, acids, bases, temperature, and pH. The results for the crosslinking agent glutaraldehyde showed that 8 mL of glutaraldehyde had a higher swelling rate compared to that of 10 mL and 12 mL.
Conclusion:
In this work the authors studied the swelling degree in different acids and bases. It is concluded that the degree of swelling decreases with increases in the concentration of glutaraldehyde and also depending on the concentrations of the acids. The swelling degrees of PVA/SA hydrogels gradually increase with increases in the concentrations of acids (low pH). The swelling of hydrogels decreases with increases in pH (>7) or at high alkaline. Based on the results for salt solutions the swelling behavior was found to be in the order: K+>Na+>Ca2+>Mg2+.
Keywords: Swelling, pH, crosslinking agent, buffer solution, sodium alginate, PVA
INTRODUCTION
Hydrogels have been used in various chemical and biomedical applications in ophthalmology as contact lenses and surgical sutures, as well as in numerous other areas like agricultural applications.
Sodium alginate (SA) is an anionic copolymer composed of 1,4-linked β-D-mannuronic acid (M-blocks) and α-L-guluronic acid (G-blocks), interspersed with regions of alternating structure. Gel formation and three-dimensional network structures occur when divalent ions (Ca2+, Ba2+, Fe2+, Si2+, etc.) or trivalent ions (Al3+, etc.) crosslink with G-blocks in the polymer chain. Such binding zones between G-blocks are often referred to as “egg boxes”. These crosslinked ions stabilize alginate chains, forming a gel structure, with more freely movable chains that bind and entrap large quantities of water or biological fluids. The gel formation (gelification) process is characterized by the eviction of water.1 The softer, more fragile, and lower porosity gels are made of M-rich alginate groups. This is due to the lower binding strength between the polymer chains and to the higher flexibilities of the molecules. The gel formation process is highly dependent on diffusion of gel formation ions into the polymer network. Visco-elasticity and transmittance of alginate structures are highly affected by the M/G ratio. Alginic acid and its salts of sodium and calcium are used in the medical, pharmaceutical, cosmetic, and food industries because of its nontoxicity and biocompatibility.2 The main advantage of hydrogels is that they possess a degree of flexibility very similar to that of natural tissues, due to their significant water content. Their stimuli-sensitive behavior makes hydrogels interesting for the design of smart devices applicable to a variety of technological fields.
Hydrogels are, in general, materials composed of three-dimensional hydrophilic polymer networks and water that fills the free spaces inside this network. Hydrogels are able to absorb and retain 10-20% up to 1000 times the water or biological fluids than their dry weight can. Hydrogels respond reversibly to slight changes in the properties of surrounding media; hence they are called “intelligent materials”. This ability means hydrogels have found many applications in industry and in pharmaceuticals, for example as controlled drug delivery systems.3 The swelling and dehydration behavior is one of the most important properties of hydrogels.4 Applications of hydrogels include drug delivery systems (slow drug release), wound dressings, dental applications, transdermal implants, injectable polymers, contact lenses, superabsorbents, and environmentally sensitive hydrogels.5,6,7,8,9 Hydrogels interact with aqueous solutions and swell to a certain equilibrium and retain a significant proportion of water within their structure.10 SA is a hydrophilic polysaccharide, a natural polymer, composed of a mannuronic acid unit. This compound has been used for a long time in various industries, such as agriculture, food, medicine, textile, cosmetics, and printing. SA is also used as a thickener, stabilizer, and emulsifier and for microencapsulation, as well as in slow release drug delivery systems and fertilizers.11 SA has a molecular structure similar to collagen; therefore it can make the skin smooth and elastic and can accelerate wound healing, and it can be used as a natural alternative product for health care and cosmetics.12,13,14 SA is of biological origin and has good characteristics, such as biocompatibility, biodegradability, and gel-forming ability. Poly(vinyl alcohol) (PVA) is a hydrophilic polymer and is of great interest for use as a biomaterial because of its good biocompatible properties. It has chemical stability, high durability, and a high degree of swelling in water or biological fluids. PVA is nontoxic to viable cells, noncarcinogenic, has high biocompatibility, and has a consistency similar to soft tissue, film forming with high mechanical strength, and long-term temperature stability. Its three-dimensional network enables diffusional exchange of nutrients and waste products with the surrounding environment, and it is used in various biomedical, pharmaceutical, biotechnological, and other industrial fields.15,16,17,18,19,20 However, even though PVA is a biomaterial it is brittle in nature; therefore, it needs to be blended with other polymers or by copolymerization, e.g., with SA, to obtain a better property that can be used to encapsulate or entrap or to immobilize an enzyme or drug in micron or submicron (nano) size, to keep the constancy of its activity, or to prevent activity decreases drastically. Hence it works more effectively and efficiently compared to when it is in free condition.6,21,22 The crosslinked alginate hydrogels have been used as a controlled release medium for drugs,23,24,25,26,27 pesticides,28 superabsorbent filament fibers,29 and flocculants.30
In the presence of an aqueous solution, the polymer chains absorb water and the association, dissociation, and binding of various ions to polymer chains cause the hydrogel to swell. The shrinking and swelling properties of hydrogels are currently being exploited in a number of applications including the control of microfluidic flow,31 muscle-like actuators,32,33 filtration/separation,34 and drug delivery.35,36 The structure and properties of hydrogels are similar to those of many biological tissues such as cartilage and the corneal stroma in the eye.37,38 Hydrogels are accomplished through large reversible deformation in response to changes in several environmental factors.39 For example, hydrogel size is sensitive to pH, temperature, concentration of salts, and electric fields.
EXPERIMENT
MATERIALS AND METHODS
Materials
SA, PVA molecular weight 125.000, and glutaraldehyde (25%) were purchased from S.D. Fine Chem Limited, Mumbai, India. Hydrochloric acid, perchloric acid, sodium hydroxide, and acetic acid were purchased from Reachem Laboratory Chemicals Private Ltd, Chennai, India. Calcium chloride, magnesium chloride, sodium chloride, and potassium chloride were purchased from E-Merck Limited, Mumbai, India, and double distilled water was used throughout the experiment.
Methods
Preparation of SA/PVA hydrogels
First 7 g of SA was dissolved in 100 mL of water with constant stirring. Then 9 g of PVA was dissolved in the same solution with stirring for about 3 h at 80°C to 85°C, 12 mL of glutaraldehyde (25%) was added to the same solution, and this solution was kept at 80°C for 3 h. After 3 h the obtained hydrogel was washed with distilled water and ethanol to remove the excess monomer and crosslinking agent. After washing 2-3 times the hydrogel was dried at 40°C in an oven. Likewise the different SA/PVA hydrogels synthesized with varying volume of glutaraldehyde (10 and 8 mL) were used for swelling studies.
CHARACTERIZATION
Fourier-transform infrared spectroscopy
The FTIR spectra of the SA, PVA, and the crosslinked hydrogel samples were recorded in the range of 4000 to 500 cm-1 to provide the proof of hydrogels (Figure 1).
Figure 1.
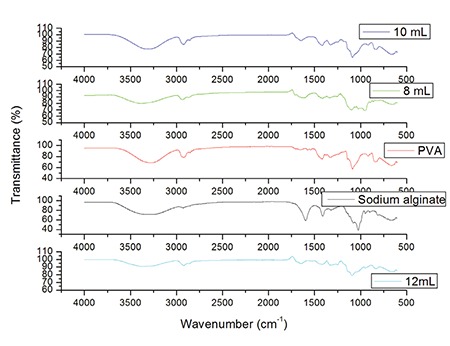
FTIR spectra of pure SA, PVA, 12% glutaraldehyde crosslinked, 10% glutaraldehyde crosslinked, and 8% glutaraldehyde crosslinked hydrogels
FTIR: Fourier transform infrared spectroscopy, SA: Sodium alginate, PVA: Poly(vinyl alcohol)
Surface morphology of hydrogels
The surface morphology of the SA, PVA, and glutaraldehyde crosslinking hydrogels were investigated using scanning electron microscopy (SEM) (SEM Zeiss, LS15) (Figure 2).
Figure 2.
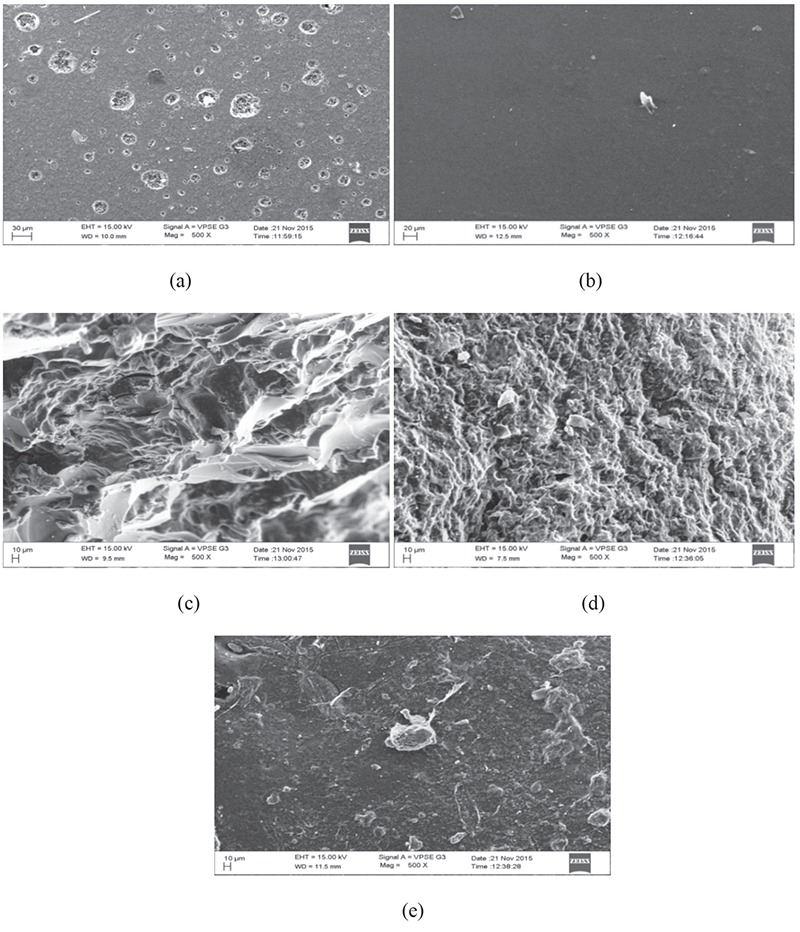
SEM images of (a) pure sodium alginate, (b) pure PVA, (c) 8 mL glutaraldehyde crosslinked SA/PVA hydrogel, (d) 10 mL glutaraldehyde crosslinked SA/PVA hydrogels, (e) 12 mL glutaraldehyde crosslinked SA/PVA hydrogels
SEM: Scanning electron microscopy, SA: Sodium alginate, PVA: Poly(vinyl alcohol)
Swelling behavior of hydrogels
For the swelling behavior of hydrogels, the swelling ratio of the hydrogel samples was measured at different temperatures in different solvents by gravimetric method. Pre-weighed dry hydrogel samples were immersed in excessive different solutions and left undisturbed for 24 h, 48 h, and 72 h at different temperatures like room temperature, 30°C, 37°C, and 40°C until constant values were obtained. Degree of swelling rate can be calculated by the following equation:
% DS = (W2 - W1) / W1 × 100......................... (Equation 1)
% DS is the degree of swelling expressed in a percentage and W1 and W2 are the masses of sample before and after swelling, respectively (Figure 3 to 5) (Table 1).
Figure 3.
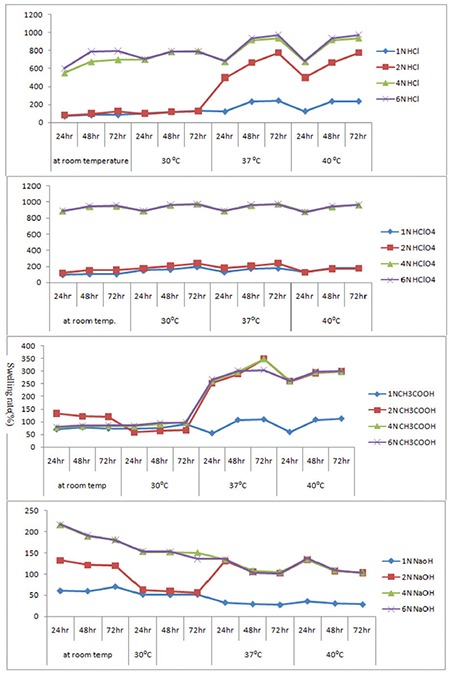
Swelling rate of 8 mL glutaraldehyde crosslinked hydrogels
Figure 5.
Swelling rate of 12 mL glutaraldehyde crosslinked hydrogels
Table 1. Data of swelling rate of 8 mL glutaraldehyde, 10 mL glutaraldehyde, and 12 mL glutaraldehyde crosslinked hydrogels at 37°C.
Swelling at various pHs
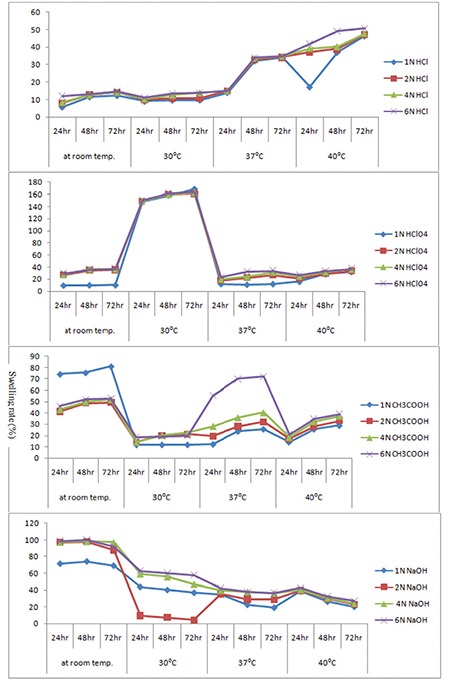
The solution was adjusted to acidic, basic, and neutral pH by diluting with phosphate buffer (pH 3.2, pH 7, pH 2, and pH 10) solutions at room temperature, 30°C, 37°C, and 40°C. The pH values were checked by pH meter. The dried hydrogel samples were used for the swelling measurement according to Equation (1) (Figure 6).
Figure 6.
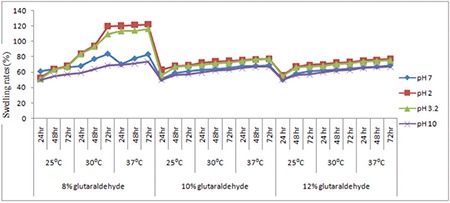
Swelling studies of hydrogels at different pH values of phosphate buffer solutions
Swelling in salt solutions
The swelling capacity of hydrogels was determined in different salt solutions (KCl, NaCl, CaCl2, and MgCl2) and also with various concentrations like 0.4, 0.6, 0.8, 1, and 1.2 N according to the above method (Figure 7).
Figure 7.
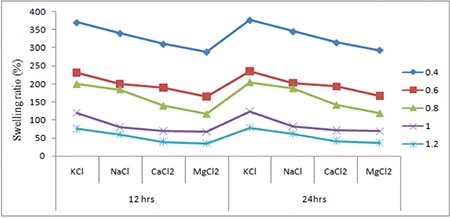
Swelling studies of different concentrated salt solutions at different times
RESULTS
The authors reported the swelling effects on hydrogels of different salts, acids, bases, temperatures, and pH values. A higher swelling rate was shown by 8 mL of glutaraldehyde compared to 10 mL and 12 mL. Figure 1 shows the FTIR spectra of the molecular interaction between SA and PVA. The surface morphology of the hydrogels was studied using SEM (SEM Zeiss, LS15) as shown in Figure 2; Figure 3 to 5 and Table 1 represent the swelling behavior of the hydrogels at different temperatures, i.e. 30°C, 37°C, 40°C, and room temperature, with different salts. Figure 6 shows the behavior of the hydrogels at different pH values.
DISCUSSION
Fourier transformed infrared spectral analysis
Figure 1 represents the FTIR spectra, characterizing the molecular interaction of SA and PVA hydrogels. The FTIR spectra of SA show the characteristic absorption peak at 3270.47 cm-1 is for the hydroxyl (-OH) group. The asymmetric and symmetric stretching vibration of the carboxylic (COO-) group is found to be at 1597.36 and 1412 cm-1, respectively.40,41 The peak at 2925.38 cm-1 represents the C-H alkyl stretching bond.42 An absorption peak around 2919.05 cm-1 shows the characteristic spectra of PVA. This peak arises from the C-H stretching at 1570 to 1420 cm-1 is assigned for CH2 (vinyl group), while the sharp absorption peak at 1150-1050 cm-1 is used for indication of PVA.43 In addition, it was found that the peak at 1549-1453 cm-1 is a stretching band for the CH2 group. This band is also found in pure PVA and crosslinked SA/PVA hydrogels. The decrease in wave number of the carbonyl peak from 1652.14 to 1635.24 cm-1 is for the crosslinking of SA/PVA hydrogels. Crosslinking of glutaraldehyde takes place at 2863 to 2750 cm-1.
SEM images
SEM describes the surface morphology of SA/PVA hydrogels in Figure 2. According to the SEM images (Figure 2a), the pure SA shows a very smooth surface nearly devoid of any surface feature.43 In Figure 2b the SEM images of pure PVA show a very smooth, uniform, and nonporous surface structure, which may be attributed to the crystallization of PVA.44 However, the addition of SA to PVA hydrogel in different portions provides very tiny pores at the surface and these pores decrease with an increase in glutaraldehyde concentration (Figures 2c to 2e).
Effect of pH on the swelling of hydrogels
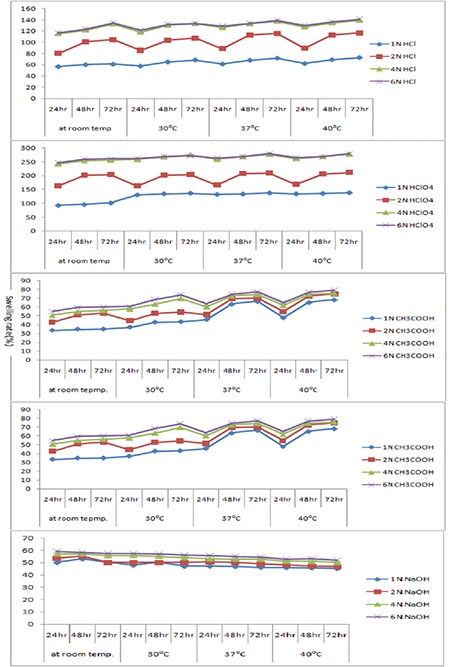
The sensitivity of the hydrogels was measured from pH 2 to pH 10. No additional ions (through buffer solutions) were added to the medium for setting pH because the absorbency of an absorbent is strongly affected by ionic strength. Therefore, stock HCl (pH 1.0) and NaOH (pH 10.0) solutions were diluted with distilled water to achieve the preferred acidic or basic pH values, respectively. In Table 2 and Figure 6, the swelling capacity of hydrogel at pH 2 can be accredited to the high repulsion of anion–anion COO- groups. At basic conditions (pH ≥7), most of the carboxylate groups are protonated and the low swelling values of hydrogels can be attributed to the presence of nonionic hydrophilic -OH and -COOH groups in the PVA and alginate backbones, respectively. The swelling capacity is decreased with further increase in pH (pH 10 or pH >7). Again the swelling loss is due to the counter ions, i.e. Na+, that shield the charge of the carboxylate anions and prevent efficient anion–anion repulsion. As a result, a remarkable decrease in equilibrium swelling is observed.
Table 2. Data of swelling studies of hydrogels in different pH solutions at 37°C.
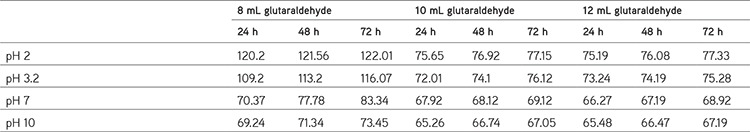
pH Dependence of the swelling rate of water
The swelling rate decreased with increasing pH from 2 to 10; it was evident from the data given. The negatively charged ionic backbones of PVA and SA are more expanded because the protonation of COO- groups is negatively charged. This expanded form makes for easy diffusion of water molecules into the hydrogel network. On the other hand, the -OH groups are mostly in the protonated form and show less polar character at pH ≥7. Therefore, this results in a polymer with lower affinity to water. Thus, hydrogels are less expanded at pH ≥7. The increases in swelling ratio are responsible for the theory of electrostatic repulsion between COO- ions in the polymer chains and ionic present in the pH solution and the ionic osmotic pressure generated from mobile counter ions to charged ions in the network. Thus, the charge density of the hydrogel is diluted, because PVA is not ionic in character (Table 2). Figure 6 shows the swelling studies of phosphate buffered pH solutions. The glutaraldehyde crosslinked PVA/SA samples show a gradual increase (in swelling degree at pH 2 and 3.2) followed by a decrease in swelling degree at pH 7 and 10 at different temperatures (25°C, 30°C, 37°C). In these studies the 8% glutaraldehyde crosslinked hydrogel has a high swelling degree compared to the 10% and 12% glutaraldehyde crosslinked hydrogels.
Effect of temperature on the swelling of hydrogels
It is obvious from Figure 6 that the temperature leading to hydrogels with the highest absorbency is around 37°C. The swelling capacity of hydrogels decreased with increasing temperature above 37°C. The increase in swelling rates is dependent on the kinetic energy of the polysaccharide chains, which led to lower soluble content of the hydrogel as well as increasing concentration of glutaraldehyde diffusion rate of SA and PVA backbones. The higher reaction temperature proves the results from higher reactant movement and effective collision. At temperatures about 37°C, the possible “thermal crosslinking” reaction to polysaccharide backbones may play a major role in the creation of low-swelling hydrogels. In addition, the swelling loss may be related to the increase in crosslinked bond formation of completion of the ester and ether formations by further reaction to the possible mono-ester species with another polysaccharides chain (Scheme 1).
Scheme 1.
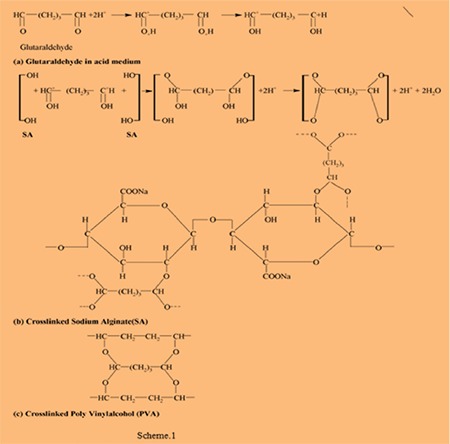
(a) Glutaraldehyde in acid medium, (b) crosslinked sodium alginate, (c) crosslinked poly(vinly alcohol)
Effect of salt solution on hydrogels
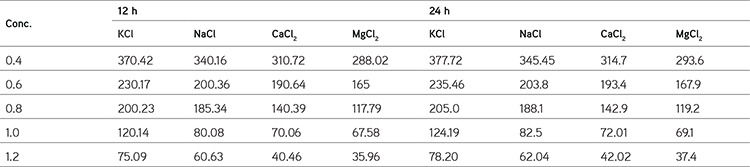
Hydrogels are considered polyelectrolytes, suggesting that their porosity should decrease as ionic strength increases. SA/PVA hydrogels with various chloride salt solutions are appreciably reduced in swelling compared to those measured in deionized water. This results from a charge screening effect of the additional cations causing anion–anion electrostatic repulsion, which leads to a decreased osmotic pressure difference between the polymer network and the external solution. At a given ionic strength, Mg2+ and Ca2+ contribute more charge than monovalent cations like Na+ and K+ and induce a bigger drop in intermolecular repulsion and increased interaction between molecules, which, in turn, cause to a large extent the hydrogel collapse. In addition, Mg2+ and Ca2+ can chelate COO- groups, leading to a compact network and causing further shrinking from the hydrogel; on the other hand, we also find that the smaller the radius of atoms of some valent monoatomic cations, the more the water absorption capacity if different cations were K+>Na+>Ca2+>Mg2+ (Table 3 and Figure 7).45
Table 3. Data of swelling studies of hydrogels in different salt solutions.
CONCLUSIONS
In this work the authors studied the swelling degree in different acids and bases. It is concluded that the degree of swelling decreases with an increase in the concentration of the glutaraldehyde and also depending on the concentration of the acids. Here the swelling degree of PVA/SA hydrogels gradually increases with increases in the concentrations of acids. The aldehyde groups are covalently bonded with the COO- and OH- groups of PVA/SA and consequently the swelling degree is significantly reduced. In different concentrations of different salts the swelling rates decreased with increasing concentrations of salts. The swelling rate is in the order of K+>Na+>Ca2+>Mg2+.
Further extension of the work
This swelling study is to be extended for biomedical and agricultural applications such as drug delivery and controlled release fertilizers.45
Figure 4.
Swelling rate of 10 mL glutaraldehyde crosslinked hydrogels
Acknowledgments
The authors are grateful to the Institution of Excellence for the FTIR and SEM analysis help.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Serp D, Mueller M, Von Stockar U, Marison IW. Low-temperature electron microscopy for the study of polysaccharide ultrastructures in hydrogels. II. Effect of temperature on the structure of Ca2+-alginate beads. Biotechnol Bioeng. 2002;79:253–259. doi: 10.1002/bit.10287. [DOI] [PubMed] [Google Scholar]
- 2.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 3.Dumitriu S. Polymeric Biomaterials, Revised and Expanded. Boca Raton; CRC Press. 2001:1–62. [Google Scholar]
- 4.Gemienhart RA, Guo C. Fast swelling hydrogel systems. In: Yui N, Mrsny RJ, Park K, eds. Reflexive Polymers and Hydrogels. New York; CRC Press. 2004:245–258. [Google Scholar]
- 5.Rosiak JM, Yoshii F. Hydrogels and medical applications. Nucl Instr Meth Phys Rev. 1999;151:56–64. [Google Scholar]
- 6.Silva GS, Fernadez LRV, Higa OZ, Vitolo M, De Queiroz ASA. Alginate-Poly (vinyl alcohol) core-shell microspheres for lipase - immobilization. Cebecimat, XVI congreso Brasileiro de Engenhariae Ciencia dos Materials. Porto Alegre – RS de 28 de novembroa 02 de dezembro de. 2004;15. [Google Scholar]
- 7.Abbas AA, Lee SY, Selvaratnam L, Yusof N, Kamaru T. Porous PVAchitosan based hydrogels as an extracellular matrix scaffold for cartilage regeneration. European Cells and materials. 2008;16(Suppl 2):50. [Google Scholar]
- 8.Ustundag GC, Karaca E, Ozbek S, Cavusoglu I. In vivo evaluation of electrospun Poly (vinyl alcohol) / sodium alginate nanofibrous mat a wound Dressing. Tekstil ve Konveksiyon. 2010;4:290–298. [Google Scholar]
- 9.Sariri R. Physicochemical characteristics and Biomedical applications of hydrogel. A review. J Phys Theor Chem IAU Iran. 2011;8:217–231. [Google Scholar]
- 10.Tombs MP, Harding SE. An Introduction to Polysaccharide Biotechnology. Taylor and Francis UK. 1999;183. [Google Scholar]
- 11.Cunha AG, Gandini A. Turning polysaccharides into hydrophobic materials: a critical review. Part 2. Hemicellulose, chitin/chitosan, starch, pectin and alginates. Cellulose. 2001;17:1045–1065. [Google Scholar]
- 12.Cha DS, Choi JH, Chinnan MS, Park HJ. Antimicrobial film based on Na-alginate and K- carrageenan. Lebensmittel Wissenschaft und Technologie. 2002;35:715–719. [Google Scholar]
- 13.Boninsegna S, Dal Toso RD, Monte RD, Carturan G. Alginate microspheres loaded with animal cells and coated by a siliceous layer. Journal of Sol-Gel Science and Technology. 2003;26:1151–1157. [Google Scholar]
- 14.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;43:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 16.Bahrami SB, Kordestani SS, Mirzadeh H, Mansoori P. Poly (vinyl alcohol) - Chitosan blends: preparation, mechanical and physical properties. Iranian Polymer Journal. 2003;12:139–146. [Google Scholar]
- 17.Nam SY, Nho YC, Hong SH, Chae GT, Jang HS, Suh TS, Ahn WS, Ryu KE, Chun HJ. Evaluations of poly (vinyl alcohol)/alginate hydrogels cross-linked by γ-ray irradiation technique. Macromolecular Research. 2004;12:219–224. [Google Scholar]
- 18.Mishra S, Bajpai R, Katare R, Bajpai AK. Radiation induced cross linking effect on semi -interpenetrating polymer networks of poly (vinyl alcohol) Express Polymer Letters. 2007;1:407–415. [Google Scholar]
- 19.Zain NAM, Suhaimi MS, Idris A. Development and modification of PVA - alginate as suitable immobilization matrix. Process Biochemistry. 2011;46:2122–2129. [Google Scholar]
- 20.Wu KY, Wisecarver KD. Cell immobilization using PVA crosslinked with boric acid. Biotechnol Bioeng. 1992;39:447–449. doi: 10.1002/bit.260390411. [DOI] [PubMed] [Google Scholar]
- 21.Dave R, Madamwar D. Polymer of poly (vinyl alcohol)- boric acid for esterification in organic media. Indian Journal of Biotechnology. 2006;5(Suppl):368–372. [Google Scholar]
- 22.Kulkarni AR, Soppimath KS, Aminabhavi TM. Controlled release of diclofenac sodium from sodium alginate beads crosslinked with glutaraldehyde. Pharm Acta Helv. 1999;74:29–36. [Google Scholar]
- 23.Ostberg T, Vesterhus L, Graffner C. Calcium alginate matrices for oral multiple unit administration. Part 2. Effect of process and formulation factors on matrix properties. Int J Pharm. 1993;97:183–193. [Google Scholar]
- 24.Pillay V, Dangor DM, Govender T, Moopanar KR, Hurbans N. Drug release modulation from cross-linked calcium alginate microdiscs, 2: swelling, compression, and stability of the hydrodynamically-sensitive calcium alginate matrix and the associated drug release mechanisms. Drug Delivery. 1998;5:35–46. doi: 10.3109/10717549809052025. [DOI] [PubMed] [Google Scholar]
- 25.Pillay V, Dangor CM, Govender T, Moopanar KR, Hurbans N. Ionotropic gelation: encapsulation of indomethacin in calcium alginate gel discs. J Microencapsul. 1998;15:215–226. doi: 10.3109/02652049809006851. [DOI] [PubMed] [Google Scholar]
- 26.Pillay R, Fassihi R. In vitro release modulation from crosslinked pellets for site-specific drug delivery to the gastrointestinal tract. I. Comparison of pH-responsive drug release and associated kinetics. J Controlled Release. 1999;59:229–242. doi: 10.1016/s0168-3659(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni AR, Soppimath KS, Aminabhavi TM, Dave AM, Mehta MH. Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J Control Release. 2000;63:97–105. doi: 10.1016/s0168-3659(99)00176-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Yoon KJ. Ko SW. J Appl Polym Sci. 2000;78:1797–1804. [Google Scholar]
- 29.Tripathy T, Pandey SR, Karmakar NC, Bhagat RP. Singh RP. Eur Polym J. 1999;35:2057–2072. [Google Scholar]
- 30.Beebe DJ, Moore JS, Bauer JM, Liu Q, Yu RH, Devadoss C, Jo BH. Functional hydrogel structures for autonomous flaw control inside micro- fluidic channels. Nature. 2000;404:588–590. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- 31.Shahinpoor M. J. Micro-elecctro - mechanics of ionic polymer gels as electrically controllable artificial muscles. Intell Mater Syst Struct. 1995;6:307–314. [Google Scholar]
- 32.Brock D, Lee WJ. A dynamic model of a linear actuator based on polymer Hydrogels. Intel Mater System Struct. 1994;5:764–771. [Google Scholar]
- 33.Helfferich F. Ion exchange. New York: McGraw-Hill. 1962;5. [Google Scholar]
- 34.Grodzinsky AJ, Grinshaw PE. Elctrically and chemically controlled hydrogels, for drug delivery. Pulsed and Self-Regulated Drug Delivery. 1990:47–64. [Google Scholar]
- 35.Peppas NA, Brannon - Peppas L. Solute and Penetrant diffusion in swellable polymers. IX. The mechanism of drug release from pH – sensitive swelling –controlled systems. J Control Release. 1989:267–274. [Google Scholar]
- 36.Eisenberg SR. The kinetics of chemically induced non equilibrium swelling of articular cartilage and corneal stroma. J Biomed Eng. 1987;109:79–89. doi: 10.1115/1.3138647. [DOI] [PubMed] [Google Scholar]
- 37.Myers ER, Lai WM, Mow VC. A continuum theory and an experiment for the ion-induced swelling behavior of articular cartilage. J Biomech Eng. 1984;106:151–158. doi: 10.1115/1.3138473. [DOI] [PubMed] [Google Scholar]
- 38.Okano K, Bac YH, Kim SW. “Temperature responsive controlled drug delivery.” Pulsed and self- regulated drug delivery pulsed and selfregulated. Drug Delivery. 1990:17–46. [Google Scholar]
- 39.Kim JO, Park JK, Kim JH, Jin SG, Yonga CS, Li Dx, Choi HG. Development of Poly (vinyl alcohol) - sodium alginate gel-matrix –based wound dressing system Containing Nitrofurazone. Int J Pharm. 2008;359:79–86. doi: 10.1016/j.ijpharm.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Chhatri A, Bajpai AK, Shandhu SS, Jain N, Biswas J. Cryogenic fabrication of salvon loaded macroporous blends of alginate and Poly(vinyl alcohol) (PVA). Swelling, deswelling and antibacterial behaviors. Cabohydr Polym. 2011;83:876–882. [Google Scholar]
- 41.Mansur HS, Orefice RL, Mansur AAP. Characterization of Poly (vinyl alcohol)/ Poly (ethylene glycol) hydrogels and PVA – derived hybrids by small –angle x-Ray Scattering and FTIR spectroscopy. Polymer. 2004;45:7193–7202. [Google Scholar]
- 42.Sonali K, Udayabhanu MJ, Karthik KT, Rebecca G, David KM. Permance evalution of nanoclay enriched anti-microbial hydrogels for biomedical application. Heliyon. 2016;2:e00072. doi: 10.1016/j.heliyon.2016.e00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamouna EA, Kenawy ERS, Tamer TM, Meligy MAE, Eldin MSM. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arabian Journal of Chemistry. 2015;8:38–47. [Google Scholar]
- 44.Zho Y, Su H, Fang L, Tan T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer. 2005;46:5368–5376. [Google Scholar]
- 45.Raafat AI, Eid M, El-Arnaouty MB. Radiation synthesis of superabsorbent CMC based hydrogels for agriculture applications. Nuclear Instrument and Methods in Physics Research Section B Beam Interactions with Materials and Atoms. 2012;283:71–76. [Google Scholar]


