Abstract
Objectives:
Natural products originating from plants have been used for many years in the treatment of various diseases, including cancer. Centaurea solstitialis subsp. solstitialis is used in Turkish folk medicine. This study was the first to determine the in vitro biological effects of ethanolic extract from the flowering parts of C. solstitialis L. subsp. solstitialis collected from Muğla Province.
Materials and Methods:
The cytotoxic effect was evaluated against Daudi, A549, and HeLa cancer cells and one normal BEAS-2B cell line using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- dipenyltetrazolium bromide) assay. Flow cytometric analysis and the caspase-3 activity assay were performed to detect apoptotic cell death. Angiogenic factor [vascular endothelial growth factor (VEGF)] secretion and the release of interleukin (IL)-1α, IL-6, and tumor necrosis factor (TNF)-α by cells treated with the extract were measured using enzyme-linked immunosorbent assay.
Results:
The extract exhibited cytotoxic effects against all the cancer cell lines used but HeLa and Daudi were the most sensitive cells, with IC50 values of 63.18 μg/mL and 69.27 μg/mL, respectively. Selective cytotoxicity was observed between the HeLa and normal BEAS-2B cell lines. The extract arrested the cell cycle at the S and G2 phases. In addition, apoptotic cell death was detected in HeLa and A549 cells. Moreover, the plant extract caused a significant decrease in VEGF secretion in A549 cells and a fluctuation in IL-1α, IL-6, and TNF-α secretion in A549 and Daudi cells.
Conclusion:
These observations suggest that the flowering parts of C. solstitialis may be a potential source in the development of natural drugs for the treatment of cancer and modulation of cytokine secretion.
Keywords: Centaurea solstitialis, cancer cell lines, anti-cancer, anti-inflammatory
INTRODUCTION
Cancer is one of the most common diseases in both developed and developing countries. Plant products have been used throughout history to treat and prevent diseases because of their large number of different phytochemicals with different biological activities.1 In fact, the compounds derived from plants play an important role in the development of anticancer agents to be used in clinical practice.2 Since substantial evidence has proved that plant secondary metabolites are a potential source of anticancer compounds and cancer cells may develop resistance to existing drugs, today extensive research is being carried out all over the world to discover new plant species with anticancer properties.
Cancer is a multistage disease. Angiogenesis, defined as the formation of new blood vessels, is an essential process in tumor development and prevention of tumor vasculation is a crucial strategy in cancer treatment.3,4 Even though there are a variety of angiogenic factors, vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis. Increased VEGF secretion promotes invasion and metastasis and so targeting of VEGF is pivotal in the prevention of tumor metastasis. Therefore, the discovery of a new plant extract with anti-angiogenic activity is necessary to serve as an alternative to toxic chemotherapeutics.
Inflammation is a common cause of many diseases and alone it is not sufficient to cause cancer, but epigenetic events and mutations caused by environmental exposure or immune modulation contribute significantly to the cancer process.5 In addition, the cytokine release-mediated inflammatory mechanisms were reported to facilitate cancer metastasis.6 Various plants or their bioactive compounds can inhibit or stimulate different enzymes associated with inflammatory and immune response regulating pathways.7 Because anti-inflammatory drugs may be effective in cancer therapy or prevention,8 it is important to evaluate the anti-inflammatory potential of plant extracts as well.
The genus Centaurea L., belonging to the family Asteraceae, is the third largest genus in Turkey.9 Some Centaurea species are used as remedies against various diseases in Turkish folk medicine.10Centaurea solstitialis is known in Turkish as “gelin dikeni” and it has been used to treat hemorrhoids, peptic ulcers, common colds,11,12 malaria,13 and herpes infections around the lips of children.14 Previous studies examined the pharmacological and biological properties of Centaurea species and some Centaurea species exhibited cytotoxic effects against some cancer cell lines.15 The major constituents of Centaurea species were reported to be sesquiterpene lactones, flavonoids, and fatty acids.16,17 Nevertheless, to the best of our knowledge, there were no adequate reports about the anticancer or anti-inflammatory effects of C. solstitialis. Therefore, the present study has scientific importance for the anticancer and anti-inflammatory potential of ethanolic extract from the flowering parts of C. solstitialis collected from Muğla.
MATERIALS AND METHODS
Plant material
The plant C. solstitialis was collected during the flowering period from June to July 2015 from Muğla, in the southwest of Turkey. The plant species was identified in the Herbarium Laboratory, Department of Biology, Muğla Sıtkı Koçman University.
Plant extraction
The flowering parts of C. solstitialis were washed with distilled water and air-dried under shade for about 15 days. Air-dried flowers were ground into powder in a porcelain mill. The powder (10 g) was soaked in absolute ethanol (96°, Merck, USA) and placed in a Soxhlet apparatus for 10 h to obtain ethanolic extract. After filtration of the extract using Whatman filter paper no. 1, the ethanol was removed using a rotary evaporator (IKA, RV 10, USA). The solvent was evaporated by keeping the extracts at 37°C for 7 days. The powdered crude extract was stored at 4°C in an air-tight container until used. The extract was dissolved in 10% dimethyl sulfoxide (DMSO) as stock solution and further diluted to obtain working solutions. DMSO in the final concentrations of the extract was less than 1% and showed no effect on the examined parameters.
Cell lines and culture conditions
Daudi(Burkitt’s lymphoma, CCL-213), A549 (lung carcinoma), HeLa (cervix adenocarcinoma), and BEAS-2B (normal bronchial epithelium) cell lines were originally obtained from ATCC. The cells were maintained in RPMI 1640 medium premixed with stable L-glutamine (Biochrom, Germany) and supplemented with 10% heat inactivated fetal bovine serum (Biochrom, Germany), penicillin (100 U/mL), and streptomycin sulfate (100 mg/mL) (Biochrom, Germany). All cell lines were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
In vitro cytotoxicity assay
The cytotoxic effects of ethanolic extracts from the flowering parts of C. solstitialis on Daudi, A549, HeLa, and Beas-2B were determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- dipenyltetrazolium bromide) assay. In this assay, the reduction of yellow soluble MTT to insoluble blue formazan crystals by mitochondrial dehydrogenase reflects cell viability.18 A total of 4×103 cells/well were seeded in 96-well plates (Greiner, Germany) in triplicate and incubated for 24 h. Plant extracts were added to the wells at 7 different final concentrations between 1000 µg/mL and 15.625 µg/mL followed by incubation for 72 h. Then 10 µL of 5 mg/mL MTT reagent (Applichem, USA) in phosphate-buffered saline (PBS) was added to each well. After 4 h of incubation, the medium was gently discarded and 100 µL of pure DMSO was added to each well to dissolve the formazan blue crystals formed in the cells. The absorbance of reduced MTT in each well was measured at 540 nm using a microplate reader (Thermo Scientific, Multiskan FC, USA). The cytotoxic effects of the extracts were determined by comparing the optical density of treated cells against that of untreated cells.
Cell cycle analysis
Cells at 5×105/well were seeded in 6-well plates and treated with plant extracts at 500 µg/mL and 200 µg/mL for 24 h. After treatment, the cells were washed with ice-cold PBS, fixed in 4 mL of absolute ethanol, and stored at -20°C for 48 h. After that, the cells were centrifuged at 1200 rpm for 10 min at 4°C and the cell pellets were washed in ice-cold PBS. The cells were resuspended in 1 mL of PBS containing 0.1% (v/v) Triton X-100 (Amresco, USA) and then 100 µL of RNase A (200 µg/mL) (Applichem, USA) was added to each of the cell suspensions. After incubation for 30 min at 37°C, 100 µL of propidium iodide (PI) (1 mg/mL in ddH20) was added to each cell suspension and the cells were incubated in the dark for 15 min at room temperature. The cells were analyzed by BD FACSCanto flow cytometer using ModFit LT 3.0 software for cell cycle phases.
Apoptosis assay
Exponentially growing A549 and HeLa cells were cultured at 5×105 cells/well in 6-well plates (Greiner, Germany) and incubated for 24 h. The cells were treated with plant extract at final concentrations of 200 µg/mL and 500 µg/mL for 24 h. Annexin V-FITC/PI staining was carried out using the Annexin V-FITC Apoptosis Detection Kit (eBioscience, USA) protocol. Briefly, treated cells were washed with PBS, trypsinized, washed, and resuspended in binding buffer. Then 5 µL of Annexin V-FITC and 10 µL of PI at 20 µg/mL were added to each cell suspension and the cells were incubated for 15 min in the dark. After 500 µL of binding buffer was added, 10,000 cells per group were analyzed by flow cytometry (BD FACSCanto A, BD Biosciences) using BD FACSDiva software v6.13.
Caspase-3 activity assay
Caspase-3 activity of the cell lysates was determined by colorimetric assay kits (Abcam, Cambridge, UK). A549 and HeLa cells were plated at 2×106 cells/well in 6-well plates and incubated for 24 h. Then the cells were treated with plant extract at 500 µg/mL for 36 h. After centrifugation, the cells were resuspended in 50 µL of cell lysis buffer and incubated on ice for 10 min. The cell lysates were centrifuged at 10.000 × g for 1 min and the protein concentration of each cell lysate was determined by Bradford assay (1976).19 Later, 200 µg of protein from each sample was mixed with 50 µL of 2X reaction buffer containing 10 mM DTT and 5 µL of the caspase-3 substrate (4 mM DEVD-p-NA), followed by incubation at 37°C for 2 h. Absorbance of p-NA light emission was read at 405 nm in a microplate reader. Fold increase in caspase-3 activity was determined by comparing the absorbance of p-NA from an apoptotic sample with that of untreated control cells.
Quantitative detection of human VEGF by enzyme-linked immunosorbent assay
To determine the effect of plant extract on VEGF secretion, A549 cells were cultured at a density of 2×105 cells/well in a 6-well plate and incubated for 1 h. Then the cells were treated with plant extract at 200 µg/mL and incubated for 6 h. The supernatants were collected after centrifugation and stored at -20°C until analysis. The untreated cells served as the control. The concentrations of VEGF in the cell culture supernatants were detected by ELISA as described in the manufacturer’s procedure (VEGF ELISA kit; Boster Biological Technology, USA). The absorbance of each well was measured using a microplate reader at 450 nm within 30 min. The VEGF concentrations of the cell culture supernatants were interpolated from the standard curve.
Quantitative detection of human interleukin-1α, interleukin-6, and tumor necrosis factor-α by ELISA
In order to examine the effects of plant extract on inflammation, 2×105 cells/well of A549 or Daudi cells were plated in triplicate in 6-well plates. The cells were treated with 200 µg/mL C. solstitialis extract for 6 h or left untreated to serve as the control. The supernatants were collected and 100 µL of each supernatant was tested for inflammatory cytokine production by ELISA based on the manufacturer’s instructions using commercial human ELISA kits for interleukin (IL)-1α, IL-6, and tumor necrosis factor (TNF)-α (Boster Biological Technology, USA). The amount of each cytokine in the supernatants was calculated from the formula of the calibration curve of standard cytokine.
Statistical analysis
The data were analyzed using GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA, USA). Comparisons of treatments among the groups were performed using one-way or two-way ANOVA and post-hoc analysis. Significance was presented as ***(p<0.01) and ****(p<0.0001). The data are the mean ± standard deviation of three replicates.
RESULTS
Cytotoxic activity of plant extract on different cancer cell lines
The cytotoxicity of the crude ethanolic extract from the flowering parts of C. solstitialis at seven different concentrations was investigated on the A549, Daudi, HeLa, and Beas-2B cell lines to determine the IC50 value (µg/mL) that causes 50% cell death. The results demonstrated that the percentage of viable cells changed according to the cell lines used (Figure 1). The viability of all the cancer cells was significantly reduced by the extract in a concentration-dependent manner. However, the extract at concentrations of 15.6 and 31.2 µg/mL did not exert significant cytotoxicity on the normal BEAS-2B cell line, indicating the selectivity of the extract against cancer cells (Figure 1d). The highest cytotoxicity, with an IC50 value of 63.18 µg/mL, was observed against HeLa cells (Figure 1c), whereas the IC50 values of A549 and Daudi cells were 252.5 µg/mL and 69.27 µg/mL, respectively (Figure 1a and 1b). On the other hand, the extract exhibited a lower cytotoxic effect on normal BEAS-2B cells, with an IC50 value of 75.25 µg/mL, when compared with the effects on the HeLa and Daudi cancer cell lines (Figure 1). In other words, in terms of cytotoxicity, HeLa and Daudi cells were the most sensitive cell lines against the extract.
Figure 1.
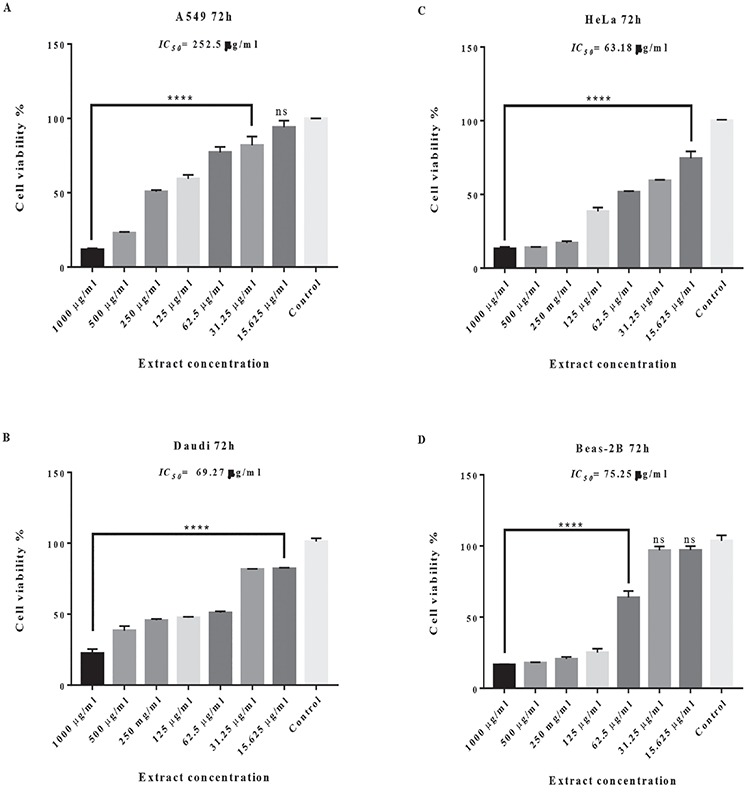
Cytotoxic activity of plant extract against different cancer cell lines. Human cancer cells A549 (A), Daudi (B), HeLa (C), and Beas-2B (D) were treated with ethanolic extract from the flowering parts of Centaurea solstitialis for 72 h. Cell viability was determined based on the MTT assay. Data are the means (±standard deviation) of three independent experiments
****: p<0.0001 compared with untreated cells
The effect of plant extract on cell cycle distribution
Since the plant extract at 250 µg/mL was cytotoxic against all the cell lines tested, the rest of the analyses for different parameters were performed with the extract at 200 µg/mL and 500 µg/mL. The changes in cell-cycle progression of the HeLa and A549 cancer cells after treatment with plant extracts at 200 µg/mL and 500 µg/mL for 24 h were analyzed using flow cytometry with PI staining. Plant extract at 200 µg/mL showed virtually no effect in the cell cycle phases of HeLa cells (Figure 2A; a, b, d). At 500 µg/mL, there was a slight increase in the percentage of HeLa cells in the G2 phase and it was accompanied by a decrease in the percentage of cells in the G1 phase from 48.97% to 41.57% (Figure 2A; a, c, d). In addition, treatment of A549 cells with plant extracts at 200 and 500 µg/mL for 24 h resulted in 7.1% and 12.5% increases in cells in the S phase, respectively, and it caused a concomitant decrease in the percentage of cells in the G1 phase (Figure 2B; a-d). Furthermore, the percentage of G2 phase cells increased from 4.8% to 15.6% and 9.6% in A549 cells treated with the extract at 200 and 500 µg/mL, respectively. These results suggest that the plant extract might inhibit cell proliferation by arresting both cells especially in the G2 phase.
Figure 2.
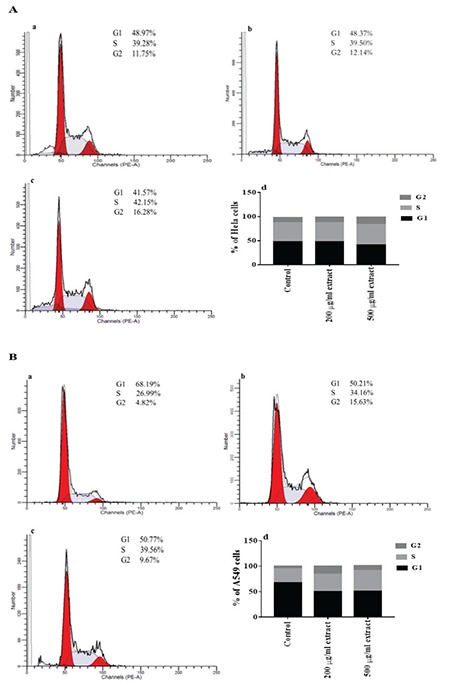
Effect of plant extract on cell cycle distribution of cancer cells. Histograms present a cell cycle distribution of HeLa (A) and A549 (B) cells after treatment with no extract (a), 200 μg/mL (b), and 500 μg/mL (c) extract for 24 h. The percentages of cells at different cell cycle phases are shown (d)
Apoptotic effect of plant extract
As cell cycle regulation and apoptosis are closely related, disruption of cell cycle progression may result in apoptotic/necrotic death.20 Therefore, it was investigated whether or not apoptosis was initiated in cells treated with plant extracts for 24 h. As shown in Figure 3A, the percent of apoptotic HeLa cells (quadrants 2 and 4) increased from 1% to 9.3% and 11.8% after treatment with the extract at 200 and 500 µg/mL, respectively (Figure 3A; a-d). In addition, the percentage of apoptotic A549 cells (quadrants 2 and 4) increased from 3.7% to 4.5% and 31.8% after treatment with 200 and 500 µg/mL extract, respectively (Figure 3B; a-d). These findings demonstrate that the plant extract at 500 µg/mL induces apoptosis in both cell lines, especially in A549. These data were consistent with the results obtained from the cell cycle analysis.
Figure 3.
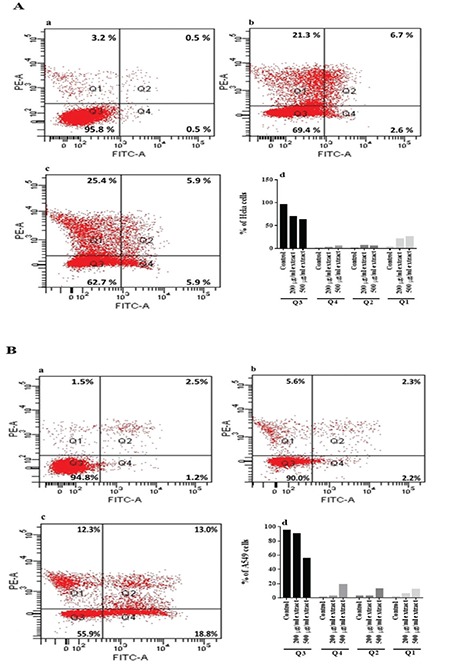
Plant extract induces apoptosis in cancer cells. HeLa (A) and A549 (B) cells were treated with no extract (a), 200 μg/mL (b), and 500 μg/mL extract (c) for 24 h. Cells were distributed into four quadrants: viable cells (Q3), early apoptotic cells (Q4), late apoptotic cells (Q2), and necrotic cells (Q1). The percentage of apoptotic cells (d)
Caspase 3 activation
Caspases play an important role in mediating various apoptotic signaling pathways. In the present study, we analyzed the activity of caspase 3 in A549 and HeLa cells treated with the extract at 500 µg/mL for 36 h. As shown in Figure 4, the extract increased caspase-3 activity about 1.65- and 1.5-fold compared to the control in HeLa and A549 cells, respectively. These results indicate that the plant extract induces apoptosis in both of these cell lines.
Figure 4.
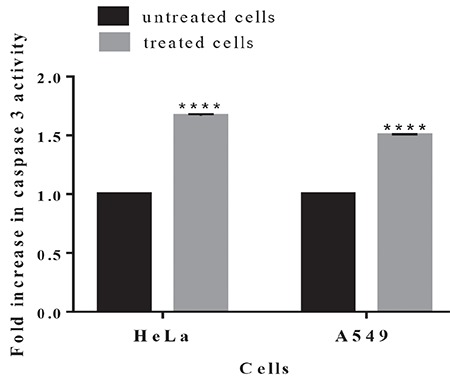
Caspase 3 activity in HeLa and A549 cells after treatment with the plant extract. Cells were treated without or with the extract at 500 μg/mL for 36 h. Caspase 3 activity in untreated cells was taken as 1-fold and the change in the treated cells was expressed by comparing untreated cells. The results are the means (± standard deviation) of three independent experiments
****: p<0.0001
VEGF secretion of A549 cells
VEGF is a potent cytokine produced by many cell types including most cancer cells and it has critical roles in physiological and pathological angiogenesis.21 Because VEGF protein expression was determined in the airway epithelial cancer cell line A549 by Koyama et al.,22 VEGF secretion of A549 cells was investigated after treatment with the extract at 200 µg/mL by human VEGF ELISA assay. The plant extract caused a 2.5-fold decrease in VEGF secretion of A549 cells compared to untreated control cells (Figure 5), indicating the antiangiogenic function of the extract.
Figure 5.
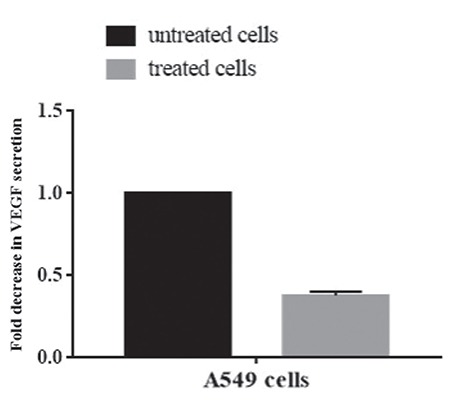
Effects of plant extract on vascular endothelial growth factor secretion of A549 cells. Cells were treated with 200 μg/mL extract for 6 h and vascular endothelial growth factor concentration in supernatants was detected by enzyme-linked immunosorbent assay. Results are presented as fold of change in relation to the control cells. Data are the means (±standard deviation) of three independent experiments
VEGF: Vascular endothelial growth factor
Effect of plant extract on IL-1α, IL-6, and TNF-α secretion
It is known that different cytokines and growth factors may contribute to cancer progression.23 In the present study, the IL-1α, IL-6, and TNF-α concentrations in A549 and Daudi cell culture supernatants after treatment with plant extract at 200 µg/mL were determined. The effect of plant extract on cytokine secretion varied according to the cell lines used. The highest level of inhibition on the release of cytokines was observed in A549 for IL-6 and Daudi for IL-1α compared to untreated control cells (Figure 6). In contrast, there was a slight increase in the release of IL-1α and TNF-α in A549 cells and IL-6 in Daudi cells. In other words, the plant extract caused a significant change in the cytokine levels of cancer cells.
Figure 6.
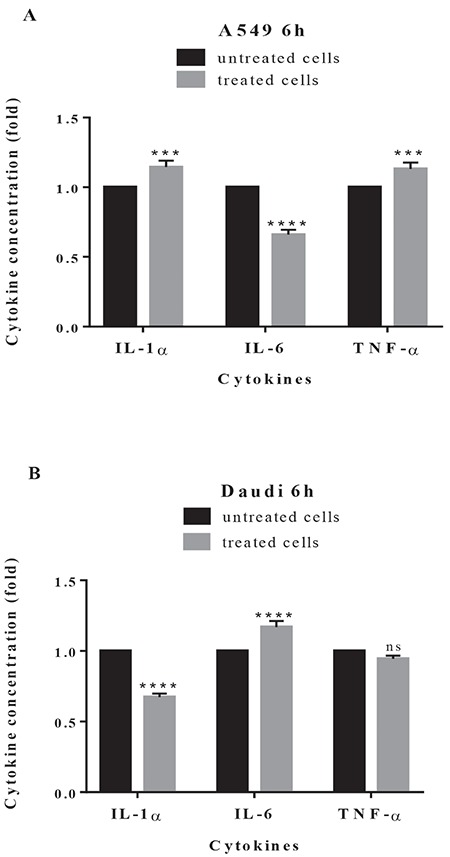
Effects of plant extract on cytokine secretion. A549 cells (A) and Daudi cells (B) were treated with plant extract at 200 μg/mL for 6 h. The concentrations of IL-1α, IL-6, and tumor necrosis factor-α in the supernatants of cancer cells were detected by enzyme-linked immunosorbent assay. Results are presented as fold of change in relation to the control cells. Data are the means (±standard deviation) of three independent experiments
****: p<0.0001, ***: p<0.01, ns: nonsignificant, TNF: tumor necrosis factor, IL: interleukin
DISCUSSION
Cancer is one of the major causes of death in the world.24 It has been known for centuries that plants have anticancer properties and they are important resources for new anticancer drugs.25 The genus Centaurea has been the subject of many phytochemical and biological studies because of its widespread application in folk medicine to treat various diseases. Different biological activities such as antioxidant,26 antimicrobial,27 antipyretic,28 and anti-ulcerogenic functions29 were reported for C. solstitialis. However, to the best of our knowledge not much information is available about the anticancer and anti-inflammatory activities of C. solstitialis in the literature. Therefore, such biological activities of ethanolic extract from the flowering parts of C. solstitialis were examined in the present study.
Investigation of the cytotoxic effect of a plant extract against cancer cells is an important step for the development of plant-based drugs for cancer treatment. Likewise, the cytotoxic effect of ethanolic extract from the flowering parts of C. solstitialis on different cancer cell lines was tested. The findings indicated that plant extract showed cytotoxic effects at different levels according to the type of cell lines used. The extract exhibited the highest cytotoxicities in HeLa cells, with an IC50 value of 63.18 µg/mL, and Daudi cells, with an IC50 value of 69.27 µg/mL, whereas the IC50 value in the BEAS-2B normal cell line was 75.25 µg/mL. However, plant extract showed the lowest cytotoxic effect against A549 cells (IC50 value of 252.5 µg/mL). Erenler et al.30 investigated the antiproliferative activities of methanol extract of the root, stem, and flowering parts of C. solstitialis L. subsp. solstitialis on C6 cells and HeLa cells in vitro and found that the methanol extract of the stem exhibited the most antiproliferative activity. In contrast to their study, our previous investigation demonstrated that the flowers were a more effective plant part compared to the stem (unpublished data) and so ethanolic extract only from the flowering parts was used in the present study. The reason for this may have been the type of solvent used for extract preparation. In fact, different solvents result in extraction of chemical compounds at different scales.
Similar to this study, there are publications related to different Centaurea species that have cytotoxic effects against the A549 and HeLa cell lines. Tugba Artun et al.31 reported that among 14 plant extracts the methanol extract of Centaurea nerimaniae exhibited the highest cytotoxic effect against the Vero normal cell line and methanolic extract of the endemic Centaurea antiochia Boiss. var. praealta showed a selective cytotoxic effect against the HeLa cell line, with an IC50 value of 427±3.06 µg/mL. In another study, chloroform extracts of Centaurea cadmea showed the most inhibitory activities against the HeLa (IC50: 14.24 µg/mL), A549 (IC50: 35.00 µg/mL), and U20S (IC50: 43.10 µg/mL) human cancer cell lines and the 293HEK (IC50: 23.50 µg/mL) noncancer cell line.32 In addition, Zater et al.33 stated that chloroformic extract of C. diluta Ait. subsp. algeriensis exhibited more significant cytotoxic effects on the cancer cells A549, MCF-7, and U373 than the isolated pure compounds. Taken together, these studies indicate that the cytotoxicity level changes depending on the different Centaurea species and solvents used for extract preparation and the type of cell lines used for the in vitro cytotoxicity test.
Because cell cycle inhibition is a main target in the development and discovery of a drug against cancer, the effect of plant extract on the cell cycle progression of the HeLa and A549 cell lines after 24 h treatment was investigated in the present study. The results indicated that the plant extract blocked cancer cell proliferation by arresting both cell lines especially in the G2 phase of the cell cycle. In contrast to our results, Ghantous et al.34 reported that inhibition of the cell proliferation of the papilloma and squamous cell carcinoma cell lines by crude extract of Centaurea ainetensis and the compound salograviolide A isolated from this plant was due to G0/G1 cell cycle arrest. Other researchers demonstrated that crude extract of Centaurea ainetensis induced a progressive increase in the proportion of sub-G1 cells in the HCT-116 cell line.35
Apoptosis is an important physiological process that plays a critical role in development and homeostasis in normal tissues; however, the balance between cell division and apoptosis is lost in cancer.36,37 Therefore, targeting apoptosis in cancer treatment is crucial. In cells undergoing apoptosis, phosphatidylserine (PS) translocates toward the extracellular side of the membrane. Annexin V is a phospholipid-binding protein and so translocation of PS to the outside of the membrane is detected by Annexin V staining and it shows early stage apoptosis.38 In the literature, only two studies investigated the apoptotic effects of extracts from Centaurea ainetensis34 and Centaurea fenzlii Reichardt39 on different cancer cell lines and they showed the presence of apoptotic cell death. In the present study, Annexin V staining along with flow cytometric analysis was carried out to reveal the mechanism in the cytotoxicity of plant extract on the A549 and HeLa cancer cells. Similar to previous studies, treatment of HeLa and A549 cells with C. solstitialis extract induced apoptosis and increased apoptotic cell number in a dose-dependent manner (Figure 3).
Caspases, a family of proteases, play an essential role in the apoptotic pathway and become activated during the early stages of apoptosis.40 Because elevation in caspase-3 activity is regarded as an apoptotic marker, caspase-3 activity in treated and untreated cancer cell lines was examined. The results indicated that ethanolic extract of the flowering parts of C. solstitialis caused an increase in caspase-3 activity in both the HeLa and A549 cell lines (Figure 4). In addition, Yırtıcı et al.39 reported that dichloromethane extracts-ethyl acetate fractions from C. fenzlii Reichardt exhibited an apoptotic effect on MCF-7 cells using flow cytometry and western blot analysis of an apoptosis-related protein, adenosine diphosphate ribose polymerase.
Angiogenesis is defined as the formation of new microvessels from preexisting ones and is required for tumor growth and distribution of tumor cells to distant locations.41 VEGF is known to be one of the most potent angiogenic factors. Previous studies indicated that inhibition of VEGF secretion suppresses tumor growth, tumor invasion, and metastasis.41 A549, an airway epithelial cancer cell line, releases VEGF constitutively.22 Therefore, the angiogenic potential of the extract on the A549 cell line was investigated by measuring VEGF secretion after 6 h of treatment. A significant inhibition of VEGF secretion in A549 cells implies that the plant extract has potential as an anti-angiogenic agent in cancer therapy.
Inflammatory cytokines play a role in different stages of tumor development and many cytokines such as TNF, IL-1, and IL-6 can be induced by hypoxia, one of the well known properties of cancer cells.42,43 Here we tested the effect of plant extract on the secretion of IL-1α, IL-6, and TNF-α in A549 and Daudi cells. The plant extract at 200 µg/mL did not decrease TNF-α production in either cell line (Figure 6). The plant extract significantly inhibited the release of IL-6 in A549 and the release of IL-1α in Daudi cells. According to a previous study, production of angiogenic factors such as VEGF could be induced by TNF, IL-1, and IL- 6.42 A decrease in VEGF production in A549 cells may be associated with decreased IL-6 production in A549 cells in the present study. Similar to our result, Talhouk et al.44 reported that water extract of C. ainetensis inhibited IL-6 production in a dose-dependent manner. In addition, in vivo anti-inflammatory effects of some Centaurea species were reported as well by Erel et al.45 and Koca et al.46 The present study indicates that induction or inhibition of inflammatory cytokines by ethanolic extract of C. solstitialis is cell-type dependent.
Study limitations
Crude ethanolic extract from the flowering parts of C. solstitialis was investigated for its anticancer and anti-inflammatory potential. Isolation of pure compounds in a future study will show if each constituent alone or in different combinations may exhibit increased anticancer or anti-inflammatory activities.
CONCLUSIONS
Ethanolic extract from the flowering parts of C. solstitialis showed significant anticancer and anti-inflammatory potential against different cancer cell lines, indicating that the flowering parts of C. solstitialis are a potential source of active compounds for the development of natural drugs against cancer.
Acknowledgments
This work was supported by grants 15/006 and 15/247 from Scientific Research Projects (BAP) of Muğla Sıtkı Koçman University.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O’Neal JM, Cornwell T, Pastor I, Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 2.Unnati S, Ripal S, Sanjeev A, Niyati A. Novel anticancer agents from plant sources. Chin J Nat Med. 2013;11:16–23. [Google Scholar]
- 3.Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Tahergorabi Z, Khazaei M. A Review on Angiogenesis and Its Assays. Iran J Basic Med Sci. 2012;15:1110–1126. [PMC free article] [PubMed] [Google Scholar]
- 5.Schottenfeld D, Beebe-Dimmer J. Chronic Inflammation: A Common and Important Factor in the Pathogenesis of Neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheim JJ, Murphy WJ, Chertox O, Schirrmacher V, Wang JM. Prospects for Cytokine and Chemokine Biotherapy. Clin Cancer. Res.1997;3:2682–2686. [PubMed] [Google Scholar]
- 7.Hollman PC, Katan MB. Dietary Flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 8.Thun MJ, Patrono C. Nonsteroidal Anti-inflammatory Drugs as Anticancer Agents: Mechanistic, Pharmacologic, and Clinical Issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 9.Güner A, Özhatay N, Ekim T, Başer K. Flora of Turkey and the East Aegean Islands. Vol 11. Edinburgh: Edinburgh University. 2000. [Google Scholar]
- 10.Altundag E, Ozturk M. Ethnomedicinal studies on the plant resources of east Anatolia. Procedia Soc Behav Sci. 2011;19:756–777. [Google Scholar]
- 11.Honda G, Yeşilada E, Tabata M, Sezik E, Fujita T, Takeda Y, Takaishi Y, Tanaka T. Traditional medicine in Turkey VI. Folk medicine in West Anatolia: Afyon, Kütahya, Denizli, Muğla, Aydın provinces. J Ethnopharmacol. 1996;53:75–87. doi: 10.1016/S0378-8741(96)01426-2. [DOI] [PubMed] [Google Scholar]
- 12.Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol. 2001;75:95–115. doi: 10.1016/s0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- 13.Bulut G, Tuzlaci E. An ethnobotanical study of medicinal plants in Turgutlu (Manisa-Turkey) J Ethnopharmacol. 2013;149:633–647. doi: 10.1016/j.jep.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Sezik E, Tabata M, Yeşilada E, Honda G, Takeda Y, Tanaka T, Takaishi Y. Traditional Medicine in Turkey VII. Folk Medicine in Middle and West Black Sea Regions. Econ Bot. 1995;49:406–422. [Google Scholar]
- 15.Khammar A, Djeddi S. Pharmacological and Biological Properties of some Centaurea Species. Eur J Sci Res. 2012;84:398–416. [Google Scholar]
- 16.Kaïj-a-Kamb M, Amoros M, Girre L. The chemistry and biological activities of the genus Centaurea. Pharm Acta Helv. 1992;67:178–188. [PubMed] [Google Scholar]
- 17.Aktumsek A, Zengin G, Guler GO, Cakmak YS, Duran A. Screening for in vitro antioxidant properties and fatty acid profiles of five Centaurea L. species from Turkey flora. Food Chem Toxicol. 2011;49:2914–2920. doi: 10.1016/j.fct.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 21.Chung AS, Ferrara N. Developmental and Pathological Angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 22.Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, Nagai S, Izumi T. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J. 2002;20:1449–1456. doi: 10.1183/09031936.02.00089802. [DOI] [PubMed] [Google Scholar]
- 23.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. World health statistics 2017: monitoring health for the SDGs, Sustainable Development Goals. Geneva: World Health Organization. 2017. [Google Scholar]
- 25.Cragg GM, Newman DJ. Natural products: A continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekeli Y, Sezgin M, Aktümsek A. Antioxidant property of Centaurea solstitialis L. from Konya, Turkey. Asian J Chem. 2008;20:4831–4835. [Google Scholar]
- 27.Özçelik B, Gürbüz I, Karaoglu T, Yeşilada E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol Res. 2009;164:545–552. doi: 10.1016/j.micres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Akkol EK, Arif R, Ergun F, Yesilada E. Sesquiterpene lactones with antinociceptive and antipyretic activity from two Centaurea species. J Ethnopharmacol. 2009;122:210–215. doi: 10.1016/j.jep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Gürbüz İ, Yesilada E. Evaluation of the anti-ulcerogenic effect of sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis by using various in vivo and biochemical techniques. J Ethnopharmacol. 2007;112:284–291. doi: 10.1016/j.jep.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Erenler R, Sen O, Yaglioglu AS, Demirtas I. Bioactivity-Guided Isolation of Antiproliferative Sesquiterpene Lactones from Centaurea solstitialis L. ssp. solstitialis. Comb Chem High Throughput Screen. 2016;19:66–72. doi: 10.2174/1386207319666151203002117. [DOI] [PubMed] [Google Scholar]
- 31.Tugba Artun F, Karagoz A, Ozcan G, Melikoglu G, Anil S, Kultur S, Sutlupinar N. In vitro anticancer and cytotoxic activities of some plant extracts on HeLa and Vero cell lines. J BUON. 2016;21:720–725. [PubMed] [Google Scholar]
- 32.Astari KA, Erel ŞB, Köse FA, Köksal Ç, Karaalp C. Cytotoxic and Antibacterial Activities of Centaurea cadmea Boiss. Turk J Pharm Sci. 2014;11:101–106. [Google Scholar]
- 33.Zater H, Huet J, Fontaine V, Benayache S, Stévigny C, Duez P, Benayache F. Chemical constituents, cytotoxic, antifungal and antimicrobial properties of Centaurea diluta Ait. subsp. algeriensis (Coss. & Dur.) Maire. Asian Pac J Trop Med. 2016;9:554–561. doi: 10.1016/j.apjtm.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Ghantous A, Tayyoun AA, Lteif GA, Saliba NA, Gali-Muhtasib H, El-Sabban M, Darwiche N. Purified Salograviolide A isolated from Centaurea ainetensis causes growth inhibition and apoptosis in neoplastic epidermal cells. Int J Oncol. 2008;32:841–849. [PubMed] [Google Scholar]
- 35.El-Najjar N1, Dakdouki S, Darwiche N, El-Sabban M, Saliba NA, Gali-Muhtasib H. Anti-colon cancer effects of Salograviolide A isolated from Centaurea ainetensis. Oncol Rep. 2008;19:897–904. [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 200;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 37.Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb). 2011;3:279–296. doi: 10.1039/c0ib00144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deepa M, Sureshkumar T, Satheeshkumar PK, Priya S. Purified mulberry leaf lectin (MLL) induces apoptosis and cell cycle arrest in human breast cancer and colon cancer cells. Chem Biol Interact. 2012;200:38–44. doi: 10.1016/j.cbi.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Yırtıcı Ü, Göger F, Sarımahmut M, Ergene A. Cytotoxic and apoptotic effects of endemic Centaurea fenzlii Reichardt on the MCF-7 breast cancer cell line. Turk J Biol. 2017;41:370–377. [Google Scholar]
- 40.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 41.Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth. Cancer Metastasis Rev. 2012;31:143–162. doi: 10.1007/s10555-011-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes JV, Cobucci RN, Jatobá CA, Fernandes TA, de Azevedo JW, de Araújo JM. The Role of the Mediators of Inflammation in Cancer Development. Pathol Oncol Res. 2015;21:527–534. doi: 10.1007/s12253-015-9913-z. [DOI] [PubMed] [Google Scholar]
- 44.Talhouk RS, El-Jouni W, Baalbaki R, Gali-Muhtasib H, Kogan J, Talhouk SN. Anti-inflammatory bio-activities in water extract of Centaurea ainetensis. J Med Plant Res. 2008;2:24–33. [Google Scholar]
- 45.Erel SB, Demir S, Nalbantsoy A, Ballar P, Khan S, Yavasoglu NU, Karaalp C. Bioactivity screening of five Centaurea species and in vivo anti-inflammatory activity of C. athoa. Pharm Biol. 2014;52:775–781. doi: 10.3109/13880209.2013.868493. [DOI] [PubMed] [Google Scholar]
- 46.Koca U, Süntar IP, Keles H, Yesilada E, Akkol EK. In vivo anti-inflammatory and wound healing activities of Centaurea iberica Trev. ex Spreng. J Ethnopharmacol. 2009;126:551–556. doi: 10.1016/j.jep.2009.08.017. [DOI] [PubMed] [Google Scholar]


