Summary
Cryptococcus neoformans is a global human fungal pathogen that causes fatal meningoencephalitis in mostly immunocompromised individuals. During pulmonary infection, cryptococcal cells form large polyploid cells that exhibit increased resistance to host immune attack and are proposed to contribute to the latency of cryptococcal infection. These polyploid titan cells can generate haploid and aneuploid progeny that may result in systemic infection. What triggers cryptococcal polyploidization and how ploidy reduction is achieved remain open questions. Here we discovered that Cryptococcus cells polyploidize in response to genotoxic stresses that cause DNA double strand breaks. Intriguingly, meiosis-specific genes are activated in C. neoformans and contribute to ploidy reduction, both in vitro and during infection in mice. Cryptococcal cells that activated their meiotic genes in mice were resistant to specific genotoxic stress compared to sister cells recovered from the same host tissue but without activation of meiotic genes. Our findings support the idea that meiotic genes, in addition to their conventional roles in classic sexual reproduction, contribute to adaptation of eukaryotic cells that undergo dramatic genome changes in response to genotoxic stress. The discovery has additional implication for evolution of sexual reproduction and the paradox of the presence of meiotic machinery in asexual species. Finally, our findings in this eukaryotic microbe mirror the revolutionary discoveries of the polyploidization and meiosis-like ploidy reduction process in cancer cells, suggesting that the reversible ploidy change itself could provide a general mechanism for rejuvenation to promote individual survival in response to stress.
Keywords: Cryptococcus, ploidy reduction, meiosis, pathogenesis, Dmc1, Rec8
Graphical Abstract
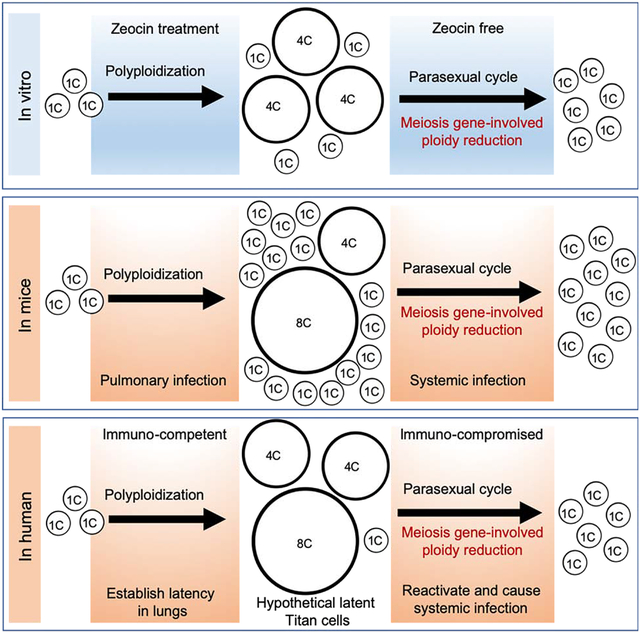
In Brief
Zhao et al. show that meiosis genes facilitate ploidy reduction in the global fungal pathogen Cryptococcus neoformans and they are activated during infection. Progeny isolated from the host that have meiotic genes activated show increased resistance to specific genotoxic stress, indicating the occurrence of meiosis may empower adaptative mutations.
Introduction
Cryptococcus neoformans is a global pathogen causing meningoencephalitis in mostly immunocompromised individuals [1]. This fungus is responsible for 15% of deaths in AIDS patients [2]. Its bisexual cycle involving partners of a and α mating types has been defined for decades [3]. However, the α mating type predominates among natural cryptococcal populations, particularly in the serotype A clade which is responsible for the vast majority of cryptococcosis (MATα > 99%). Our previous discoveries that a single C. neoformans strain can complete a sexual cycle in the absence of a partner of the opposite mating type (unisexual reproduction) [4] and that MATα alleles enhance unisexual reproduction in vitro [5] offer a plausible explanation for its dominance in nature. Both unisexual and bisexual reproduction naturally entail changes in nuclear DNA content (1C-2C-4C-1C), due to nuclear fusion or endoreplication, premeiotic replication, and meiosis. Although unisexual reproduction differs from bisexual reproduction in that the non-self-recognition system for cell fusion is dispensable [6], both sexual reproduction modes require meiosis to achieve ploidy reduction and have only been observed in vitro.
Meiosis is a specialized reductive cell division. A single round of DNA replication followed by two consecutive DNA divisions enable reduction in the nuclear DNA content. During the first meiotic division (meiosis I), extensive DNA recombination takes place between two parental homologous chromosomes, which are then segregated from one another. The second round of meiotic DNA division (meiosis II) more closely resembles mitotic DNA division, in which sister chromatids are segregated from each other. Diversification of genotypes during meiosis is primarily attributed to re-shuffling the pre-existing genetic sequences. Generation of novel genetic information, however, is the less known but critical feature of meiosis due to a much higher rate of de novo mutations that are dependent on DNA double strand breaks (DSBs) [7–10].
C. neoformans mostly exists in the haploid yeast state when cultured under standard laboratory conditions. During pulmonary infection, a portion of C. neoformans cells become polyploid in DNA content (typically tetraploid and sometimes higher) and larger in cell size (>10 m) than standard haploid yeast cells (4–7 μm) [11, 12]. These large titan cells can evade host immune surveillance and are more resistant to various stresses, enhancing C. neoformans survival in the hostile pulmonary environment [11, 13, 14]. Cell gigantism can be potentially promoted by many different stimuli, such as serum, CO2, phospholipids or nutrient starvation [15–18]. Isolated polyploid cells can generate haploid progeny under permissive conditions in vitro [14]. Whether meiotic machinery is involved in this ploidy reduction process remains an open question. Here, we observed the activation of meiotic genes in cryptococcal cells during infection with a single isolate. We showed that genotoxic stresses that cause DSBs induce cryptococcal polyploidization and that meiotic genes contribute to the subsequent ploidy reduction process. Remarkably, cryptococcal cells that activated their meiotic genes during infection are more resistant to specific genotoxic stress that causes DSBs compared to sister cells recovered from the same animal tissue.
Results
Meiosis-specific genes in C. neoformans are activated during infection
Bisexual and unisexual reproduction in Cryptococcus are associated with yeast-to-hypha transition under in vitro conditions. Ploidy increase is achieved either through cell fusion followed by nuclear fusion (bisex) or mostly through endoreplication (unisex, Figure 1A). Meiosis occurs in the basidial heads formed at the apexes of aerial hyphae to halve the DNA content prior to sporulation. Meiosis-specific factors, such as the meiosis-specific recombinase Dmc1 and the meiosis-specific component of the cohesin complex Rec8, are highly conserved from yeasts to humans and they are critical for ploidy reduction in a sexual cycle [19–21]. Deletion of DMC1 (CNAG_07909) or REC8 (CNAG_04404) abolishes sporulation without affecting cryptococcal hyphal and basidia development [4, 22] (Figure 1B), consistent with their specific roles in meiosis.
Figure 1. . Meiosis-specific genes are activated in C. neoformans cells during infection.
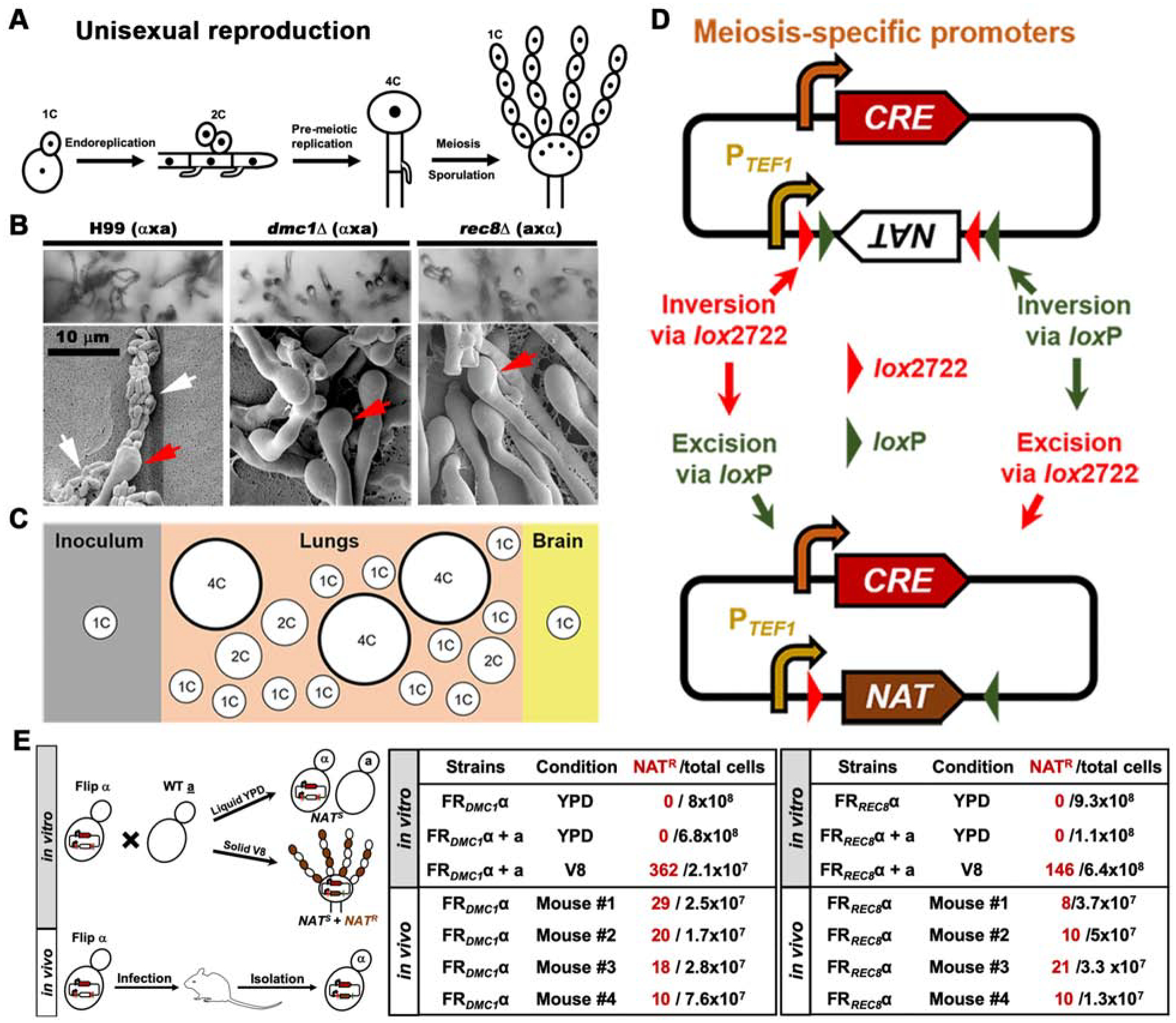
A, Diagram of cryptococcal unisexual reproduction and concomitant ploidy changes. B, Deletion of DMC1 or REC8 blocks cryptococcal sporulation. Bilateral crosses of WT H99 (axα), dmc1Δ (axα), and rec8Δ (axα) were cultured on V8 agar medium and imaged under a light (upper) or scanning electron microscope (bottom), respectively. White arrows point at spore chains while red arrows point at basidium heads. C, Diagram of the distribution of cell size and ploidy during cryptococcal infection. D, Diagram of the double-floxed Cre-mediated flip reporter to detect activation of meiosis-specific genes. The double-floxed (loxP and lox2722) dominant drug marker NAT is placed inverted relative to the constitutively active promoter of TEF1 (PTEF1). The expression of the site-specific recombinase Cre is driven by the promoter of a meiosis-specific gene (DMC1 or REC8). Activation of meiosis-specific genes induces the expression and production of Cre. Cre recognizes the two pairs of lox sites, resulting in inversion of the NAT drug marker and excision of one loxP site and one lox2722 site. The incompatibility of remaining unpaired loxP and lox2722 sites impedes further Cre-mediated inversion. This ensures correct orientation between PTEF1 and NAT, and concomitant transcription of NAT and consequent resistance to nourseothricin. E, Diagram of testing the flip reporter strains in vitro and in vivo (left panel). The two tables to the right show the number of flip events detected with the FRDMC1 or the FRREC8 reporter system when the cells were cultured in vitro (upper panel) or in vivo (infected mice were euthanized at DPI 13 to measure the flip events, bottom panel). The in vitro experiments were repeated at least three times and all showed a consistent pattern. Results from one batch of experiment were shown here. The in vivo flip events were detected independently from the experiments conducted in Lin lab at the University of Georgia and Xue lab at Rutgers University. See also Figure S1.
Given that a portion of Cryptococcus cells become polyploid in vivo (Figure 1C) and these cells can then undergo ploidy reduction to produce haploid progeny when cultured in vitro [14], we wondered if meiotic machinery contributes to the ploidy reduction during infection. It is, however, challenging to monitor the occurrence of meiosis or the activation of meiotic genes in vivo for the following reasons: (i) Only a small portion of the cryptococcal population in mouse lungs become polyploid and only a subset of these polyploid cells will likely undergo de-polyploidization in vivo [13, 14]. (ii) Among this subset of cells, ploidy reduction could be achieved through mitotic cycles where chromosomes are randomly lost (parasexual cycle); (iii) Even if the meiotic machinery is activated, the expression of meiosis-specific genes are likely transient; (iv) The clinical reference strain H99 amplifies rapidly in mice [23–26] and mitotic divisions from highly proliferative haploid cells likely dominate in this host.
In order to detect potential transient expression of meiotic genes occurring at a very low frequency, we employed a flip reporter (FR) using a modified recombination-based in vivo expression technology [27, 28]. The FR approach links the activation of meiosis-specific genes to the production of a site-specific recombinase Cre by driving the expression of CRE with the promoter of the meiosis genes (Figure 1D). There are two pairs of Cre recognition sites bordering a drug selection marker that is inversely placed after a promoter of a house keeping gene TEF1 (PTEF1). Consequently, the drug selection marker is not expressed in the original strain. If meiosis genes are activated, the CRE will be expressed and the resulting Cre enzyme causes inversion at its recognition loci and correct orientation of the drug marker gene with the PTEF1 promoter, resulting in the expression of a dominant drug resistance selection marker. Thus, even transient activation of a meiosis-specific gene will yield a heritable genotypic change that leads to a permanent phenotypic change conferring nourseothricin (NAT) resistance.
We chose to monitor the activation of meiosis-specific genes, DMC1 and REC8. We next constructed FR strains in which CRE is driven by the promoter of the DMC1 or the REC8 gene. The FRDMC1 and FRREC8 strains showed no differences from the wild type (WT) in terms of growth, thermo-tolerance, capsule production or melanization (Figure S1A&B). To examine if the appearance of NAT-resistance in FRDMC1 and FRREC8 is coupled to meiosis in vitro, we first tested the FRDMC1 α and FRREC8 α strains cultured alone in media that favor yeast mitotic growth (e.g. YPD) (left upper panel, Figure 1E). No NAT-resistant colonies were recovered from either FRDMC1 α or FRREC8 α under this culture condition (middle and right panels, Figure 1E). Likewise, no NAT-resistance was detected when FRDMC1 α or FRREC8 α was co-cultured with a WT a strain on media that favor yeast mitotic growth (Figure 1E). By contrast, NAT-resistant progeny were recovered from a cross between the FRDMC1 α or the FRREC8 α strain and a WT a mating partner on the mating-inducing V8 agar medium (Figure 1E). The recovery of relatively small number of cells with DMC1 and REC8 activated from the cross on V8 agar medium is not unexpected because a cryptococcal mating colony is highly heterogeneous, with the vast majority of cells dividing mitotically and only a small fraction of cells at the periphery of the mating colony engaging in sexual reproduction (especially so for strains of the H99 background). Additionally, the FRDMC1 and FRREC8 systems likely report strong but not all biologically relevant activation of these meiotic genes occurring during meiosis. This postulation is based on the observation that the number of meiotic progeny yielded by a control cross were 50–100 times higher than the detected flip events. For negative controls, we generated a promoterless flip reporter and a flip reporter without Cre (Figure S1C). We found extremely low levels of false positive activation of FR caused by leaky expression of the promoterless Cre or spontaneous flip without Cre. Furthermore, their activation was not associated with sexual reproduction (Figure S1D). Collectively, these findings indicate that the activation of DMC1 and REC8 detected in these FRDMC1 α and FRREC8 α strains is coupled with meiosis in vitro and is likely an underestimation of meiotic events.
To determine if DMC1 and REC8 are activated during infection, we inoculated mice with the FRDMC1 α or the FRREC8 α strain using the intranasal infection model. The FRDMC1 α and FRREC8 α reporter strains were as virulent as the unmarked WT α strain based on lung fungal burden (Figure S1E). NAT-resistant colonies were recovered from the lungs of every mouse infected with the FRDMC1 α strain or the FRREC8 α strain (Figure 1E and Figure S1F), although the frequency of NAT-resistant isolates recovered was low and varied among individual mice and the batch of the animal experiment (Figure 1E and Figure S1F). Collectively, our findings show that the meiosis-specific genes DMC1 and REC8 are activated during infection in the murine model.
Deletion of meiosis-specific genes increases the proportion of large cryptococcal cells during infection
Given the conserved role of meiosis in ploidy reduction, the activation of meiosis-specific genes during cryptococcal infection raises the possibility that meiotic machinery is activated in vivo and this could contribute to depolyploidization of titan cells. To test the effects of deletion of these meiosis-specific genes on cryptococcal gigantism in vivo, we inoculated mice intranasally with WT H99, dmc1Δor rec8Δ cells. We then collected cryptococcal cells from bronchoalveolar lavage (BAL) fluid at day 3 post infection (DPI 3) to analyze their cell size. We observed an increased proportion of titan cells from the BAL fluid infected with the dmc1Δ mutant and the rec8Δ mutant compared to WT (Figure 2A&B). An increased proportion of titan cells in the lungs in the absence of DMC1 or REC8 was observed in the α as well as the a mating type background (Figure 2A&B). Histopathological examination of fungal cells by Grocott’s methenamine silver (GMS) staining revealed an increase in large cryptococcal cells in the lungs of mice infected with the dmc1Δ or the rec8Δ mutant (brown-black staining in Figure 2A&B). As the ploidy of titan cells is known to be proportional to their cell size [16], these observations suggest that these core meiosis-specific genes contribute to changes in cryptococcal cell size and ploidy during cryptococcal infection.
Figure 2. Deletion of meiosis-specific genes increases the proportion of large cells during cryptococcal infection.
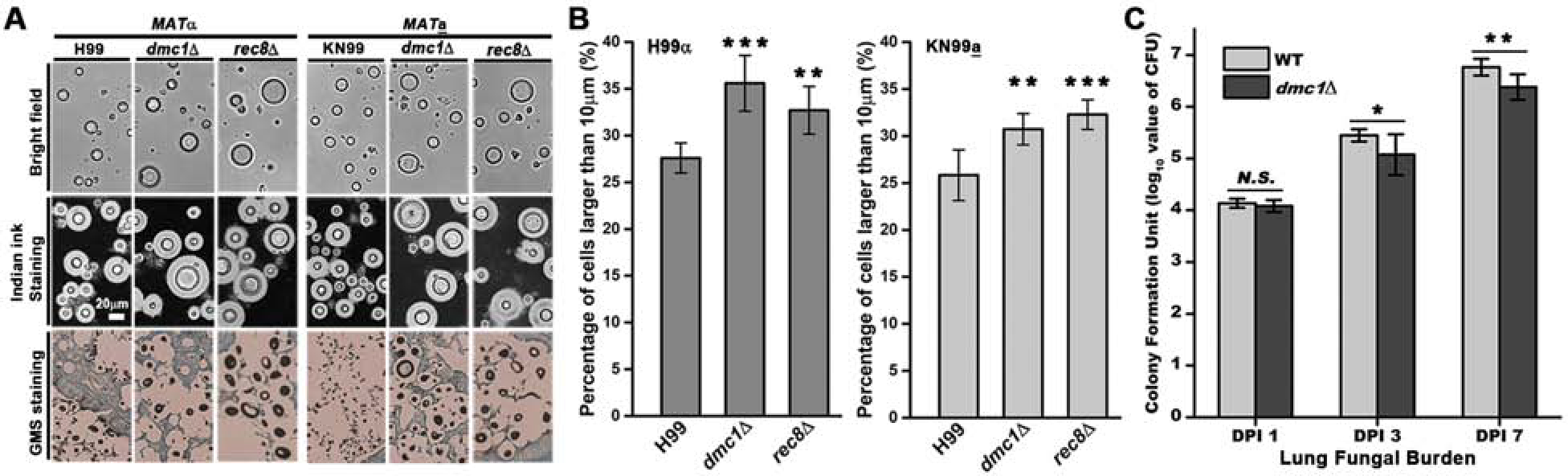
A, BAL fluid and histopathological examination of WT (H99α or KN99a strain alone), dmc1Δ (α or a strain alone), and rec8Δ (α or a strain alone) during lung infection. 5×106 cells of each of these six strains were intranasally inoculated into mice. Cryptococcal cells were recovered from BAL fluid at DPI 3. These fungal cells were examined under microscope with or without India ink negative staining (the white halo surrounding yeast cells reflects the capsule). For histological examination, the lungs were fixed and stained with GMS. Fungal cells appear dark brown or black with GMS staining. B, Quantification of the percentage of cells larger than 10 μm in WT (α or a), dmc1Δ (α or a), and rec8Δ (α or a) during lung infection. For each of the six strains, diameters of 400 cells were quantified based on the microscopic images. Five independent cell size quantifications for each strain were conducted for statistical analysis. Each error bar indicates the standard deviation among the five-independent quantifications. **, P<0.01 (t-test); ***, P<0.001 (t-test). C, Lung fungal burden of mice infected with WT H99 and dmc1Δ. 1×104 of haploid cryptococcal cells were intranasally inoculated into mice and lung fungal burden was analyzed at DPI 1, 3, and 7. Five mice were used for each group and the error bars represent the standard deviations. N.S., p=0.21 (t-test); *, p<0.05 (t-test); **, p<0.01 (t-test).
An increase in the polyploid subpopulation could lead to reduced proliferation due to decreased haploid subpopulation, resulting in delayed progression of disease or reduced fungal burden. To test this hypothesis, we inoculated mice with haploid WT and dmc1Δ mutant strains and compared their lung fungal burden at DPI 1, 3 and 7. No difference was observed at DPI 1, indicating consistent original inoculum (Figure 2C). At both DPI 3 and DPI 7, however, the dmc1Δ mutant showed a subtle but significant reduction in the lung fungal burden relative to WT in the murine model (Figure 2C). The modest reduction in fungal burden of the dmc1Δ mutant in this animal model is expected: mice are hyper-susceptible to this highly aggressive C. neoformans reference strain H99 and most of the haploid cells that do not become titan cells likely dominate in this model host, consequently diminishing the virulence attenuation effects from the deletion of the meiosis-specific gene. Nonetheless, the result indicates that DMC1 contributes to cryptococcal disease progression.
Deletion of DMC1 retards ploidy reduction without affecting polyploidization induced by zeocin in vitro
Titan cell formation can be potentially promoted by many stimuli present in the host, such as serum, CO2, phospholipids, and nutrient starvation [15–18]. However, the nature of the stress that ultimately triggers Cryptococcus polyploidization remains unknown. In some eukaryotes, polyploidization is known to serve as a response to genotoxic stress [29–33]. Here we found that γ-irradiation, which caused DNA DSBs and high levels of cell death at the tested doses (Figure S2A), indeed induced polyploidization of C. neoformans based on propidium iodide (PI) staining (Figure S2B). The result supports the hypothesis that cryptococcal polyploidization is a response to genotoxic stress that causes DSBs. To further test this hypothesis, we treated Cryptococcus cells with different genotoxic stresses, including different doses of ultraviolet radiation (UV), methyl methanesulfonate (MMS), and zeocin (Figure S2C). Zeocin is a radiomimetic DNA-damaging chemical known to cause DNA DSBs in different organisms [33–36]. In C. neoformans, treatment with zeocin at a high concentration resulted in cell death (Figure S2C&S3A) and DNA DSBs, which were detected by the TUNEL assay (Figure S3B). After treating cryptococcal cells with various genotoxic stresses, we observed cell size enlargement only in response to γ-irradiation and zeocin, which cause DSBs, but not to UV or MMS (Figure S2D&F), even though all these treatments at the tested high doses resulted in a poor survival rate (Figure S2C). Consistent with the enlargement of cell size, C. neoformans populations increased in ploidy following zeocin treatment (Figure S2E and Figure S3C), further supporting the idea that genotoxic stresses that cause DSBs induce polyploidization. In this process, we noticed that PI staining of zeocin-treated cells gave some signal in the cytosol, suggesting that PI staining may not be the best approach to monitor DNA content in zeocin-treated cells. Therefore, we decided to use the GFP labeled histone H2B subunit, which is widely used in various eukaryotes to reflect nuclear DNA content [33, 37] (Figure S3D). We then treated the H2B-GFP-labeled strain with zeocin and assayed changes in cell size and ploidy. As shown in Figure 3A–D, zeocin treatment yielded enlarged cells with an approximately 4C nuclear DNA content, consistent with our observation with PI staining in the non-labeled strain (Figure S3C). Unlike two-peak FACS profiles observed in normal cryptococcal populations, it notable that only one peak was observed in the FACS profile of cells treated with zeocin. Similar flow cytometry profiles were observed when analyzing Cryptococcus populations induced to undergo cell enlargement in vitro in other studies [38–40]. This could be attributable to zeocin treatment itself, a population composed of cells in different stages of the cell cycle or ploidy, and/or the use of H2B-GFP which cannot accurately reflect cell cycle compared to direct measurement of DNA content. Nonetheless, the upshift in ploidy and cell size was obvious upon zeocin treatment or γ- irradiation. Given that C. neoformans in the host is subject to genotoxic insults (e.g. external ROS from host immune attack or internal ROS induced by starvation or hypoxia), it is conceivable that cryptococcal polyploidization is a response to genotoxic stress that causes DNA DSBs during infection.
Figure 3. Deletion of DMC1 does not affect the increase in cell size and ploidy induced by zeocin, but it delays the size and ploidy reduction process.
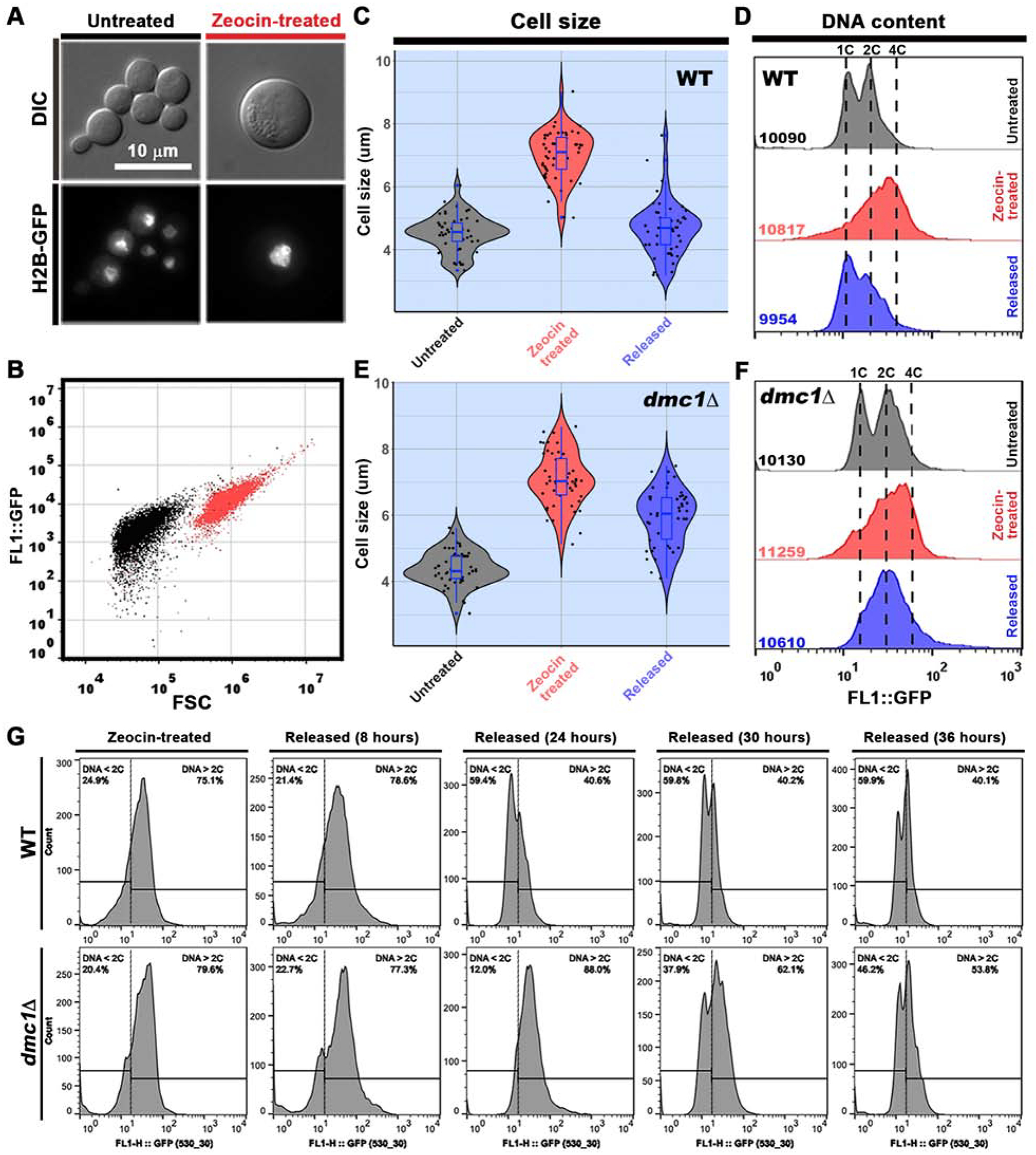
A, Microscopic images of cryptococcal cells with or without zeocin treatment. Overnight cultures of H2B-GFP-tagged H99 cells in liquid YPD medium were treated with zeocin (80 μg/ml) for 5 hours at 30°C with shaking. An aliquot of cells was fixed for microscopy (A) and flow cytometry (B) analyses. The remaining cells were washed twice with PBS buffer and re-cultured in liquid YPD at 30°C with shaking for the time course assay (C-F). B, Flow cytometry analysis using the forward scatter signal (proxy for cell size) and the H2B-GFP signal (proxy for nuclear DNA content) of cryptococcal cells with or without zeocin treatment. C, Diameters of untreated cells (n=50), zeocin-treated cells (n=52), and released cells (n=51) of WT H99 were measured based on microscopic images. The zeocin-released cell populations shown here in C, D, E, and F were collected at 24 hours after being transferred to the zeocin-free medium. Violin plots in C and E were made with the R package of ggplot2 [50]. D, Flow cytometry analysis of H2B-GFP labeled WT cells in response to zeocin treatment and 24 hours after released from zeocin. The total numbers of events analyzed by flow cytometry are shown in the panel. E, Diameters of untreated, zeocin-treated, and released dmc1Δ cells (n= 50 for all) were measured based on microscopic images. F, Flow cytometry analysis of H2B-GFP labeled dmc1Δ cells in response to zeocin treatment and 24 hours after released from zeocin. The total numbers of events analyzed by flow cytometry are shown. Experiments were independently repeated three times. G, A time course flow cytometry analysis of H2B-GFP labeled WT and dmc1Δ cells in response to zeocin (80 μg/ml) treatment and after released from zeocin for 8 hrs, 24 hrs, 30 hrs, and 36 hrs. Zeocin-treated cells were released to zeocin-free YPD media and released-cells were collected at the indicated time points for FACS analysis of H2B-GFP intensity. The vertical lines in each panel indicate the H2B-GFP intensity representing the 2C DNA content. The percentages of the population that has less than 2C DNA content (DNA < 2C) or greater than 2C DNA content (DNA > 2C) were presented in each panel above the horizontal indicator lines. More than 10,000 cells were analyzed for each sample. See also Figure S2 and S3.
The majority of the dmc1Δ population became polyploid (4C) and large in cell size after zeocin treatment to a level comparable to the zeocin-treated wild type population (Figure 3E&F), indicating that Dmc1 does not affect the cryptococcal polyploidization process. After transferring the zeocin-treated wild-type cells to zeocin-free YPD media, we observed a reversal of cell size and ploidy in the population based on flow cytometry assays (Figure 3C, D&G). Deletion of DMC1 obviously delayed the reduction of both cell size and ploidy after the enlarged cells were released into zeocin-free YPD medium (Figure 3E, F&G). The dmc1Δ mutant cells eventually reversed back to the haploid status after prolonged incubation in zeocin-free media (Figure 3G). These results indicate that meiosis-specific factor Dmc1 contributes to de-polyploidization of these large polyploid cryptococcal cells.
Cryptococcal cells that activated their meiosis-specific genes during infection show resistance specific to zeocin
The evidence presented above demonstrates that meiosis-specific genes are activated during infection and they contribute to cryptococcal pathogenesis. Because we infected mice with a single isolate of only one mating type, we expect that cells with activated meiosis-specific genes would appear clonal to the parental strain both genotypically and phenotypically even if meiosis occurred in these cells during infection. Here, we decided to compare cryptococcal cells with or without these meiosis genes being activated during infection. As activation of the meiosis genes is rare in vivo, we resorted to our flip reporter system to identify these progeny. We refer to cells that activated their meiosis-specific genes as “flipped” strains. The flipped strains are NAT-resistant while the original strain and non-flipped progeny are NAT-sensitive (Figure 1D&E). We picked 68 flipped progeny recovered from the lungs and brains of mice infected with either the FRDMC1 α strain or the FRREC8 α strain. For comparison, we picked 24 non-flipped sister strains recovered from the same host tissues. We then examined these strains for their stress tolerance, including resistance to the antifungal fluconazole, H2O2, UV, zeocin, and MMS. Overall, the non-flipped strains behaved similarly as the original WT H99 in all the stress tests, although there were subtle variations (Figure 4A and Figure S4C). Surprisingly, the flipped strains showed a significant wider range of resistance level to zeocin compared to the non-flipped sister strains or the original H99 strain (Figure 4A), but not to the DNA damaging stress caused by H2O2 (Figure S4C), UV irradiation or MMS (Figure 4A&B). To confirm the variation in zeocin-resistance among H99, the non-flipped strains, and the flipped strains, we conducted a time course growth assay. As shown in Figure 4C, flipped progeny showed a wider range of resistance to zeocin while H99 and all the non-flipped progeny were sensitive to zeocin (Figure 4C). Collectively, these results reveal phenotypic diversity among the flipped progeny and implicate specific DNA-damaging stress in activating meiosis-specific genes during cryptococcal infection.
Figure 4. Phenotypic diversity of the flipped progeny recovered from infected mice.
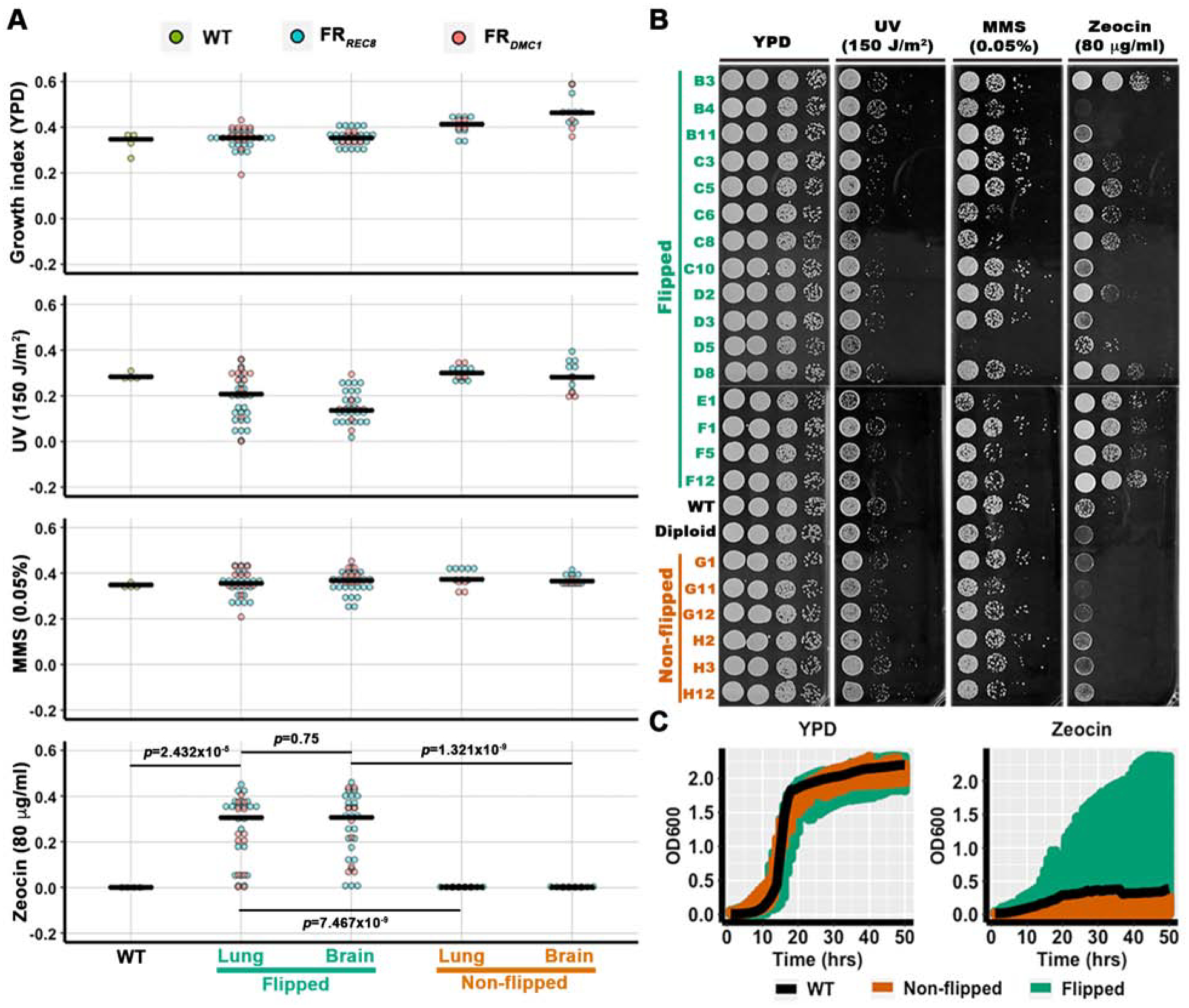
A, A quantitative representation of phenotypic assays of the flipped and the non-flipped progeny recovered from animal lungs and brains after animals were euthanized at DPI 13. Cells were diluted into OD600=0.03 and spotted onto YPD agar media in a 96-spot format with the indicated stress factors at the indicated concentrations. Images were taken after four days of incubation at 30°C and analyzed using Colonyzer [51]. The median growth index values are shown as black bars. See Figure S4 and Table S1&S2 for the raw resource images, data, and the strain list. Mann-Whitney test was used to analyze the growth difference between WT, the flipped, and the non-flipped strains under zeocin condition. The p values of the pairwise comparisons were shown. B, Serial dilutions of the selected flipped and non-flipped progeny were spotted onto YPD agar media supplemented with the indicated stress factors at the indicated doses. Cells were then incubated at 30°C for four days. The parental haploid H99 strain and a diploid strain (H99α/H99α) served as controls. The strain labels correspond to their plate positions in Figure S4B. C, A time course growth assay of the flipped, non-flipped, and WT strains cultured in liquid YPD with or without zeocin. The overnight cultures of the strains used in panel B were diluted into OD=0.05 and inoculated into liquid YPD or YPD with 10 μg/ml of zeocin in a 48-well plate. The plates were cultured at 30°C with continuous shaking and growth was monitored by measuring OD600 every hour. The growth of the WT, the non-flipped, and the flipped strains based on OD600 measurement was plotted against the time after inoculation (growth curve assay). The colored range shows the distribution of growth curves of the WT (black), the non-flipped (orange), and the flipped (green) strains. See also Figure S4.
Discussion
Cryptococcosis is one of the leading causes of deaths among patients with HIV in sub-Saharan Africa [2]. Epidemiological evidence, however, indicates a high prevalence of non-symptomatic cryptococcal infections in the general immunocompetent population. C. neoformans can respond to the hostile environment in the lungs of an immunocompetent host by polyploidization. This fungus can return to normal ploidy under permissive conditions in vitro. Here, we discovered that C. neoformans polyploidizes in response to specific genotoxic stresses that cause DSBs, a type of stress that this fungus likely experiences in the host, based on other studies [41]. DMC1 and REC8, two highly conserved meiosis-specific genes, are activated during cryptococcal disease progression in mice and they contribute to ploidy reduction of polyploid cells when growth conditions become permissive. Disruption of DMC1 reduces cryptococcal virulence in the murine model. As immunocompetent humans are much more resistant to Cryptococcus than mice, we postulate that polyploid cells likely represent a much larger proportion of pulmonary cryptococcal populations in humans than in mice. We speculate that in patients with polyploid cryptococcal cells, ploidy reduction occurs when the host immunity is impaired due to HIV infection or immunosuppressive therapies (e.g. in organ transplant recipients). Meiotic machinery is activated in polyploid cells and contributes to their ploidy reduction. The generated proliferative haploid progeny can cause fatal systemic infections. The impact of meiotic genes on cryptococcosis might be much more dramatic in humans than what is observed in mice. Our findings open a new avenue to investigate the key and yet poorly understood aspects of cryptococcal disease progression (i.e. latency and subsequent reactivation).
At a broader level, the reversible ploidy changes associated with the progression of cryptococcosis is strikingly similar to that of cancer, in which various cancer cells become polyploid in response to genotoxic chemotherapy or radiation therapy. These polyploid tumor cells can de-polyploidize through a meiosis-like process, giving rise to rejuvenated and proliferative cancer cells with normal ploidy, which are often resistant to the therapy [30, 42–44]. The same conserved meiotic genes such as DMC1 and REC8 are activated in TP53 mutant tumors, enhanced by genotoxic treatments or spindle inactivation and associated with reversible polyploidy [45–48]. Such reversible ploidy events are rare (in the scale of 1–10 out of a million), as most cancer cells die due to exposure to the genotoxic treatment. However, few surviving polyploid cells successfully reverse ploidy and become proliferative. The consequence of such events, however, is dire and not rare.
One advantage of using the eukaryotic microbial pathogen C. neoformans for such research is its genetic amenability and robust growth. For instance, here we could select the low proportion of progeny that activated their meiotic genes during cryptococcal infection in mice with the Cre-loxP-mediated flip reporter system. It facilitates phenotypically dissection of these rare populations of cells. An intriguing observation from this study is the increased resistance to zeocin of the progeny that activated their meiotic genes in mice compared to the sister non-flipped progeny isolated from the same host tissue. These cells had never been exposed to zeocin prior to the stress-test. We do not think that the insertion of the flip reporter system into the cryptococcal genome itself caused zeocin resistance in the flipped strains for the following reasons: (i) The non-flipped progeny are sensitive to zeocin and they carry the same constructs as the flipped ones; (ii) FRDMC1 was inserted randomly into the genome and FRREC8 was inserted into the safe haven region [49], and yet flipped strains derived from both reporters showed a similar phenotype; and (iii) not all flipped strains are resistant to zeocin despite carrying the same reporter system. Because we inoculated mice with a single haploid cryptococcal isolate H99, recombination or assortment of chromosomes, typically observed during classic meiosis in a heterozygous zygote derived from mating of two genetically distinct parental strains, is unlikely to account for such phenotypic diversity observed in our study. However, meiosis is a mutagenic process that can generate mutations de novo with much higher rates than mitosis [7].It is possible that the phenotypic diversity and specific resistance to zeocin observed in the flip strains might have resulted from increased mutations due to bona fide meiosis or atypical mitosis with some involvement of the meiotic machinery. Future in-depth comparative genomic analysis of the flipped and the non-flipped progeny may reveal the underlying genetic bases. Our findings provide a new avenue to explore the molecular mechanisms underlying the mutagenic property of meiosis, the genetic bases for the preservation of meiotic machinery in asexual organisms/cells, and the impact of meiotic genes on the evolution and adaption of species.
The findings reported here from a clinically important eukaryotic microbe together with the mounting literature on polyploidization and ploidy reduction via a meiosis/meiosis-like process in cancer cells suggest a general mechanism for rejuvenation in eukaryotes in response to genotoxic stress to promote individual survival. It is our conviction that eukaryotic cells possess an inherent capability of gametogenesis. Whether it is a bona fide meiotic process or a meio-mitotic process [48] involving some of the meiotic machinery warrant further investigation. This and future investigations will yield novel insights into a defining feature of eukaryotes and will have far-reaching implications for our understanding of a wide range of diseases.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xiaorong Lin (xiaorong.lin@uga.edu). Plasmids used in these studies can be made available upon request following the signing of a material transfer agreement with the University of Georgia. All materials generated in the current study are available from the lead contact upon reasonable request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fungi
The C. neoformans strains used in this study are listed in the key resources table. Yeast cells were maintained on YPD medium unless specified otherwise. Mating assays were conducted on V8 juice agar medium in the dark at 22°C. Transformants obtained by biolistic transformation [52] or by TRACE [53] were selected on YPD with 100 μg/ml of nourseothricin (NAT), 100 μg/ml of neomycin (NEO), or 200 μg/ml of hygromycin (HYG). For in vivo experiments, C. neoformans strains were cultured in YPD liquid medium overnight at 30°C with shaking at 220 rpm. The fungal cells were washed with sterile saline three times and resuspended to 1×106 cell/ml in saline.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals | ||
| Hygromycin | Research Products International Corp. | Cat. NO.: H75000 |
| G418 | Research Products International Corp. | Cat. NO.: G64000 |
| Nourseothricin | Jena Bioscience | Cat. NO.: AB-102–25G |
| Zeocin | VWR | Cat. NO.: AAJ67140–8EQ |
| Propidium iodide | Sigma- Aldrich | Cat. NO.: P4864–10ML |
| Experimental Models: Organisms/Strains | ||
| Mouse: A/Jcr | The Jackson Laboratory | CRL:563 |
|
Cryptococcus neoformans strain XL280α (MFα, mild type serotype D strain) |
[5] | Strain ID: XL280α |
|
Cryptococcus neoformans strain H99 (MFα, wild type serotype A strain) |
[23] | Strain ID: H99 |
|
Cryptococcus neoformans strain KN99 (MFa, wild type serotype A strain) |
[56] | Strain ID: KN99 |
|
Cryptococcus neoformans strain YZ83 (MFα, DMC1::NAT serotype A) |
This manuscript | Strain ID: YZ83 |
|
Cryptococcus neoformans strain YZ463 (MFa, DMC1::NAT serotype A) |
This manuscript | Strain ID: YZ463 |
|
Cryptococcus neoformans strain YZ478 (MFα, REC8::NAT serotype A) |
This manuscript | Strain ID: YZ478 |
|
Cryptococcus neoformans strain YZ486 (MFa, REC8::NAT serotype A) |
This manuscript | Strain ID: YZ486 |
|
Cryptococcus neoformans strain YZ175 (MFα, PH2B-H2B-GFP-HYG serotype A) |
This manuscript | Strain ID: YZ175 |
|
Cryptococcus neoformans strain YZ187 (MFα, DMC1::NAT, PH2B-H2B-GFP-HYG serotype A) |
This manuscript | Strain ID: YZ187 |
|
Cryptococcus neoformans strain YZ63 (MFα, FRDMC1, Serotype A) |
This manuscript | Strain ID: YZ63 |
|
Cryptococcus neoformans strain YZ659 (MFα, FRREC8, Serotype A) |
This manuscript | Strain ID: YZ659 |
|
Cryptococcus neoformans strain YZ661 (MFα, FRREC8, Serotype A) |
This manuscript | Strain ID: YZ661 |
| Oligonucleotides | ||
| See Table S3 | This manuscript | N/A |
| Recombinant DNA | ||
| pYZ-FRDMC1 (pXL-PDMC1-CRE-PTEF1-NAT-NEO) |
This manuscript | Plasmid ID: pYZ-FRDMC1 |
| pYZ-FRREC8 (pXL-PREC8-CRE-PREC8-NAT-NEO) |
This manuscript | Plasmid ID: pYZ-FRREC8 |
| pYZ99 (pXL1-PH2B-H2B-GFP-HYG) |
This manuscript | Plasmid ID: pYZ99 |
| pYZ179 (pXL1-PTUB4-TUB4--tdTomato-NEO) |
This manuscript | Plasmid ID: pYZ179 |
| Software and Algorithms | ||
| R 3.6 | The R Foundation | https://www.rproject.org/ |
| ggplot2 | [48] | https://ggplot2.tidyverse.org/ |
| Colonyzer | [49] | https://research.ncl.ac.uk/colonyzer/ |
Animal studies
Clinical observation in animals indicates no sex predisposition of developing cryptococcosis (e.g. cats, dogs, and koalas) [54, 55]. Here we used female A/J mice to minimize the number of mice needed for this study and also to facilitate direct comparison of our findings to published work that is typically based on results obtained from female mice. Six to eight weeks old A/Jcr mice were purchased from the Jackson Laboratory. All mice were healthy before the initiation of those studies. Animals were supplied with hardwood chips as bedding and housed in a temperature-controlled, air-conditioned room on a 12-hr light-dark cycle. The animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and IACUC regulation at University of Georgia and at Rutgers University.
METHOD DETAILS
Flip reporter systems
The coding sequence of the site-specific recombinase Cre fused with the terminator sequence of GPD1 was amplified from the template plasmid pJL519 [56] with primers Linlab3140/YZ and Linlab3141/YZ. The resulting PCR amplicon was digested with restriction enzymes EcoRV and NotI, and cloned into the vector pXL1-PTEF1 [57], which was digested with the same restriction enzymes. The promoter of the meiosis-specific gene DMC1 was amplified with primers Linlab3144/YZ and Linlab3145/YZ using the H99 genome as the template. PDMC1 was then cloned onto the upstream of the coding sequence of Cre after digestion with NotI and XbaI to generate the plasmid pXL1-PDMC1-CRE. Two pairs of Cre-recognition sites, loxP and lox2722 [27, 28, 58], were designed in a convergent orientation flanking the nourseothricin-resistant gene NAT, and FseI and PacI cutting sites were designed at the end of the double-floxed inverted NAT cassette for subsequent cloning. The whole double-floxed inverted NAT cassette was synthesized by GenScript (Piscataway, NJ), which was then cloned into pXL1-PDMC1-CRE with FseI and PacI digestion to generate the flip reporter plasmid pYZ-FRDMC1. The flip reporter plasmid pYZ-FRREC8 was constructed in a similar way, except that the promoter of REC8 was amplified using the primers Linlab4619/YZ and Linlab4620/YZ.
Gene manipulation
To delete the DMC1 open reading frame (ORF) in the H99 background, approximately 1 kb 5’ and 3’ flanking sequences were amplified with primer pairs Linlab3660/YZ-Linlab3661/YZ and Linlab3662/YZ-Linlab3663/YZ. The 5’ and 3’ flanking sequences were then fused with the split dominant NAT drug resistance marker through overlap PCR with primer pairs Linlab3664/YZ-Linlab1539/Wang and Linlab3665/YZ-Linlab1540/Wang, respectively. The split deletion constructs were introduced into the indicated recipient strain by biolistic transformation as described previously [52]. Transformants generated were screened by two rounds of diagnostic PCR. The first round of PCR was to detect integration of the construct into the correct genetic locus (primer pair Linlab3660/YZ and P-actin reverse). The second round of PCR was to confirm the replacement of the DMC1 ORF by the drug-selection cassette (primer pair Linlab3660/YZ and Linlab3663/YZ). A similar strategy was used to delete the REC8 ORF. The primers used in REC8 deletion and mutant confirmation are listed in the key resource table. The deletion mutants in the MATa background were obtained by dissecting the meiotic progeny from crosses between the MATα deletion strains and the corresponding congenic WT a strain. The mating type and the gene deletion of the dissected progeny were confirmed through PCR. All the primers used were listed in key resources table.
To introduce the flip reporters into C. neoformans cells, M13F and M13R were used to amplify the flip reporter cassette with the drug selection marker from the plasmid pYZ-FRDMC1 or pYZ-FRREC8. The FRDMC1 cassette was introduced into recipient cryptococcal cells through biolistic transformation. The FRREC8 cassette was introduced into the safe haven [49] region of the H99α strain through the recently developed TRACE method [53]. Stable transformants were confirmed by drug resistance after 5 passages on non-selective media. To test the specificity of the flip reporter system in vitro, cryptococcal cells were cultured under the yeast-growth-promoting rich YPD medium and mating-promoting V8 medium as shown in Figure 1E. The original inoculum was similar either in YPD or on V8 media for the flip tests. The final numbers of cells examined under these two different conditions were also similar. That means the numbers of cell replication were similar. The incubation time was different because cells grow much faster in YPD medium compared to V8 medium. Stable transformants that did not yield NAT-resistance after overnight cultures either alone or with KN99a [59] cells in liquid YPD were then crossed with KN99a cells on V8 juice agar media (pH=5.0). Mating colonies on V8 plates were cultured for 10 days at 22°C in the dark. The edge of the mating colonies containing the mixture of yeasts, hyphae, and spores were collected and plated onto YPD plates with NAT. Candidates that generate NAT-resistant progeny after mating with the a partner on V8 agar medium, but not in liquid YPD medium, are considered the positive flip reporter strains. Those were stored for the next step of the flip reporter assay.
To tag H2B with GFP, the ORF of H2B-coding gene and 578 bp of its upstream sequence were amplified and cloned into the pXL1-based GFP vector with HYG as the fungal selection marker to generate pYZ99. To tag Tub4 with tdTomato, the ORF of TUB4 and about 1 kb of its upstream sequence were amplified and cloned into the pXL1-based tdTomato vector with NEO as the fungal selection marker to generate pYZ179. Both vectors were linearized with BglII digestion and introduced into Cryptococcus cells through biolistic transformation. Positive transformants were confirmed by diagnostic PCR and fluorescent signals.
Murine cryptococcosis and fungal burden analysis
C. neoformans strains were cultured in YPD liquid medium overnight at 30°C with shaking at 220 rpm. The fungal cells were washed with sterile saline three times and resuspended in saline. Female A/Jcr mice (The Jackson Laboratory, Bar Harbor, ME, USA) of 8 to 10 weeks old were sedated with ketamine and xylazine via intraperitoneal injection. Sedated mice were then inoculated intranasally with 50 μl fungal cell suspension. After infection, animals were monitored daily for disease progression, including weight loss, gait changes, labored breathing, or fur ruffling. Infected mice were euthanized at days as indicated. Lungs or brains were dissected and homogenized in 2 ml cold PBS buffer. The tissue suspensions were serially diluted, plated onto YNB agar medium, and incubated at 30°C for two days to count colony forming units (CFUs). The remaining tissue suspensions were plated onto YPD medium with NAT for the selection of flipped progeny. For flip event detection, the mice inoculated with 5×106 cells in 50 μl saline and the infected mice were euthanized at DPI 13. For assessment of the virulence level of meiotic gene deletion mutants, the mice were inoculated with 1×104 cells in 50 μl saline and infected mice were euthanized at DPI 1, 3, and 7.
Bronchoalveolar lavage (BAL) fluid and histopathology analysis
Groups of 6 to 8 weeks old female A/Jcr mice (Jackson Laboratory, Bar Harbor, ME, USA) were infected intranasally with 5×106 C. neoformans cells in 50 μl PBS. At DPI 3, mice were euthanized, and BAL fluid was harvested and processed as previously described [60]. In brief, a catheter was inserted into the trachea of each animal after euthanasia, and airway-infiltrating cells were obtained by lavage with 1 ml of 1×PBS each time for three times. Cells in the lavage fluid were pelleted at 16,000 g, resuspended in 3.7% formaldehyde, and incubated at 22°C for 30 min. Cells were then washed once with PBS, and >300 cells per animal were analyzed for size by light microscopy. Cells were classified as regular cells (diameter <10 μm) or titan cells (diameter >10 μm). Cells were also visualized with India ink negative staining and observed with an Olympus CX41 microscope equipped with an Infinity digital camera (Olympus).
For histopathological analysis, lungs were immersed in 10% buffered formalin, paraffin embedded, and stained with modified Grocott’s methanamine silver (GMS) stain according to the manufacturer’s instructions (Richard-Allan Scientific, Kalamazoo, MI, USA).
To determine the size of cells isolated from mice, we measured the pixel size of the cell and converted it into actual size according to the scale of the images. The ratio of cells that are larger than 10 μm was calculated. For each strain background, the size of 400 cells from three microscopic images was determined and five-independent quantifications were conducted for each strain background for statistical analysis.
Crosses and the estimation of meiotic events
Mating partners (a and α) were grown separately in YPD at 30°C. Cells were collected and equal numbers of a and α cells were mixed and co-cultured on V8 juice agar medium (pH=5.0) in the dark at 22°C. Successful mating led to the formation of mating hyphae and chains of basidiospores at the periphery of the mating colony. The mating process was monitored microscopically.
For crosses between NAT-resistant MATα and G418-resistant MATa strains, cells from the periphery of the mating patches were collected and suspended in 1×PBS. The cell suspension was diluted and spread onto YPD plates with NAT and G418 to select double-drug-resistant colonies. After 3–4 days of incubation on the selective medium at 30°C, colony forming units (CFUs) were counted. The total number of meiotic progeny yielded from the cross should be approximately four times of the CFUs of the double-drug-resistant colonies. For crosses between FRDMC1 α and WT a strains, cells collected from the periphery of the mating patches were plated onto YPD plates with NAT to select the NAT-resistant flipped progeny. The CFUs on the NAT containing YPD plates indicate the meiotic frequency detected by the flip reporter. This frequency from the crosses between FRDMC1 α and WTa strains was compared to the meiotic events calculated from the crosses between NAT-resistant MATα and G418-resistant MATa strains. The comparison indicated the percentage of meiotic events detected by the FRDMC1 α reporter system.
γ irradiation
C. neoformans cells suspended in PBS (1×108 cells/mL) in microcentrifuge tubes were γ-irradiated (J. L. Shepherd Irradiator) with 500, 1000, and 1500Gy at 22°C. Irradiated samples were then aliquoted into two tubes. Cells from one tube were diluted and plated onto YPD plates to calculate survival rates; Cells from the other tube were fixed for PI staining and FACS analysis.
DNA damage stresses
In addition to γ irradiation, Cryptococcus cells were challenged with other DNA damage stresses, including UV, MMS, and zeocin. For UV irradiation, 1×107 cells were plated onto YPD agar medium, air dried, and then exposed to UV at a dose of 150, 300, and 450 J/m2. For MMS treatment, 1×107 cells in liquid YPD medium were treated with MMS at a final concentration of 0.04% or 0.08% for five hours at 30°C with shaking at 220 rpm. For zeocin treatment, 1×107 cells in liquid YPD medium were treated with zeocin at 10, 20, 40, or 80 μg/ml for five hours at 30°C with shaking at 220 rpm. After treatment, cells were collected and washed with PBS twice. An aliquot of the washed cells was diluted and plated onto YPD agar medium to count CFUs.The survival rate of control cells without any treatment was set as 100% and the relative survival rate of cells under different treatments were calculated. The remaining cells were fixed with 3.7% formaldehyde for microscopy and flow cytometry analyses.
TUNEL Assay
An overnight culture of the wild-type H99 strain was washed twice with PBS. Cells were then inoculated into 10 ml of fresh YPD or YPD plus zeocin (80 μl/ml) to reach OD600=1 and cultured for 5 hours at 30°C with shaking at 220 rpm. DNA strand breaks induced by zeocin were examined by the TUNEL assay with the In Situ Cell Death Detection kit, fluorescein (Catalog No. 11684795910; Roche). Briefly, cryptococcal cells were collected, fixed with 3.7% (vol/vol) formaldehyde for 30 min at 21°C, and washed three times with PBS. Cell wall of the fixed cells was digested with 20 mg/ml lysing enzymes (Lysing enzymes from Trichoderma harzianum, Sigma-Aldrich, in a spheroplasting solution: 1M sorbitol, 10 mM EDTA, and 100mM sodium citrate pH 5.8) at 37°C for 120 min. Digested cells were centrifuged at 800 g for 2 minutes and gently washed three times with PBS. The digested cryptococcal cells were then incubated in the permeabilization solution [0.1% (vol/vol) Triton X-100 and 0.1% (wt/wt) sodium citrate] on ice for 2 min and washed twice with PBS. The permeabilized spheroplasts were subsequently incubated with 50 μl of TUNEL reaction mixture, containing terminal deoxynucleotidyl transferase and fluorescein isothiocyanate dUTP, at 37°C for 60 min. Finally, the spheroplasts were washed three times with PBS and observed under an epi-fluorescence microscope Zeiss Imager M2 (Carl Zeiss, Jena, Germany). Images were acquired with an AxioCam camera using Zen pro software (Carl Zeiss Microscopy).
Microscopy
For fluorescent microscopy, the mCherry-tagged or GFP- tagged cells, or the PI-stained cells were observed under Zeiss Imager M2 microscope. Images were acquired with an AxioCam MRm camera and processed with the software Zen pro.
For scanning electron microscopy (SEM), MATa and MATα cells of WT H99, dmc1Δ, and rec8Δ were cultured in liquid YPD at 30°C with shaking overnight. Cells were collected and diluted in sterile water into a final density of OD600=3. The same number of MATa and MATα cells of H99, dmc1Δ, or rec8Δ were mixed, and 3 μl of the a-α mixture were spotted onto V8 agar and incubated at 22°C in dark for 14 days for sporulation. Areas ~2 mm2 of the mating colonies were excised using a razor blade, placed in vials of fixative containing 2.5% v/v glutaraldehyde in 0.1 M potassium phosphate buffer (pH=7.2) and stored overnight at 4°C. Samples were washed with buffer and post-fixed for at 4°C for 2 h in similarly buffered 1% osmium tetroxide. These samples were then rinsed in distilled water and dehydrated in a graded ethanol series (25%, 50%, 75%, 95%, and 100%). The samples were then critical point dried using a Samdri model 780-A Critical Point Dryer (Tousimis, Inc, Rockville, MD, USA). Samples were mounted on sticky carbon tabs on top of aluminum stubs, sputter-coated with gold-palladium (Leica EM Ace 600 Sputter Coater, USA), and viewed using a FEI FE-SEM Teneo Scanning Electron Microscope (Thermo Fisher Scientific, Hillsboro, OR, USA) operating at 10 kV.
Flow cytometry analysis
Flow cytometry analysis was performed as described previously [61]. Briefly, H2B-GFP-labelled cells were washed and resuspended in PBS buffer for flow cytometry analysis. Cells used for PI staining were first fixed in ice-cold 70% ethanol overnight at 4°C. The fixed cells were washed, resuspended in 0.5 ml of NS buffer (10 mM Tris-HCl [pH=7.2], 0.25 M sucrose, 1 mM EDTA, 1 mM MgCl2, 0.1 mM ZnCl2, 0.4 mM phenylmethylsulfonyl fluoride, 7 mM β-mercaptoethanol). RNase A (0.5 mg/ml) and propidium iodide (10 μg/ml) were added into the suspension and incubated for 2 h at 37°C or overnight at 4°C in the dark. The cells were sonicated for 10 s before analysis with a CyAn ADP cell analyzer (Beckman Coulter, Hialeah, FL) or BD Acuri™ C6 flow cytometer (BD Biosciences). We noted that different cell analyzers may yield FACS patterns with subtle differences. Profiles obtained from the same cell analyzer should be used for direct comparison. Data were analyzed with FlowJo software (Treestar, Inc., Ashland, OR, USA).
Phenotypic assays
Strains were grown overnight in liquid YPD at 30°C with shaking. The cells were washed, adjusted to the same optical density (OD600=0.03), and serially diluted. To test these strains for sensitivity to UV radiation, an equal number of cells were spotted onto YPD agar medium, air dried, exposed to 150 J/cm2 of UV, and then incubated at 30°C. To test the tolerance of fungal cells to other stressors, an equal number of cells were spotted onto YPD agar or YPD agar supplemented with fluconazole (15 μg/ml), zeocin (80 μg/ml), or MMS (0.05%). Cells were cultured at 30°C and images of the plates were taken at day 3 and day 4 of incubation. The colony growth was quantified with Colonyzer [51] and visualized with the R package Platetools [62].
Funding Statement
This work was supported by National Institutes of Health (R21AI132125 and R01AI140719 to XL and R01AI123315 to CX) and the University of Georgia (fund to XL). Dr. Lin holds an Investigator Award in the Pathogenesis of Infectious Disease from the Burroughs Wellcome Fund (1012445). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
QUANTIFICATION AND STATISTICAL ANALYSIS
All in vitro experiments were conducted at least in triplicates unless otherwise stated in the text or figure legends. Statistics were measured by Student’s t-test or Mann-Whitney test for significance using R version 3.6. Statistical information for each experiment can be found in the corresponding figure legend.
DATA AND CODE AVAILABILITY
The authors declare that the data supporting the findings of this study are available within the manuscript and its Supplementary Information. All materials and datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Supplementary Material
Highlights.
Meiosis-specific genes are activated in C. neoformans cells during infection
Deletion of meiotic genes increases the proportion of large cells during infection
Deletion of DMC1 delays the ploidy reduction of C. neoformans cells
Progeny with meiotic genes activated show increased resistance to genotoxic stress
Acknowledgments
We thank Beth Richardson and Dr. John Shields at the Georgia Electron Microscopy laboratory for SEM analyses, Julie Nelson at the UGA Cytometry Shared Resource laboratory for her help with flow cytometry analysis using the CyAn ADP cell analyzer (NIH grant 1S10RR027814), and Dr. Wendy Watford for her help with γ-irradiation. We thank Drs. Douda Bensasson, Ence Yang, Alexander Idnurm, Joseph Heitman, Bing Zhai and the Lin lab members for their inputs on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Casadevall A, and Perfect JR (1998). Cryptococcus neoformans, (Washington, D.C.: ASM Press; ). [Google Scholar]
- 2.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, and Boulware DR (2017). Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon-Chung KJ (1976). A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 68, 943–946. [PubMed] [Google Scholar]
- 4.Lin X, Hull CM, and Heitman J (2005). Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434, 1017–1021. [DOI] [PubMed] [Google Scholar]
- 5.Lin X, Huang JC, Mitchell TG, and Heitman J (2006). Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATalpha allele enhances filamentation. PLoS Genet 2, e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyawali R, Zhao YB, Lin JF, Fan YM, Xu XP, Upadhyay S, and Lin XR (2017). Pheromone independent unisexual development in Cryptococcus neoformans. PLoS Genet 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rattray A, Santoyo G, Shafer B, and Strathern JN (2015). Elevated mutation rate during meiosis in Saccharomyces cerevisiae. PLoS Genet 11, e1004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbeithuber B, Betancourt AJ, Ebner T, and Tiemann-Boege I (2015). Crossovers are associated with mutation and biased gene conversion at recombination hotspots. Proc Natl Acad Sci U S A 112, 2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halldorsson BV, Palsson G, Stefansson OA, Jonsson H, Hardarson MT, Eggertsson HP, Gunnarsson B, Oddsson A, Halldorsson GH, Zink F, et al. (2019). Characterizing mutagenic effects of recombination through a sequence-level genetic map. Science 363. [DOI] [PubMed] [Google Scholar]
- 10.Arbel-Eden A, and Simchen G (2019). Elevated mutagenicity in meiosis and its mechanism. Bioessays 41, e1800235. [DOI] [PubMed] [Google Scholar]
- 11.Zaragoza O, and Nielsen K (2013). Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol 16, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, and Casadevall A (2010). Fungal cell gigantism during mammalian infection. PLoS Pathog 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, and Nielsen K (2010). Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6, e1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstein AC, Fu MS, Mukaremera L, Li Z, Ormerod KL, Fraser JA, Berman J, and Nielsen K (2015). Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, and Casadevall A (2011). Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog 7, e1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dambuza IM, Drake T, Chapuis A, Zhou X, Correia J, Taylor-Smith L, LeGrave N, Rasmussen T, Fisher MC, Bicanic T, et al. (2018). The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trevijano-Contador N, de Oliveira HC, García-Rodas R, Rossi SA, Llorente I, Zaballos Á, Janbon G, Ariño J, and Zaragoza Ó (2018). Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog 14, e1007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hommel B, Mukaremera L, Cordero RJB, Coelho C, Desjardins CA, Sturny-Leclère A, Janbon G, Perfect JR, Fraser JA, Casadevall A, et al. (2018). Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog 14, e1006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop DK, Park D, Xu L, and Kleckner N (1992). DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69, 439–456. [DOI] [PubMed] [Google Scholar]
- 20.Kagawa W, and Kurumizaka H (2010). From meiosis to postmeiotic events: Uncovering the molecular roles of the meiosis-specific recombinase Dmc1. FEBS J 277, 590–598. [DOI] [PubMed] [Google Scholar]
- 21.Mehta GD, Kumar R, Srivastava S, and Ghosh SK (2013). Cohesin: functions beyond sister chromatid cohesion. FEBS Lett 587, 2299–2312. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, He GJ, Chen L, Zheng J, Chen Y, Shen L, Tian X, Li E, Yang E, Liao G, et al. (2018). Genetic basis for coordination of meiosis and sexual structure maturation in Cryptococcus neoformans. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perfect JR, Lang SD, and Durack DT (1980). Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 101, 177–194. [PMC free article] [PubMed] [Google Scholar]
- 24.Janbon G, Ormerod KL, Paulet D, Byrnes EJ 3rd, Yadav V, Chatterjee G, Mullapudi N, Hon CC, Billmyre RB, Brunel F, et al. (2014). Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet 10, e1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittington A, and Wang P (2010). The RGS protein Crg2 is required for establishment and progression of murine pulmonary cryptococcosis. Medical mycology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngamskulrungroj P, Chang Y, Sionov E, and Kwon-Chung KJ (2012). The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohal VS, Zhang F, Yizhar O, and Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, and Lichtman JW (2007). Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62. [DOI] [PubMed] [Google Scholar]
- 29.Erenpreisa J, Cragg MS, Salmina K, Hausmann M, and Scherthan H (2009). The role of meiotic cohesin REC8 in chromosome segregation in gamma irradiation-induced endopolyploid tumour cells. Exp Cell Res 315, 2593–2603. [DOI] [PubMed] [Google Scholar]
- 30.Illidge TM, Cragg MS, Fringes B, Olive P, and Erenpreisa JA (2000). Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage. Cell Biol Int 24, 621–633. [DOI] [PubMed] [Google Scholar]
- 31.Chumduri C, Gillissen B, Richter A, Richter A, Milojkovic A, Overkamp T, Muller A, Pott C, and Daniel PT (2015). Apoptosis resistance, mitotic catastrophe, and loss of ploidy control in Burkitt lymphoma. J Mol Med (Berl) 93, 559–572. [DOI] [PubMed] [Google Scholar]
- 32.Fox DT, and Duronio RJ (2013). Endoreplication and polyploidy: insights into development and disease. Development 140, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adachi S, Minamisawa K, Okushima Y, Inagaki S, Yoshiyama K, Kondou Y, Kaminuma E, Kawashima M, Toyoda T, Matsui M, et al. (2011). Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci U S A 108, 10004–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chankova SG, Dimova E, Dimitrova M, and Bryant PE (2007). Induction of DNA double-strand breaks by zeocin in Chlamydomonas reinhardtii and the role of increased DNA double-strand breaks rejoining in the formation of an adaptive response. Radiat Environ Biophys 46, 409–416. [DOI] [PubMed] [Google Scholar]
- 35.Shimada K, Filipuzzi I, Stahl M, Helliwell SB, Studer C, Hoepfner D, Seeber A, Loewith R, Movva NR, and Gasser SM (2013). TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol Cell 51, 829–839. [DOI] [PubMed] [Google Scholar]
- 36.Seeber A, Dion V, and Gasser SM (2013). Checkpoint kinases and the INO80 nucleosome remodeling complex enhance global chromatin mobility in response to DNA damage. Genes Dev 27, 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Gong FC, Lambert GM, and Galbraith DW (2005). Cell type-specific characterization of nuclear DNA contents within complex tissues and organs. Plant Methods 1, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dambuza IM, Drake T, Chapuis A, Zhou X, Correia J, Taylor-Smith L, LeGrave N, Rasmussen T, Fisher MC, Bicanic T, et al. (2018). The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hommel B, Mukaremera L, Cordero RJB, Coelho C, Desjardins CA, Sturny-Leclere A, Janbon G, Perfect JR, Fraser JA, Casadevall A, et al. (2018). Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. Plos Pathog 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trevijano-Contador N, de Oliveira HC, Garcia-Rodas R, Rossi SA, Llorento I, Zaballos A, Janbon G, Arino J, and Zaragoza O (2018). Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson GJ, Hernon C, Austriaco N, Shapiro RS, Belenky P, and Bennett RJ (2019). Metabolism-induced oxidative stress and DNA damage selectively trigger genome instability in polyploid fungal cells. EMBO J 38, e101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erenpreisa J, Salmina K, Huna A, Jackson TR, Vazquez-Martin A, and Cragg MS (2015). The “virgin birth”, polyploidy, and the origin of cancer. Oncoscience 2, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erenpreisa J, Salmina K, Huna A, Kosmacek EA, Cragg MS, Ianzini F, and Anisimov AP (2011). Polyploid tumour cells elicit paradiploid progeny through depolyploidizing divisions and regulated autophagic degradation. Cell Biol Int 35, 687–695. [DOI] [PubMed] [Google Scholar]
- 44.Erenpreisa J, and Cragg MS (2013). Three steps to the immortality of cancer cells: senescence, polyploidy and self-renewal. Cancer Cell Int 13, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalejs M, Ivanov A, Plakhins G, Cragg MS, Emzinsh D, Illidge TM, and Erenpreisa J (2006). Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC Cancer 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ianzini F, Kosmacek EA, Nelson ES, Napoli E, Erenpreisa J, Kalejs M, and Mackey MA (2009). Activation of meiosis-specific genes is associated with depolyploidization of human tumor cells following radiation-induced mitotic catastrophe. Cancer Res 69, 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitale I, Senovilla L, Jemaa M, Michaud M, Galluzzi L, Kepp O, Nanty L, Criollo A, Rello-Varona S, Manic G, et al. (2010). Multipolar mitosis of tetraploid cells: inhibition by p53 and dependency on Mos. EMBO J 29, 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salmina K, Huna A, Kalejs M, Pjanova D, Scherthan H, Cragg MS, and Erenpreisa J (2019). The cancer aneuploidy paradox: In the light of evolution. Genes 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Upadhya R, Lam WC, Maybruck BT, Donlin MJ, Chang AL, Kayode S, Ormerod KL, Fraser JA, Doering TL, and Lodge JK (2017). A fluorogenic C. neoformans reporter strain with a robust expression of m-cherry expressed from a safe haven site in the genome. Fungal Genet Biol 108, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wickham H (2016). ggplot2: Elegant graphics for data analysis, (Verlag New York: Springer; ). [Google Scholar]
- 51.Lawless C, Wilkinson DJ, Young A, Addinall SG, and Lydall DA (2010). Colonyzer: automated quantification of micro-organism growth characteristics on solid agar. BMC Bioinformatics 11, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toffaletti DL, Rude TH, Johnston SA, Durack DT, and Perfect JR (1993). Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol 175, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan YM, and Lin XR (2018). Multiple Applications of a Transient CRISPR-Cas9 Coupled with Electroporation (TRACE) System in the Cryptococcus neoformans Species Complex. Genetics 208, 1357–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Brien CR, Krockenberger MB, Wigney DI, Martin P, and Malik R (2004). Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Medical mycology 42, 449–460. [DOI] [PubMed] [Google Scholar]
- 55.Krockenberger MB, Canfield PJ, and Malik R (2003). Cryptococcus neoformans var. gattii in the koala (Phascolarctos cinereus): a review of 43 cases of cryptococcosis. Med Mycol 41, 225–234. [DOI] [PubMed] [Google Scholar]
- 56.Patel RD, Lodge JK, and Baker LG (2010). Going green in Cryptococcus neoformans: the recycling of a selectable drug marker. Fungal Genet Biol 47, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao YB, Upadhyay S, and Lin XR (2018). PAS domain protein Pas3 interacts with the chromatin modifier Bre1 in regulating cryptococcal morphogenesis. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolb AF (2001). Selection-marker-free modification of the murine β-casein gene using a lox2722 site. Anal Biochem 290, 260–271. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, and Heitman J (2003). Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect Immun 71, 4831–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, and Pamer EG (2006). Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity 25, 665–675. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka R, Taguchi H, Takeo K, Miyaji M, and Nishimura K (1996). Determination of ploidy in Cryptococcus neoformans by flow cytometry. J Med Vet Mycol 34, 299–301. [PubMed] [Google Scholar]
- 62.Warchal S (2018). An R package for working with multi-well plates.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the manuscript and its Supplementary Information. All materials and datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.


