Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are a diverse class of industrial chemicals with widespread environmental occurrence. Exposure to long-chain PFAS is associated with developmental toxicity, prompting their replacement with short-chain and fluoroether compounds. There is growing public concern over the safety of replacement PFAS.
Objective:
We aimed to group PFAS based on shared toxicity phenotypes.
Methods:
Zebrafish were developmentally exposed to 4,8-dioxa-3H-perfluorononanoate (ADONA), perfluoro-2-propoxypropanoic acid (GenX Free Acid), perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid (PFESA1), perfluorohexanesulfonic acid (PFHxS), perfluorohexanoic acid (PFHxA), perfluoro-n-octanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), or 0.4% dimethyl sulfoxide (DMSO) daily from 0–5 d post fertilization (dpf). At 6 dpf, developmental toxicity and developmental neurotoxicity assays were performed, and targeted analytical chemistry was used to measure media and tissue doses. To test whether aliphatic sulfonic acid PFAS cause the same toxicity phenotypes, perfluorobutanesulfonic acid (PFBS; 4-carbon), perfluoropentanesulfonic acid (PFPeS; 5-carbon), PFHxS (6-carbon), perfluoroheptanesulfonic acid (PFHpS; 7-carbon), and PFOS (8-carbon) were evaluated.
Results:
PFHxS or PFOS exposure caused failed swim bladder inflation, abnormal ventroflexion of the tail, and hyperactivity at nonteratogenic concentrations. Exposure to PFHxA resulted in a unique hyperactivity signature. ADONA, PFESA1, or PFOA exposure resulted in detectable levels of parent compound in larval tissue but yielded negative toxicity results. GenX was unstable in DMSO, but stable and negative for toxicity when diluted in deionized water. Exposure to PFPeS, PFHxS, PFHpS, or PFOS resulted in a shared toxicity phenotype characterized by body axis and swim bladder defects and hyperactivity.
Conclusions:
All emerging fluoroether PFAS tested were negative for evaluated outcomes. Two unique toxicity signatures were identified arising from structurally dissimilar PFAS. Among sulfonic acid aliphatic PFAS, chemical potencies were correlated with increasing carbon chain length for developmental neurotoxicity, but not developmental toxicity. This study identified relationships between chemical structures and in vivo phenotypes that may arise from shared mechanisms of PFAS toxicity. These data suggest that developmental neurotoxicity is an important end point to consider for this class of widely occurring environmental chemicals. https://doi.org/10.1289/EHP5843
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a structurally diverse class of industrial chemicals that contain aliphatic chains with all or some of the carbons bonded to fluorines () and carboxylic acid or sulfonic acid terminal moieties (OECD 2018). There are 4,370 unique PFAS structures (OECD 2018) with 602 compounds currently in commercial use in the United States (U.S. EPA 2019). PFAS have flame-retardant, water-resistant, and surfactant-like properties (Banks et al. 1994; Kissa 2001). This class of compounds is therefore widely used as protectants in paper and packaging products, water- and grease-repellent textiles, nonstick cookware coatings, and firefighting foams (Lindstrom et al. 2011). PFAS are extremely stable due to the carbon–fluorine bond strength (Banks et al. 1994; Kissa 2001). Based on their structurally inherent thermal and chemical stability, PFAS persist in the environment where they are generally resistant to biodegradation, photooxidation, direct photolysis, and hydrolysis (Schultz et al. 2003). As a result, they are widely detected in the environment (Dauchy et al. 2019; Pan et al. 2018), wildlife (Cui et al. 2018; Escoruela et al. 2018; Route et al. 2014), drinking water (Guelfo and Adamson 2018; Guelfo et al. 2018), and humans (Daly et al. 2018; Hurley et al. 2018; Jain 2018).
Since the voluntary phaseout of perfluoro-n-octanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in the early 2000s, time trends of National Health and Nutrition Examination Survey (NHANES) PFOS and PFOA serum levels are generally indicative of reduced human exposures (Jain 2018). Despite reductions, exposures are still widespread, with PFAS detectable in 95% of NHANES subjects (2013–2014) (CDC 2019) and in pregnant women, maternal serum levels for PFOS () and PFOA () have been reported (Fei et al. 2007). Of additional concern, an examination of these compounds in U.S. children 3–11 years of age, most of whom were born after PFOS and PFOA were phased out of use, revealed detectable levels of 14 PFAS, including PFOS and PFOA, in more than 60% of study subjects (Ye et al. 2018). A longitudinal study in Finnish children and adolescents showed that although serum levels of PFOS, PFOA, perfluorohexanesulfonic acid (PFHxS), and perfluorohexanoic acid (PFHxA) decreased over the study period, calculated body burdens generally remained constant and, in some cases, increased (Koponen et al. 2018). In humans, PFAS exposure has been associated with reduced birth weight (Apelberg et al. 2007; Fei et al. 2007), although weak associations with low birth weight or conflicting data have also been reported (Manzano-Salgado et al. 2017; Shoaff et al. 2018; Whitworth et al. 2012). In animal studies, early life stage exposure to PFOS or PFOA have been linked to developmental toxicity in chickens and mice (Jiang et al. 2012; Tucker et al. 2015), immunotoxicity in mice (reviewed by DeWitt et al. 2009), and developmental (Huang et al. 2010; Padilla et al. 2012; Truong et al. 2014) and reproductive toxicity in zebrafish (Jantzen et al. 2017).
To address toxicity concerns, longer alkyl chain PFAS like PFOS and PFOA have been replaced with shorter alkyl chain compounds such as perfluorobutanesulfonic acid (PFBS) or large fluoroether PFAS such as perfluoro-2-propoxypropanoic acid (GenX) and 4,8-dioxa-3H-perfluorononanoate (ADONA). Alternative chemistries that retain the long-chain character, such as ADONA, were engineered with ether linkages and sites of hydrogenation in efforts to reduce biological half-lives (Fromme et al. 2017). Replacement PFAS are therefore increasingly detected in the environment, including in surface water (De Silva et al. 2011; McCord et al. 2018; Pan et al. 2018; Strynar et al. 2015; Wang et al. 2016) and drinking water (Kaboré et al. 2018; McCord et al. 2018). Environmental screening efforts have also identified relevant exposures to PFAS by-products, such as sulfonated fluorovinyl ethers (i.e., PFESA compounds), that are not strictly chemicals of commerce (McCord et al. 2018; Strynar et al. 2015). Growing concern over the safety of GenX and other replacement PFAS has unsurprisingly led to a greater demand for toxicity data (Blum et al. 2015; Borg et al. 2017; Scheringer et al. 2014). However, traditional mammalian toxicity assays can be costly and time consuming, and it is challenging to test multiple chemicals and concentrations of chemicals in parallel. Because PFAS exposures have been historically linked to complex toxicity outcomes involving whole organisms (e.g., developmental toxicity) or specific organ systems (e.g., immunotoxicity), the use of a rapid in vivo animal screening system is justified.
The zebrafish is a widely used in vivo model for toxicity testing (Hamm et al. 2019; Padilla et al. 2012). Development is rapid, with organogenesis complete by 3 d post fertilization (3 dpf). The zebrafish genome contains orthologs for of human genes (Howe et al. 2013) and of the genes that are known human drug targets (Gunnarsson et al. 2008). Zebrafish developmental toxicity testing can be completed in a matter of days by directly exposing the developing organism to xenobiotics. Post-hatch, automated locomotor behavior tests can be used to assess swimming behavior in response to a variety of stimuli as a functional neurodevelopmental outcome. One major limitation of the zebrafish model for toxicity testing relates to chemical dosimetry. Zebrafish embryos are exposed to xenobiotics via immersion. In most studies, nominal waterborne concentrations are generally reported when making determinations on compound toxicity (i.e., positive or negative for toxicity). However, based on physicochemical properties like LogP and differences in exposure parameters (e.g., static vs. semi-static exposures), both of which can affect the uptake, distribution, metabolism, and elimination of test chemicals, the internal tissue dose does not generally reflect nominal exposure media concentrations (Brox et al. 2014, 2016; Kirla et al. 2016; Souder and Gorelick 2017).
The developmental toxicity and developmental neurotoxicity of a subset of PFAS, such as PFOS and PFOA, have been previously evaluated in zebrafish (Hagenaars et al. 2011; Huang et al. 2010; Jantzen et al. 2016; Khezri et al. 2017; Spulber et al. 2014; Ulhaq et al. 2013a, 2013b). PFOS exposure results in failed swim bladder inflation, abnormal ventroflexion of the tail (Hagenaars et al. 2011; Huang et al. 2010; Jantzen et al. 2016; Ulhaq et al. 2013a), and hyperactivity (Hurley et al. 2018; Khezri et al. 2017; Spulber et al. 2014), whereas results for PFOA exposures are quite mixed for both developmental toxicity and behavior (Hagenaars et al. 2011; Huang et al. 2010; Jantzen et al. 2016; Khezri et al. 2017; Padilla et al. 2012; Truong et al. 2014; Ulhaq et al. 2013a, 2013b). However, because replacement PFAS such as GenX and ADONA are detected in the environment yet lack adequate data on their potential toxicity, the goal of this study was to assess the developmental toxicity, developmental neurotoxicity, and tissue doses of multiple aliphatic PFAS (e.g., PFOS, PFOA, PFHxS, and PFHxA), several emerging replacement PFAS (e.g., GenX and ADONA), and a polymer production by-product [e.g., perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid (PFESA1)] in parallel, using zebrafish as a test organism. In addition, the potential of sulfonic acid PFAS with varying alkyl chain lengths to elicit similar toxicity phenotypes was assessed.
Methods
Zebrafish Husbandry
All procedures involving zebrafish were approved by the U.S. Environmental Protection Agency (EPA) National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee and carried out in accordance with the relevant guidelines and regulations. Embryos were obtained from a mixed wild-type (WT) adult zebrafish line (Danio rerio) that was generated and maintained as previously described (Phelps et al. 2017). Briefly, to maintain genetic diversity, a minimum of one WT line (AB and/or Tupfel long fin WT strains) was added one time per year. Zebrafish adults were housed in tanks at an approximate density of . Adults were fed Gemma Micro 300 (Skretting) once daily and shell free E-Z Egg (Brine Shrimp Direct) twice daily Mondays through Fridays. Both food sources were fed once daily on weekends. U.S. EPA WT zebrafish were maintained on a 14 h:10 h light cycle at 28.5°C and bred every 2–3 weeks. For embryo collection, 60–100 adults were placed in 10- or angled static breeding tanks overnight. The following morning, adults were transferred to new angled bottom tanks containing fish facility water, and embryos were collected 30–40 min later.
Chemical Preparation
ADONA [Chemical Abstracts Service Registry No. (CASRN): 958445-44-8; Catalog No. NaDONA] was purchased from Wellington Laboratories (Table 1). GenX Free Acid (CASRN: 13252-13-6; Catalog No. 2121-3-13), PFHxA (CASRN: 307-24-4; Catalog No. 2121-3-39), PFHxS (CASRN: 3871-99-6; Catalog No. 6164-3-X4), PFOA (CASRN: 335-67-1; Catalog No. 2121-3-18), PFOS (CASRN: 1763-23-1; Catalog No. 6164-3-08), perfluorobutanesulfonic acid (PFBS; CASRN: 375-73-5; Catalog No. 6164-3-09), and perfluoroheptanesulfonic acid (PFHpS; CASRN: 375-92-8; Catalog No. 6164-3-2S) were purchased from Synquest. Perfluoropentanesulfonic acid (PFPeS; CASRN 2706-91-4; Catalog No. 6164-3-2U) was synthesized for the study by Synquest Laboratories and chlorpyrifos (CASRN: 2921-88-2; Catalog No. 45395) was purchased from Sigma-Aldrich. PFESA1 (CASRN: 29311-67-9) was obtained from Chemours (Table 1). Stock solutions ( or ) were prepared either by mixing liquid chemical or dissolving neat chemical into molecular-grade dimethyl sulfoxide (DMSO) () or deionized (DI) water, and aliquots were stored at . For each experiment, working solutions were prepared by thawing single-use stock solution aliquots and performing semi- or quarter-log serial dilutions in DMSO or DI water in a 96-well polycarbonate microtiter plate. Stock plates containing working solutions were sealed (Biorad; Catalog No. MSB1001) and stored at room temperature in the dark and used for the duration of each study (maximum storage time of 5 weeks).
Table 1.
Test chemicals.
| Chemical | Name | CASRN | MW (g/mol) | LogPa (OPERAb) | Company, catalog no. |
|---|---|---|---|---|---|
| 4,8-Dioxa-3H-perfluorononanoate | ADONA | 958445-44-8 | 400.05 | 3.96 | Wellington Laboratories, NaNODA |
| Perfluoro-2-propoxypropanoic acid | GenX Free Acid | 13252-13-6 | 330.05 | 3.21 | Synquest, 2121-3-13 |
| Perfluorobutanesulfonic acid | PFBS | 375-73-5 | 300.1 | 3.10 | Synquest, 6164-3-09 |
| Perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid | PFESA1 | 29311-67-9 | 444.12 | 6.02 | Obtained from Chemours |
| Perfluoroheptanesulfonic acid | PFHpS | 375-92-8 | 450.12 | 2.83 | Synquest, 6164-3-2S |
| Perfluorohexanoic acid | PFHxA | 307-24-4 | 314.05 | 2.78 | Synquest, 2121-3-39 |
| Perfluorohexanesulfonic acid | PFHxS | 3871-99-6 | 438.21 | 3.87 | Synquest, 6164-3-X4 |
| Perfluoro-n-octanoic acid | PFOA | 335-67-1 | 414.07 | 3.79 | Synquest, 2121-3-18 |
| Perfluorooctanesulfonic acid | PFOS | 1763-23-1 | 500.13 | 2.77 | Synquest, 6164-3-08 |
| Perfluoropentanesulfonic acid | PFPeS | 2706-91-4 | 350.11 | 3.18 | Synquest, 6164-3-2U |
Note: CASRN, Chemical Abstracts Service Registration Number; MW, molecular weight.
Partition coefficient.
OPEn structure-activity/property Relationship App (OPERA) (https://comptox.epa.gov/dashboard).
Study Design
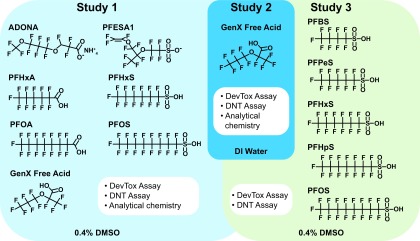
In Study 1 (Figure 1), the developmental toxicity and developmental neurotoxicity and the media and internal tissue doses of ADONA, GenX Free Acid, PFESA1, PFHxA, PFHxS, PFOA, and PFOS were determined, using DMSO as a vehicle. All chemicals except PFESA1 were tested in parallel and shared the same DMSO control samples for all three assays. PFESA1 was obtained subsequently from Chemours and therefore had unique, experiment-specific control data. In Study 1, GenX Free Acid diluted in DMSO was determined to be unstable, resulting in a null data set that was therefore excluded. In Study 2 (Figure 1), zebrafish were exposed to GenX Free Acid diluted in DI water and evaluated in the developmental toxicity (DevTox) and developmental neurotoxicity (DNT) assays. Measured media and tissue doses were also obtained. Last, in a sulfonic acid PFAS follow-up study (Study 3) (Figure 1), the ability of PFBS (4-carbon), PFPeS (5-carbon), PFHxS (6-carbon), PFHpS (7-carbon), or PFOS (8-carbon) exposure to cause developmental toxicity or developmental neurotoxicity was assessed. All chemicals tested in Study 3, except PFPeS, were exposed in parallel and have shared DMSO control data. PFPeS was synthesized for this study and tested separately, with an experiment-specific DMSO control.
Figure 1.
Study design. Zebrafish were semi-statically exposed to test PFAS daily, from dpf. At 6 dpf. developmental toxicity, developmental neurotoxicity, and PFAS tissue concentrations were assessed. Test PFAS included in Study 1, solubilized in DMSO (final concentration 0.4% DMSO), are highlighted in light blue. Because GenX Free Acid was not stable in DMSO, the compound was retested in all three assays using DI water as a diluent in Study 2 (highlighted in blue). In Study 3, a set of sulfonic acid aliphatic PFAS solubilized in DMSO were tested in the DevTox and DNT assays (shown in green). Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; DevTox, developmental toxicity; DI, deionized; DMSO, dimethyl sulfoxide; DNT, developmental neurotoxicity; dpf, days post fertilization; GenX Free Acid, perfluoro-2-propoxypropanoic acid; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHpS, perfluoroheptanesulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentanesulfonic acid.
Chemical Exposures
At 0 dpf, zebrafish embryos were bleached as previously described (Tal et al. 2017). A single embryo at the dome-to-epiboly stages (Kimmel et al. 1995) was placed into each individual well of a 96-well plate containing a nylon mesh filter (Millipore, Catalog No. MANMN4010) with of 10% Hanks’ balanced salt solution (HBSS) per well. Filter inserts containing zebrafish embryos were transferred to 96-well culture trays (Millipore, Catalog No. MAMCS9610) containing of 10% HBSS (Westerfield 2007) and of working solutions per well. A final concentration of 0.4% DMSO was used for all exposure groups and as a vehicle control. In the case of GenX Free Acid in Study 2 (Figure 1), DI water was used as a vehicle control. Daily, from dpf, plates underwent 100% media changes to refresh chemical dosing solutions by blotting (Brandel; Catalog No. FPXLR-196) and transferring mesh inserts containing zebrafish to new bottom plates (Millipore; Catalog No. MAMCS9610). To minimize evaporation, plates were sealed (Biorad; Catalog No. MSA5001) and wrapped with parafilm. Plates were maintained on a 14 h:10 h light cycle at 26.0°C and scored daily for death, malformations, hatching, and swim bladder inflation. At 6 dpf, plates were evaluated by two independent observers and DevTox or DNT assays were performed or media and tissue were collected for analytical chemistry analyses as described below.
Developmental Toxicity Assay
In Study 1 (Figure 1), zebrafish were exposed, as described in the “Chemical Exposures” section, to 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, or PFOS, PFOA, PFHxS, PFHxA, or ADONA, or 0.4% DMSO. Six 96-well plates were tested with a single chemical concentration included on each microtiter plate. Subsequently, as part of Study 1, zebrafish were exposed to 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, or PFESA1, or 0.4% DMSO. The number of biological replicates per study and additional experimental details are shown in Table 2. In Study 2, GenX Free Acid diluted in DI water was tested by exposing zebrafish to 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, or of the compound or DI water. In a follow-up study to assess the toxicity of aliphatic sulfonic acid PFAS (Study 3), a higher starting concentration was used to increase the likelihood of observing both malformations with shorter-chain compounds and malformations at multiple test concentrations. Zebrafish were exposed to 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, or of PFBS, PFHxS, PFHpS, or PFOS or 0.4% DMSO. Subsequently, zebrafish were exposed to 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, or PFPeS, or 0.4% DMSO. Chlorpyrifos was used as positive control for malformations () and lethality () (Padilla et al. 2012; Tal et al. 2017). To conduct DevTox assay assessments, at 6 dpf, two independent observers evaluated zebrafish larvae for survival, hatching, swim bladder inflation, and malformations, including curved body axis, shortened trunk, pericardial edema, yolk sac edema, necrotic yolk sac, pectoral fin abnormalities and head/jaw abnormalities. Directly after assessments, data were reviewed and, in the case of discrepancies, consensus calls were reached. Toxicity values were assigned to descriptive data (i.e., , , severely , and ), modified from a previously described approach (Padilla et al. 2012). Briefly, animals with a single malformation were scored as abnormal, whereas animals with malformations were scored as severely abnormal. A study inclusion criterion based on a previously published study (Padilla et al. 2012) was applied where microtiter plates with abnormal or dead DMSO or DI water control larvae were excluded (one plate from Study 3 was excluded).
Table 2.
Study-specific metrics.
| Study | Name | Diluent and/or vehicle | Assay | Concentrations tested () | Exposure replicatea (n) | Control replicatea (n) | 96-well plates chemicals tested acrossb (n) | |
|---|---|---|---|---|---|---|---|---|
| 1 | ADONA | DMSO | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 216 | 6 | |
| DNT | 4.4, 7.9, 14.0, 25.1, 44.8, 80.0 | 24 | 394 | 17 | ||||
| Chemistry | 25.1, 44.8, 80.0 | 5 | 5 | 3 | ||||
| 1 | GenX Free Acid | DMSO | DevTox, DNT, Chemistry | Not stable in DMSO; results not reported; see Study 2 | ||||
| 1 | PFESA1 | DMSO | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 44 | 1 | |
| DNT | 4.4, 7.9, 14.0, 25.1, 44.8, 80.0 | 24 | 339 | 7 | ||||
| Chemistry | 25.1, 44.8, 80.0 | 4 | 5 | 3 | ||||
| 1 | PFHxA | DMSO | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 216 | 6 | |
| DNT | 4.4, 7.9, 14.0, 25.1, 44.8, 80.0 | 24 | 394 | 17 | ||||
| Chemistry | 25.1, 44.8, 80.0 | 4 | 4 | 2 | ||||
| 1 | PFHxS | DMSO | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 216 | 6 | |
| DNT | 4.4, 7.9, 14.0, 25.1, 44.8, 80.0 | 24 | 394 | 17 | ||||
| Chemistry | 14.0, 25.1, 44.8 | 4 | 4 | 2 | ||||
| 1 | PFOA | DMSO | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 216 | 6 | |
| DNT | 4.4, 7.9, 14.0, 25.1, 44.8, 80.0 | 24 | 394 | 17 | ||||
| Chemistry | 25.1, 44.8, 80.0 | 4 | 4 | 2 | ||||
| 1 | PFOS | DMSO | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 216 | 6 | |
| DNT | 0.2, 0.3, 0.6, 1.0, 1.8, 3.1 | 24 | 394 | 17 | ||||
| Chemistry | 1.0, 1.8, 3.1 | 4 | 4 | 2 | ||||
| 2 | GenX Free Acid | DI water | DevTox | 0.04, 0.1, 0.4, 1.1, 3.1, 9.3, 27.2, 80.0 | 6 | 44 | 1 | |
| DNT | 4.4, 7.9, 14.0, 25.1, 44.8, 80.0 | 24 | 161 | 4 | ||||
| Chemistry | 25.1, 44.8, 80.0 | 4 | 4 | 2 | ||||
| 3 | PFBS | DMSO | DevTox | 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, 100.0 | 10 | 140 | 6 | |
| DNT | 5.5, 9.8, 17.6, 31.4, 56.0, 100.0 | 25 | 327 | 10 | ||||
| 3 | PFPeS | DMSO | DevTox | 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, 100.0 | 6 | 44 | 1 | |
| DNT | 3.1, 5.5, 9.8, 17.6, 31.4, 56.0 | 24 | 186 | 4 | ||||
| 3 | PFHxS | DMSO | DevTox | 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, 100.0 | 10 | 140 | 6 | |
| DNT | 3.1, 5.5, 9.8, 17.6, 31.4, 56.0 | 25 | 327 | 10 | ||||
| 3 | PFHpS | DMSO | DevTox | 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, 100.0 | 10 | 140 | 6 | |
| DNT | 1.7, 3.1, 5.5, 9.8, 17.6, 31.4 | 25 | 327 | 10 | ||||
| 3 | PFOS | DMSO | DevTox | 1.7, 3.1, 5.5, 9.8, 17.6, 31.4, 56.0, 100.0 | 10 | 140 | 6 | |
| DNT | 0.5, 1.0, 1.7, 3.1, 5.5, 9.8 | 25 | 327 | 10 | ||||
Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; DevTox, developmental toxicity; DI, deionized; DMSO, dimethyl sulfoxide; DNT, developmental neurotoxicity; GenX Free Acid, perfluoro-2-propoxypropanoic acid; PFBS, perfluorobutanesulfonic acid; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHpS, perfluoroheptanesulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentanesulfonic acid.
Replicate numbers indicate single animals except for chemistry samples comprising pools of 10 larvae.
Indicates total number of 96-well microtiter plates assessed for each study and/or assay. For Study 1 DevTox and DNT assays, ADONA, GenX Free Acid, PFESA1, PFHxA, PFHxS, PFOA, and PFOS were tested in parallel and shared the same 0.4% DMSO control samples. PFESA1 was obtained subsequently and had unique, experiment-specific control data. In Study 1, GenX Free Acid diluted in 0.4% DMSO was determined to be unstable, resulting in a null data set. In Study 2 (Figure 1), zebrafish were exposed to GenX Free Acid diluted in DI water and evaluated in the DevTox and DNT assays. Measured media and tissue doses were also obtained. Study 3 (Figure 1) examined the ability of PFBS (4-carbon), PFPeS (5-carbon), PFHxS (6-carbon), PFHpS (7-carbon), or PFOS (8-carbon) exposure to cause developmental toxicity or developmental neurotoxicity. All chemicals tested in Study 3, except PFPeS, were exposed in parallel and have shared DMSO control data. PFPeS was synthesized for this study and tested separately, with an experiment-specific DMSO control.
Developmental Neurotoxicity Assay
To increase the likelihood of observing behavioral effects in morphologically normal larvae, the highest concentration evaluated in the DNT assay was the lowest observed effect concentration (LOEC) determined in DevTox assay. Zebrafish were exposed in parallel to 4.4, 7.9, 14.0, 25.1, 44.8, or PFOA, PFHxS, PFHxA, or ADONA or 0.2, 0.3, 0.6, 1.0, 1.8, or PFOS or 0.4% DMSO. Subsequently, as part of Study 1, exposure to 4.4, 7.9, 14.0, 25.1, 44.8, or PFESA1, or 0.4% DMSO was evaluated. In Study 2, zebrafish were exposed to 4.4, 7.9, 14.0, 25.1, 44.8, or GenX Free Acid, or DI water. In Study 3, to increase the likelihood of observing malformations with shorter-chain compounds at multiple test concentrations, the highest concentration evaluated was . Zebrafish were exposed to 5.5, 9.8, 17.6, 31.4, 56.0, or PFBS; 3.1, 5.5, 9.8, 17.6, 31.4, or PFHxS; 1.7, 3.1, 5.5, 9.8, 17.6, or PFHpS; or 0.5, 1.0, 1.7, 3.1, 5.5, or PFOS, or 0.4% DMSO. Subsequently, as part of Study 3, zebrafish were exposed to 3.1, 5.5, 9.8, 17.6, 31.4, or PFPeS, or 0.4% DMSO. Chemical exposures and assessments were performed daily, as described above. To evaluate swimming behavior in a light/dark behavior test, microtiter plates were placed in a dark, temperature-controlled behavior testing room set to 26.0°C for at least 2 h prior to testing. At the time of testing, microtiter plates were placed on a Noldus tracking apparatus. Locomotor activity was recorded (30 frames/s) for a total of 60 min consisting of a 20-min dark acclimation period (0 lux) that was not analyzed followed by a 40-min testing period consisting of a 20-min light period (5.0 lux) and 20-min dark period (0 lux). Videos were analyzed using Ethovision software (version 3.1; Noldus Information Technology) as previously described (Jarema et al. 2015). Locomotor activity was collected for each individual fish for each 2-min period (minimum distance moved, set to ). Thus, for a 40-min test, 20 data points were collected per larvae. Based on microtiter plate inclusion criterion (i.e., abnormal or dead control larvae), two plates from Study 1 were excluded. Four additional criteria for inclusion of individual larvae were applied. One, all larvae that were identified as abnormal, severely abnormal, or dead were excluded. Two, larvae with uninflated swim bladders were removed from analyses. Three, individual larvae that moved in either 10-min dark period were removed. Four, concentrations of test PFAS with fewer than 13 animals remaining (i.e., death or malformations within the test group) were excluded from behavior analyses. Also as part of the DNT assay, media samples were collected. At 6 dpf, media samples () were collected and stored at until further analysis.
Tissue Sample Preparation for Analytical Chemistry
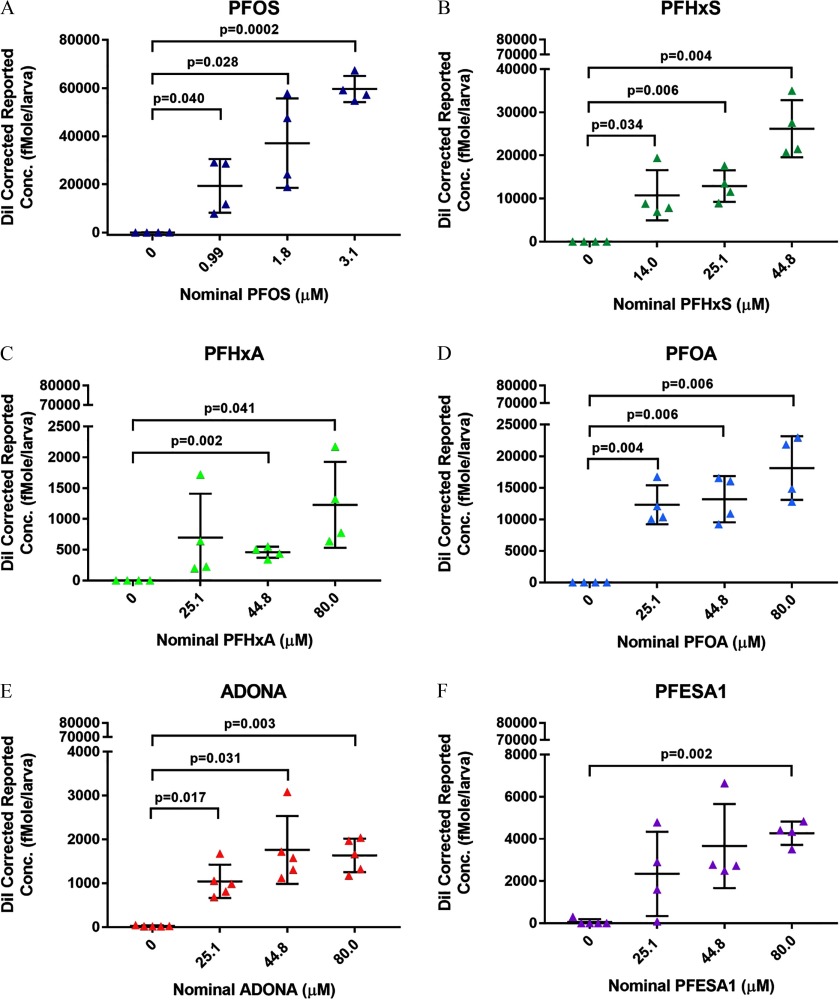
Zebrafish were exposed to 25.1, 44.8, or ADONA, PFOA, PFESA1, or PFHxA, 14.0, 25.1, or PFHxS, or 1.0, 1.8, or PFOS, or 0.4% DMSO. The highest concentration evaluated was the no observed effect concentration (NOEC) determined in Study 1. In Study 2, zebrafish were exposed to 25.1, 44.8, or GenX Free Acid or 0.4% DMSO. According to the previously described microtiter plate inclusion criterion, 15/15 plates were included in the study. Larvae were anesthetized by rapid cooling in chilled 10% HBSS. Groups of 10 anesthetized larvae were pooled to comprise one biological replicate () in of 10% HBSS, flash frozen in liquid nitrogen, and stored at . In the case of ADONA, .
Analytical Chemistry
PFAS native standards were obtained from SynQuest and Sigma-Aldrich. PFAS standards were obtained from Wellington Laboratories. Stock solutions of the PFAS were prepared in 95% methanol with 5% aqueous sodium hydroxide and stored at room temperature in plastic. Intermediate standards were prepared daily in methanol or acetonitrile.
Exposure Media Analysis
Exposure media samples (10% HBSS) and quality control (QC) samples (10% HBSS) at microgram-per-milliliter concentrations were diluted and fortified with a surrogate [i.e., perfluorononanoic acid (PFNA)] and internal standards. Calibration standards were prepared at nanogram-per-milliliter concentrations in aqueous ammonium acetate with 20% methanol. Standards and samples were analyzed with ACQUITY ultra-high-performance liquid chromatography (UPLC) system and Quattro Premier XE triple quadrupole mass spectrometer (Waters Corporation) operated in negative electrospray ionization (ESI) mode. ESI source conditions were optimized for the ion of PFAS as follows: capillary voltage , source temperature 150°C, desolvation temperature 350°C, cone gas flow , and desolvation gas flow . Compound-specific tandem mass spectrometry (MSMS) parameters were used to collect two multiple reaction monitoring (MRM) transitions for the ion of each target analyte (Table 3). UPLC separation was achieved using ACQUITY UPLC BEH C18 Column, , , (Waters Corp P/N 186,002,350) at 50°C with the gradient elution at a flow rate of using ammonium acetate in methanol and water with a injection. Data collection, integration, calibration, and quantitation were performed using MassLynx software (version 4.1; Waters Corporation). Concentration for each PFAS analyte was determined by internal standard technique using isotopically labeled internal standards and calibration standards prepared in solvent. Qualitative identification was based on relative retention time and peak abundance ratio of two MRM transitions. Exposure media analysis was verified and evaluated using blanks, calibration standards, and QC standards prepared at three concentrations across the methods range. Batch results for exposure media were evaluated based on the following criteria: the calibration curve used a minimum of seven standards with a correlation coefficient of , standards accuracy tolerance (30% at LLOQ), QC standard accuracy tolerance (30% PFOS), QC standard precision expressed as percent relative standard deviation , of QC standards satisfied accuracy criteria. Exposure media analysis method performance characteristics are listed in Excel Tables S1 and S2.
Table 3.
Compound-specific Quattro Premier XE MSMS parameters.
| Compound name | Parent (m/z) | Daughter (m/z) | Cone (V) | Collision (V) |
|---|---|---|---|---|
| GenX 1° | 329.07 | 284.06 | 10 | 5 |
| GenX 2° | 329.07 | 184.72 | 10 | 23 |
| GenX IS | 332.00 | 287.06 | 10 | 5 |
| PFHxA 1° | 312.91 | 268.81 | 15 | 9 |
| PFHxA 2° | 312.91 | 118.64 | 15 | 25 |
| PFHxA IS | 315.00 | 269.81 | 15 | 9 |
| PFHxS 1° | 398.85 | 98.57 | 55 | 37 |
| PFHxS 2° | 398.85 | 79.62 | 55 | 41 |
| PFHxS IS | 401.85 | 79.62 | 55 | 41 |
| PFOA 1° | 412.93 | 368.84 | 15 | 11 |
| PFOA 2° | 412.93 | 168.63 | 15 | 21 |
| PFOA IS | 417.00 | 372.00 | 15 | 11 |
| PFOS 1° | 499.00 | 98.57 | 60 | 41 |
| PFOS 2° | 499.00 | 79.62 | 60 | 45 |
| PFOS IS | 503.00 | 98.57 | 60 | 41 |
| ADONA 1° | 377.02 | 250.83 | 15 | 13 |
| ADONA 2° | 377.02 | 84.70 | 15 | 29 |
| PFESA1 (Nafion, BP 1) 1° | 442.98 | 146.69 | 35 | 29 |
| PFESA1 (Nafion, BP 1) 2° | 442.98 | 262.79 | 35 | 19 |
| PFNA 1° | 463.00 | 418.90 | 15 | 13 |
| PFNA 2° | 463.00 | 218.84 | 15 | 17 |
| PFNA IS | 468.00 | 423.00 | 15 | 13 |
Note: 1° denotes the primary multiple reaction monitoring (MRM) transition for a compound used for quantitative analysis and 2° denotes the secondary MRM transition for a compound used for qualitative identification confirmation. ADONA, 4,8-dioxa-3H-perfluorononanoate; GenX Free Acid, perfluoro-2-propoxypropanoic acid; MSMS, tandem mass spectrometry; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentanesulfonic acid; Quattro Premier XE, triple quadrupole mass spectrometer.
Tissue Analysis
Tissue samples were prepared by protein precipitation. Flash frozen samples, consisting of pools of ten 6-dpf zebrafish larvae, were homogenized using of 1.0-mm diameter zirconia/silica beads and a Fast-Prep-24™ homogenizer (MP Biomedicals) in formic acid fortified with the surrogate (i.e., PFNA). Protein was precipitated from the homogenate with of acetonitrile containing internal standards and separated by centrifugation at for 15 min at 4°C. Fifty microliters of the extract was diluted with aqueous ammonium formate in the LC vial for analysis. Standards and samples were analyzed with Vanquish UPLC and Orbitrap Fusion mass spectrometer (Thermo Electron) operated in negative ESI mode. ESI source conditions were optimized for the ion of PFAS as follows: spray voltage , sheath gas 25 au (arbitrary units), aux gas 6 au, sweep gas 0 au, ion transfer tube temperature 300°C, and vaporizer temperature 30°C. High-resolution accurate mass (HRAM) MS1 scans were collected with the following parameters: detector type orbitrap, orbitrap resolution 30,000 full width at half maximum (FWHM), normal mass range, Use , scan range , radio frequency (RF) lens 60%, automatic gain control (AGC) target , max injection time of , 1 microscan, data type set to profile, negative polarity, and source fragmentation disabled. Data-dependent orbitrap MSMS (ddMS2-OT) scans were collected for the ion [ for GenX]– using a target mass list for the eight PFAS and PFNA surrogate for identity confirmation. The apex detection was set to an expected peak width of 2 s at FWHM and desired apex peak window of 30%. The dynamic exclusion parameters were set to exclude after a one-time, 60-s exclusion duration, low and high mass tolerances of , and exclude isotope was set to true. The intensity threshold for collecting an MSMS scan was set to . Fragmentation was done by high-energy collisional dissociation (HCD). The ddMS2-OT scans were collected with the following parameters: orbitrap isolation mode, isolation window of , isolation offset off, stepped HCD collision energy of , scan range of auto mass-to-charge ratio normal, orbitrap resolution of 30,000 FWHM, first mass , max injection time of 54 s, AGC target , inject ions from available parallelizable time set to true, max injection time of 54 ms, 1 microscan, and data type set to centroid. UPLC separation was achieved using ACQUITY UPLC BEH C18 Column, , , (Waters Corp P/N 186,002,350) at 50°C with gradient elution at a flow rate of using ammonium formate in acetonitrile and water with a injection. Data collection, integration, calibration, and quantitation were performed using Xcalibur software (version 4.1; Thermo Electron). Concentration for each PFAS analyte was determined by internal standard technique using isotopically labeled internal standards and matrix-matched calibration standards. Quantitative analysis was performed using high-resolution MS1-extracted ion chromatograms. Qualitative identification was based on relative retention time, MS1 peak abundance ratio of to a source decomposition product, and ddMS2-OT spectra. Tissue analysis was verified and evaluated using blanks, matrix-matched calibration standards, and matrix-matched QC standards prepared at three concentrations across the method’s range. Batch results for exposure media were evaluated based on the following criteria: the calibration curve used a minimum of seven standards with a correlation coefficient of , standards accuracy tolerance (30% at LLOQ), QC standard accuracy tolerance , QC standard precision expressed as , of QC standards satisfy accuracy criteria. The tissue analysis method performance characteristics are listed in Excel Tables S3 and S4.
Statistics
For the DevTox assay, a Kruskal-Wallace nonparametric one-way analysis of variance (ANOVA) with a Dunn’s multiple comparison test was used to detect differences between exposure groups (*, **) and LOEC values were determined. If a test for linear trend was significant (), with developmental toxicity observed at the highest concentration tested, nonlinear regression was performed with a Hill slope curve fitting for half maximal effective concentration () value determinations.
For DNT assay results shown in Figures 3, 6, and 8, a repeated measures ANOVA analysis was used to detect differences in swimming behavior between exposed and control larvae (Catron et al. 2019b; Irons et al. 2013; Phelps et al. 2017; Stevens et al. 2018). These analyses were performed using SAS (version 9.4; SAS Institute Inc.). Group means and standard errors were calculated by SAS Proc Means for each 10-min period included in the 40-min testing period [i.e., light 1 (L1), light 2 (L2), dark 1 (D1), and dark 2 (D2)]. Given that individual activity values were collected for each larva for every 2-min period, there were five data points for each 10-min light or dark period. First, means were calculated by individual larva across the five time points, then concentration group means, and standard errors were calculated using the larval means. Parametric analysis of locomotor data was conducted using SAS Proc Mixed. For each test chemical, a mixed-effects repeated measures model was run separately for each light or dark period (i.e., L1, L2, D1, D2). Each larva was considered a subject and an autoregressive covariance matrix was estimated across the five time points within each 10-min light or dark period. The fixed effects included in the model were as follows: experiment, plate nested within experiment, concentration, time, and the two-way interaction concentration by time. If the concentration effect within each 10-min light or dark period was significant () (i.e., because the same larvae were tested for four 10-min time periods), then pairwise t-tests were computed, comparing each concentration group to the control group (). Dunnett’s test was used to adjust for multiple comparisons (). The parametric mixed model analysis included the ability to model design variables (e.g., plate or day of test), the correlated structure of the repeated distance measurements, and relationships among fixed effects (e.g., concentration, time). These statistics are displayed in Figures 3, 6, and 8 and Excel Tables S5–S7. To conclude that a test chemical was positive for developmental neurotoxicity, significance must have been detected at either more than one concentration within a time period or at the same concentration across multiple time periods.
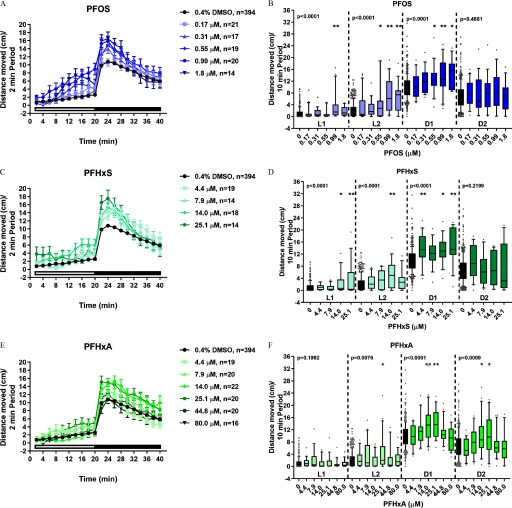
Figure 3.
Locomotor activity in zebrafish developmentally exposed to PFAS. Zebrafish were semi-statically exposed to ADONA, PFESA1, PFHxA, PFHxS, or PFOA, PFOS, or 0.4% DMSO as a vehicle control daily from dpf. At 6 dpf, larvae were assessed for developmental toxicity. Morphologically normal larvae with inflated swim bladders were subjected to behavioral testing. (A, C, E, G, I, K) Distance moved (cm) each 2-min period over the entire 40-min testing period are shown. (B, D, F, H, J, L) To make statistical comparisons, the mean distance moved during each 10-min light 1 (L1), 10-min light 2 (L2), 10-min dark 1 (D1), or 10-min dark 2 (D2) periods are shown. For all chemicals except PFESA1, 14–23 larvae were tested per chemical concentration and the same DMSO control larvae () were used. PFESA1 was tested separately ( per chemical per concentration; 339 DMSO control larvae were evaluated). Repeated measures ANOVA models were run separately by period (L1, L2, D1, or D2). If a significant effect of concentration was detected (), within-period pairwise comparisons to control were computed using t-tests with a Dunnett adjustment for multiple comparisons (*, **). Significance relative to period-specific DMSO controls are shown. Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; ANOVA, analysis of variance; D, dark period; DMSO, dimethyl sulfoxide; dpf, days post fertilization; L, light period; PFAS, per- and polyfluoroalkyl substances; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid.
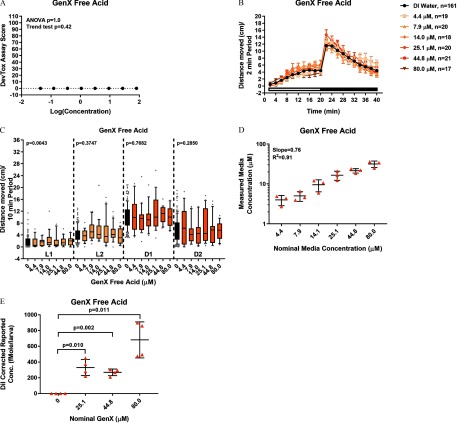
Figure 6.
Developmental and behavioral assays and media and tissue concentrations in zebrafish exposed to GenX Free Acid diluted in DI water. (A) Developmental toxicity scores at 6 dpf obtained from zebrafish developmentally exposed to GenX Free Acid diluted in DI water daily, from dpf. Significance was determined by one-way ANOVA with a Tukey’s multiple comparison test (). larvae per concentration tested. For the DNT assay, zebrafish were exposed to GenX Free Acid daily, from dpf. At 6 dpf, locomotor activity was assessed. (B) Distance moved (cm) each 2-min period or (C) mean distance moved during the light 1 (L1), light 2 (L2), dark 1 (D1), or dark 2 (D2) 10-min periods are shown. Repeated measures ANOVA models were run separately by period (L1, L2, D1, or D2). If a significant effect of concentration was detected (), within-period pairwise comparisons to control were computed using t-tests with a Dunnett adjustment for multiple comparisons (*). zebrafish per concentration and 161 DI water control larvae were assessed. (D) Media concentrations and (E) internal tissue dose at 6 dpf following daily exposure to GenX Free Acid. media replicates and biological replicates each comprising 10 pooled larvae. Significance was determined by a Welch’s ANOVA followed by a Dunnett T3 test (). Additional one-sample Student’s t-tests were performed (). Note: ANOVA, analysis of variance; D, dark phase; DevTox, developmental toxicity; DI, deionized; Dil, dilution; DNT, developmental neurotoxicity; dpf, days post fertilization; GenX Free Acid, perfluoro-2-propoxypropanoic acid; L, Light period.
Figure 8.
Locomotor activity in zebrafish exposed to alkyl sulfonic acid PFAS. Zebrafish were semi-statically exposed to PFBS, PFPeS, PFHxS, PFHpS, or PFOS daily from dpf. For all chemicals except PFPeS, 14–25 larvae were tested per chemical concentration and the same DMSO control larvae () were used. PFPeS was tested separately ( larvae per concentration; 186 DMSO control larvae were evaluated). At 6 dpf, larvae were assessed for developmental toxicity. Morphologically normal larvae with inflated swim bladders were subjected to behavioral testing. (A, C, E, G, I) Distance moved (cm) each 2-min period or (B, D, F, H, J) mean distance moved during the light 1 (L1), light 2 (L2), dark 1 (D1), or dark 2 (D2) 10-min periods are shown. Repeated measures ANOVA models were run separately by period (L1, L2, D1, or D2). If a significant effect of concentration was detected (), within-period pairwise comparisons to control were computed using t-tests with a Dunnett adjustment for multiple comparisons (*). ANOVA, analysis of variance; D, dark phase; DMSO, dimethyl sulfoxide; dpf, days post fertilization; L, light period; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFHpS, perfluoroheptanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentanesulfonic acid.
In addition to the evaluation of mean movement measures described above, linear mixed-effects models (SAS Proc Mixed) were used to examine individual movement measures from individual zebrafish as a function of time, concentration, and . Data from light and dark periods were evaluated separately, given the clear differences in time trends during each period. Nearly half of the light period data were at or below the limit of detection (LOD) (), whereas of the dark period data were . The LOD was based on the minimum distance moved value, set to in Ethovision. This means that individual larva must move, from one frame to the next, a minimum of , to be considered in motion. All values were initially assigned a value of LOD divided by the square root of 2. Inspection of QQ-plots (see Figure S1A,D) indicated that both light and dark period data were not normally distributed (Pleil 2016b). A square root transformation was therefore performed to improve the shape of the upper end of each measurement distribution (see Figure S1B,E). Because square root transformation did not sufficiently alter the lower end of each distribution, a multiple value imputation strategy was used (Pleil 2016a). This strategy is analogous to robust regression on order statistics (ROS), a widely used imputation strategy for censored data. Briefly, for the light period data, of measurements were and values were therefore imputed for this (see Figure S1C). Zebrafish increased locomotor activity in the light period over time. An inverse trend between time and the percentage of behavior measurements was observed (see Figure S2A). For chemicals that caused light-phase hyperactivity (i.e., PFOS and PFHxS), a modest inverse relationship between chemical concentration and the percentage of measurements was also observed (see Figure S2B,C). No clear trend between chemical concentration and percentage of values emerged for compounds that were negative for light period hyperactivity (see Figure S2C–G). For the dark period data, of measurements were . Given the shape of the distribution (see Figure S1E), values were ultimately imputed for the lowest 30% of the data (see Figure S1F). This ROS imputation allowed the order of the raw data to be preserved in the final corrected distribution.
To carry out the ROS technique, for both light and dark period data sets, all measurements were ordered from smallest to largest. Values were all equivalent, and were therefore extracted, randomly sorted, and placed back into the main data sets. Next, the relative position () of each measurement was determined according to the equation: , where is the rank for a given measurement and N is the total number of measurements. A z-score was then assigned to each measurement using the probit function in SAS (version 9.4). Here, the assigned z-score represents the quantile for a specific measurement, assuming it is from a standard normal distribution. In both data sets, the square root–adjusted measurements were regressed on z-scores where the regression was restricted to the upper 50% of the light period distribution and to the upper 70% of the dark period distribution. Regression equations were used to predict square root–adjusted measurements for the lower portions of the measurement distributions (see Figure S1C,F). The combined use of square root transformation and multiple value imputation allowed key regression assumptions (i.e., homoskedasticity and normality of residuals) to be met.
In all mixed models, the square root–transformed movement data were regressed on time, concentration, and the interaction of . For the light period, measurements were considered between and . For the dark period, measurements were considered between and . Data at (light period) and (dark period) minutes were considered to reflect transition periods and were, therefore, excluded from the analysis. All mixed models included a random effect for zebrafish, thus allowing partitioning of measurement variance into that which was observed between and within (over time) individual organisms. A compound symmetry covariance matrix was used, which assumes constant correlated errors between time points within organisms. Observed p-values for time indicate whether the linear effect of time on movement is significantly different than 0. The p-values for concentration indicate whether the intercept for movement (at for the light period, and for the dark period) differs across concentration groups (with DMSO set as the reference group). Finally, the p-values for indicate whether the linear relationship between time and movement changes as a function of concentration (with DMSO set as the reference). Mixed model results were used to estimate zebrafish movement at specific time points in the light and dark periods. Specifically, regression equations were used to estimate movement at and in the light period, and at and in the dark period [note: any estimates that were (occurring in the light period only) were assigned a value of LOD divided by the square root of 2]. The difference in estimated movement during each period was ultimately used to gauge the magnitude of concentration-related effects on movement across all study chemicals.
For targeted analytical chemistry, PFAS concentrations were measured across media ( replicates) and tissue samples ( replicates, each comprising 10 pooled larvae). Measured media samples that were below the method detection limit () (see Excel Table S8) were replaced with the value MDL divided by the square root of 2. To control for heteroskedasticity, log-transformation was performed followed by linear regression of log(measured media concentration) on log(nominal media concentration). Linear regression did not consider samples (i.e., ). Linear regression therefore considered measurements and, further, met assumptions of normality and homoscedasticity. Significance indicates that a linear increase in measured concentration was observed in accordance with rising nominal concentrations (). Measured tissue samples (see Excel Table S9) were replaced with the value MDL divided by the square root of 2. Welch’s ANOVA was performed followed by a Dunnett T3 test (Dunnet 1980) (). If a single sample was , all four exposure groups were considered for multiple comparison testing (this occurred for ADONA and PFESA1). If samples were at or below the MDL, only the top three exposure groups were considered in the Dunnett T3 test (this occurred for PFHxA, PFHxS, PFOA, and PFOS). Additional one-sample Student’s t-tests were then performed to compare measured concentrations to the MDL divided by the square root of 2 or to the lowest measured value above the MDL for ().
Data Availability
The data sets generated during the current study are available in Science Hub by searching for the manuscript title at https://sciencehub.epa.gov/sciencehub/.
Results
Developmental Toxicity Phenotypes in Larval Zebrafish Exposed to PFAS
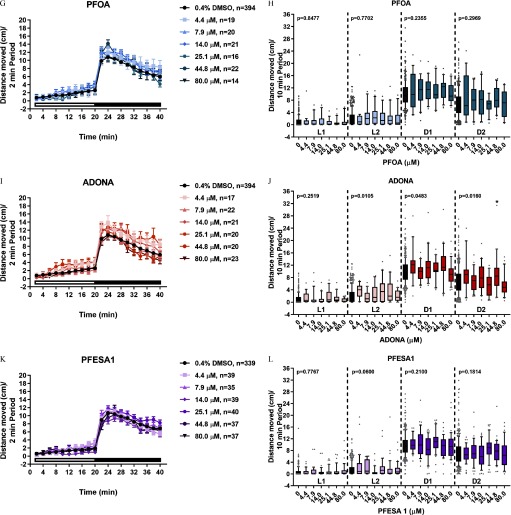
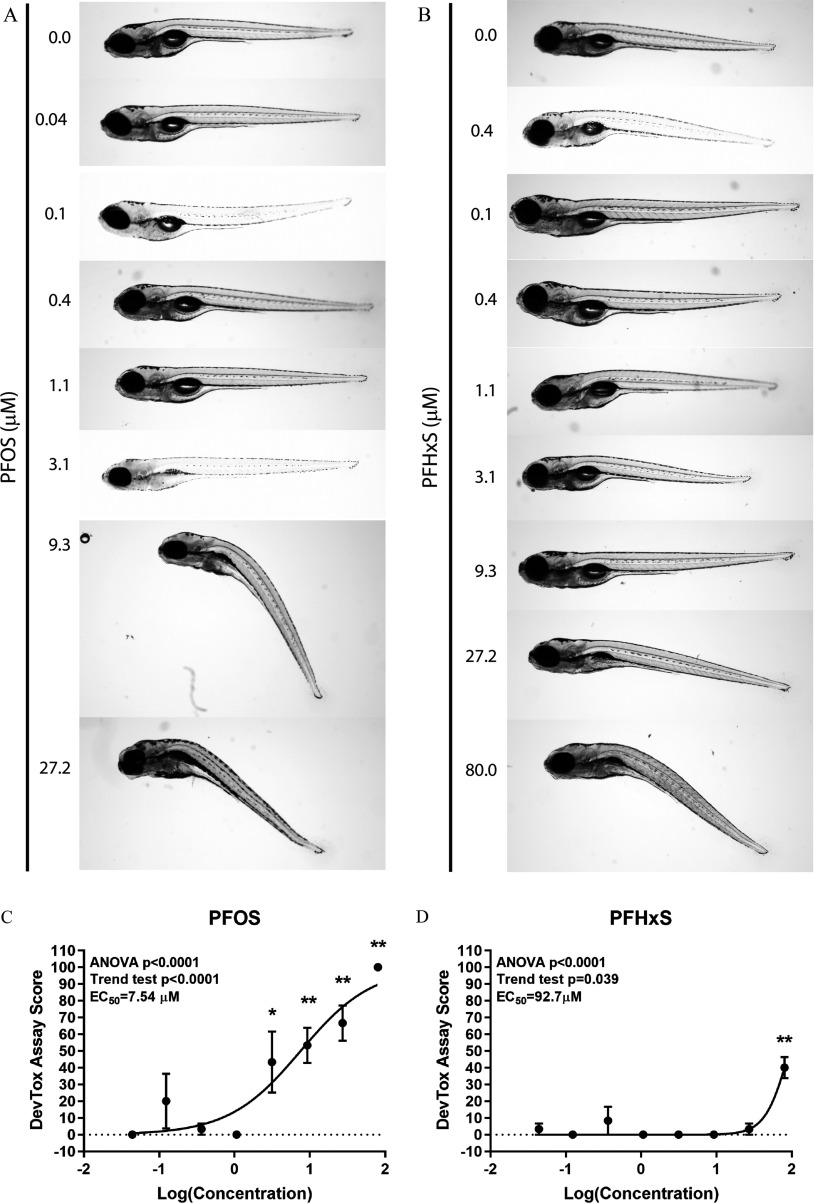
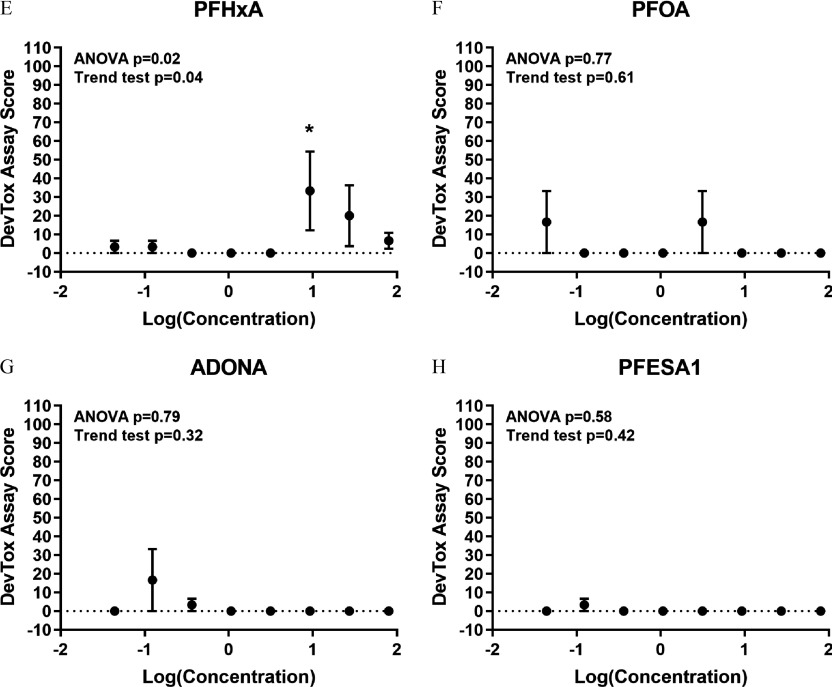
To determine whether exposure to PFAS caused developmental toxicity in larval zebrafish, embryos were exposed to PFOS, PFHxS, PFHxA, PFOA, ADONA, or PFESA1 or 0.4% DMSO daily from dpf (Study 1) (Figures 1 and 2). At 6 dpf, morphological assessments revealed developmental exposure to PFOS caused failed swim bladder inflation and ventroflexion of the tail, relative to DMSO control larvae (Figure 2A) and the value for developmental toxicity was calculated to be (Figure 2C). Exposure to PFHxS resulted in the same morphological phenotypes as PFOS (Figure 2B,D) but was less potent (), although this value was derived from a concentration–response curve with just a single positive concentration and, therefore, may not be entirely reliable. In comparison, exposure to PFHxA, PFOA, ADONA, or PFESA1 did not cause concentration-dependent effects on survival or development (Figure 2E–H).
Figure 2.
Measures of developmental toxicity in zebrafish exposed to PFAS. Zebrafish were semi-statically exposed to ADONA, GenX Free Acid, PFESA1, PFHxA, PFHxS, PFOA, or PFOS daily, from dpf. At 6 dpf, larvae were assessed for developmental toxicity. Representative images for (A) PFOS and (B) PFHxS are shown. DevTox assay scores for (C) PFOS, (D) PFHxS, (E) PFHxA, (F) PFOA, (G) ADONA, or (H) PFESA1 are shown. Significance relative to the 0.4% DMSO control was determined by a Kruskal-Wallis ANOVA with a Dunn’s multiple comparison test (*, **). If a test for linear trend was significant (), with developmental toxicity observed at the highest concentration tested, nonlinear regression was performed with Hill slope curve fitting for half-maximal value determinations. larvae per concentration per chemical tested. Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; DevTox, developmental toxicity; DMSO, dimethyl sulfoxide; dpf, days post fertilization; , half maximal effective concentration; GenX Free Acid, perfluoro-2-propoxypropanoic acid; PFAS, per- and polyfluoroalkyl substances; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid.
Developmental Neurotoxicity Phenotypes in Larval Zebrafish Exposed to PFAS
To determine whether exposure to PFAS affect neurobehavioral development, zebrafish were exposed to PFHxS, PFHxA, PFOA, ADONA, or PFESA1, PFOS or 0.4% DMSO daily, from dpf and locomotor activity was assessed at 6 dpf. Relative to DMSO, exposure to PFOS caused hyperactivity in the L1 period and exposure to PFOS caused hyperactivity in the L2 and D1 periods (Figure 3A,B). Like PFOS, developmental exposure to PFHxS caused hyperactivity in the L1 (), L2 (), and D1 (4.4, ) periods (Figure 3C,D). Finally, exposure to PFHxA resulted in hyperactivity relative to control in the L2 () and D1 () periods and, uniquely, in the D2 period () (Figure 3E,F). Zebrafish developmentally exposed to PFOA (Figure 3G,H), ADONA (Figure 3I,J), or PFESA1 (Figure 3K,L) did not exhibit differences in locomotor activity at 6 dpf. The effect of PFAS exposures on the slope of the response to light or dark stimuli was also determined (see Figures S3 and S4). Significant differences in estimated movement over the testing period were detected for all test chemicals (see Figure S3). However, the difference in estimated movement during each period, used to gauge the magnitude of concentration-related effects on movement across all study chemicals, only revealed qualitatively pronounced changes in larvae exposed to PFHxS or PFOS (see Figure S4).
Bioaccumulation of PFAS in Larval Zebrafish
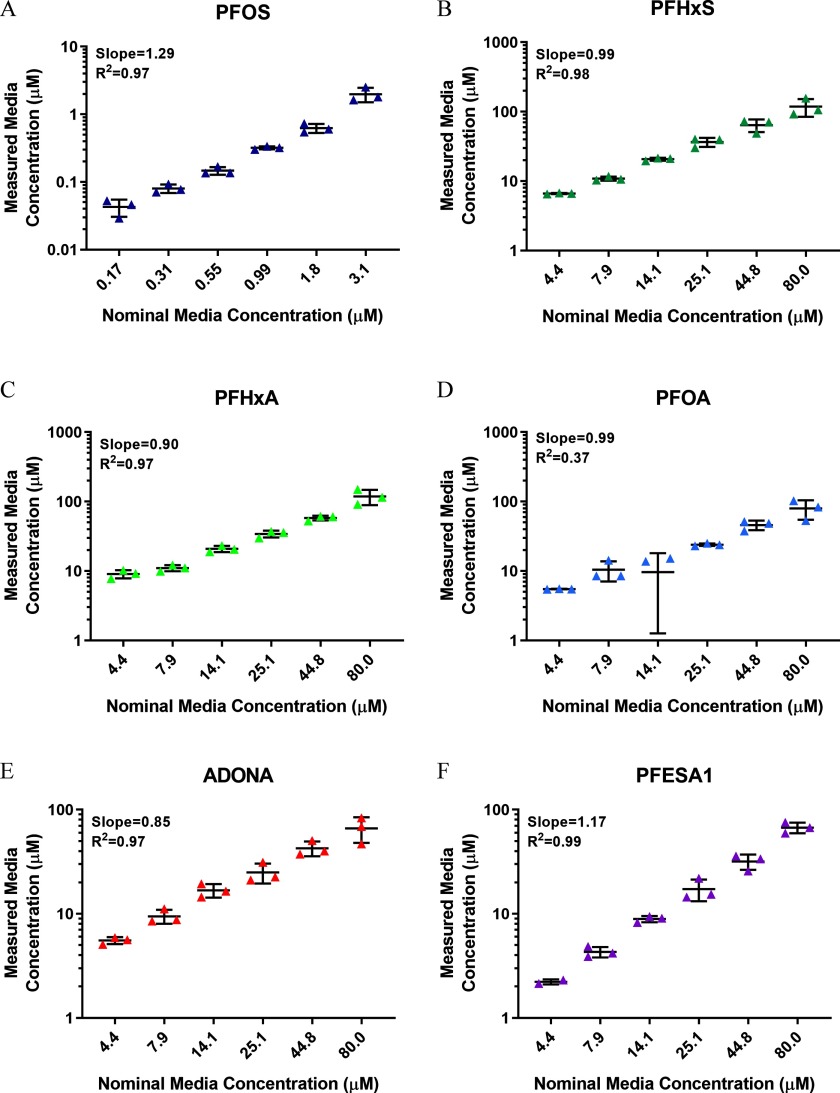
To measure media concentrations of PFAS, zebrafish were exposed to PFHxS, PFHxA, PFOA, ADONA, or PFESA1 or PFOS or DMSO daily, from dpf and at 6 dpf, exposure media was collected from wells. For all test chemicals, a linear increase in measured media concentration was observed (Figure 4). To quantitate tissue concentrations of test PFAS, zebrafish were exposed to PFHxA, PFOA, ADONA, or PFESA1, PFHxS, or PFOS or DMSO daily, from dpf and parent PFAS were measured in pools of 10 larvae () (Figure 5; see also Excel Table S9). PFOS was the most bioaccumulative compound with calculated bioconcentration factor (BCF) values ranging from 684 to 1,375, depending on test concentration (Table 4). Fluoroether PFAS (i.e., ADONA and PFESA1) and PFHxA were the least bioaccumulative chemicals assessed (Table 4).
Figure 4.
Media concentrations of test PFAS at 6 dpf. Zebrafish were semi-statically exposed to PFHxS, PFHxA, PFOA, ADONA, or PFESA1 or PFOS daily from dpf. At 6 dpf, media was collected for targeted analytical chemistry (). Measured media concentrations for (A) PFOS, (B) PFHxS, (C) PFHxA, (D) PFOA, (E) ADONA, and (F) PFESA1 are shown. One observation for PFOA nominal media concentration was and therefore not shown on the plot. However, it was included in the regression analysis using the value MDL/sqrt(2). Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; dpf, days post fertilization; MDL, method detection limit; PFAS, per- and polyfluoroalkyl substances; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid; sqrt, square-root.
Figure 5.
Internal tissue doses of test PFAS at 6 dpf. Zebrafish were semi-statically exposed to ADONA, PFOA, PFESA1, or PFHxA, or PFHxS, or PFOS. At 6 dpf, larvae were pooled and flash frozen ( biological replicates with 10 pooled larvae per replicate) for targeted analytical chemistry. Measured internal tissue doses for (A) PFOS, (B) PFHxS, (C) PFHxA, (D) PFOA, (E) ADONA, and (F) PFESA1 are shown. Significance was determined by a Welch’s ANOVA followed by a Dunnett T3 test (). Additional one-sample Student’s t-tests were performed for PFHxA, PFHxS, PFOA, and PFOS (). Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; ANOVA, analysis of variance; Dil, dilution; dpf, days post fertilization; PFAS, per- and polyfluoroalkyl substances; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid.
Table 4.
Calculated bioconcentration factors (BCFs).
| Compound name | Nominal concentration tested () | Measured tissue dose () | Measured media dose () | () |
|---|---|---|---|---|
| ADONA | 25.1 | 0.95 | ||
| 44.8 | 0.94 | |||
| 80.0 | 0.56 | |||
| GenX Free Acid | 25.1 | 0.45 | ||
| 44.8 | 0.29 | |||
| 80.0 | 0.49 | |||
| PFESA1 | 25.1 | 3.09 | ||
| 44.8 | 2.62 | |||
| 80.0 | 1.44 | |||
| PFHxA | 25.1 | 0.46 | ||
| 44.8 | 0.18 | |||
| 80.0 | 0.24 | |||
| PFOA | 25.1 | 11.68 | ||
| 44.8 | 6.52 | |||
| 80.0 | 5.17 | |||
| PFHxS | 14.0 | 11.82 | ||
| 25.1 | 8.01 | |||
| 44.8 | 9.30 | |||
| PFOS | 1.0 | 1,374.89 | ||
| 1.8 | 1,348.46 | |||
| 3.1 | 684.03 |
Note: BCFs for ADONA, GenX Free Acid, PFESA1, PFHxA, PFHxS, PFOA, and PFOS in a 6-d zebrafish toxicity assay based on measured media and tissue concentrations reported in Excel Tables S8 and S9. BCF tissue doses (mg/kg) were calculated using a dry weight for 6 dpf larvae of (Massei et al. 2015). ADONA, 4,8-dioxa-3H-perfluorononanoate; dpf, days post fertilization; GenX Free Acid, perfluoro-2-propoxypropanoic acid; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid.
Developmental Toxicity and Developmental Neurotoxicity Results in Larval Zebrafish Exposed to GenX Free Acid
Because GenX Free Acid was undetectable in media and zebrafish tissue at 6 dpf (data not shown) and, therefore, unstable in DMSO (see Figure S5), we retested the compound using DI water as a diluent. Daily exposure ( dpf) to GenX Free Acid was negative in the DevTox (Figure 6A) and DNT (Figure 6B,C; see also Figures S6 and S7) assays, relative to the DI water control. GenX Free Acid diluted in DI water resulted in detectable levels of the parent compound in media (Figure 6D; see also Excel Table S8) and larval zebrafish tissue (Figure 6E; see also Excel Table S9). Similar to other fluoroether PFAS assessed in the current study (i.e., ADONA and PFESA1) (Table 3), GenX Free Acid exposure yielded extremely low BCF values ranging from 0.29 to 0.49, depending on test concentration (Table 4).
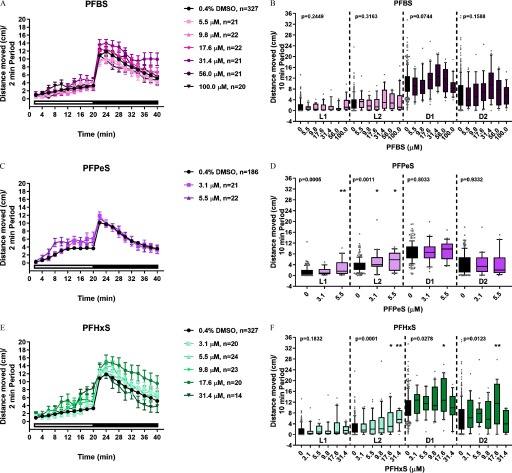
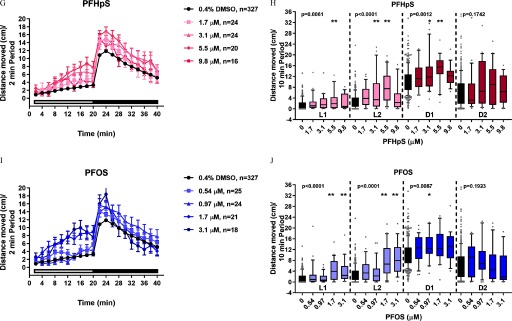
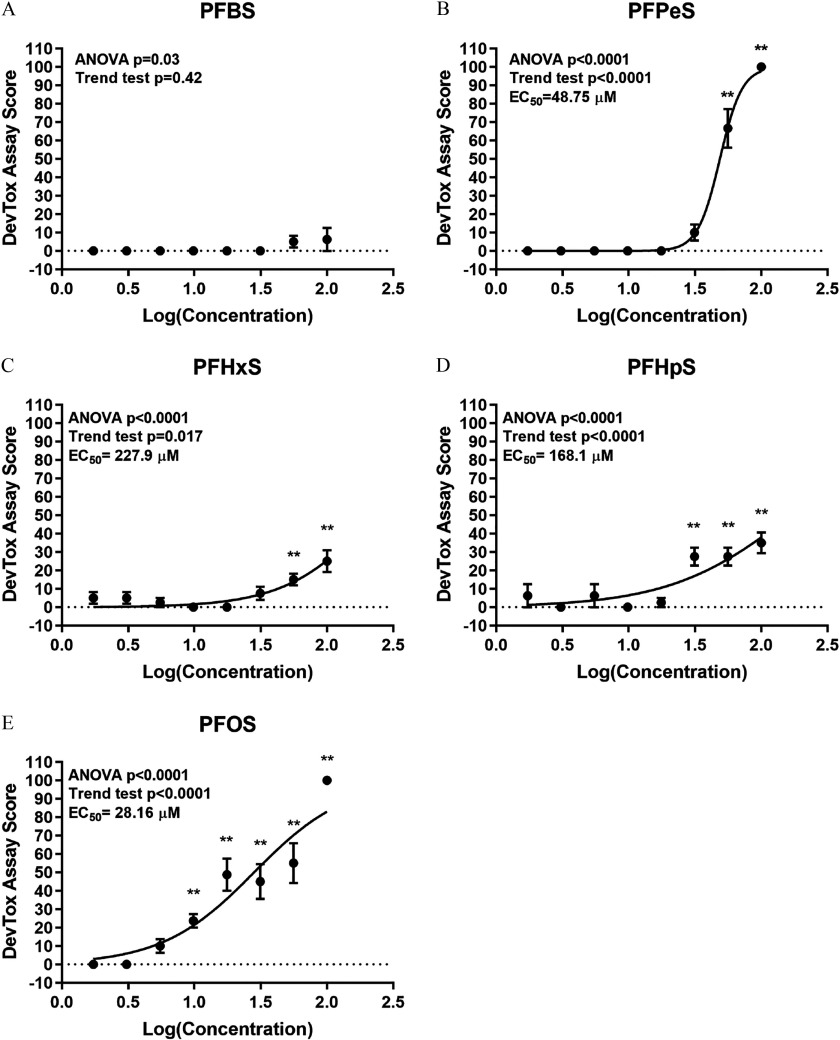
Developmental Toxicity and Developmental Neurotoxicity Phenotypes in Larval Zebrafish Exposed to Alkyl Sulfonic Acid PFAS
Because exposure to structurally similar aliphatic sulfonic acid PFAS PFOS (8-carbon) or PFHxS (6-carbon) resulted in consistent morphological (Figure 2A–D) and behavioral (Figure 3A–D) phenotypes relative to the DMSO control, we hypothesized that sulfonic acid PFAS with perfluorinated alkyl chains would elicit the same toxicity outcomes. To test this hypothesis, zebrafish were exposed to PFBS (4-carbon), PFPeS (5-carbon), PFHxS (6-carbon), PFHpS (7-carbon), or PFOS (8-carbon) (Figure 1) daily, from dpf and assessed for developmental toxicity at 6 dpf. PFBS was negative for developmental toxicity (Figure 7A). All other sulfonic acid PFAS resulted in significant developmental toxicity characterized by failed swim bladder inflation and ventroflexion of the tail (Figure 7B–E). Interestingly, PFPeS was quite potent for developmental toxicity with a calculated value of (Figure 7B). The same five alkyl sulfonic acid PFAS shown in Figure 7 were also tested in the DNT assay. Exposure to PFBS did not result in significant locomotor effects (Figure 8A,B; see also Figures S8 and S9), whereas relative to DMSO alone, exposure to PFPeS resulted in hyperactivity in the L1 () and L2 () periods but had no effect in the dark periods (Figure 8C,D; see also Figures S8 and S9). In the case of PFHxS (Figure 8E,F; see also Figures S8 and S9), and like the results from Study 1 (Figure 3C,D), exposure caused hyperactivity relative to the DMSO control. However, the observed pattern of hyperactivity was modestly different, with hyperactivity detected in the L2 (), D1 (), or D2 () periods (Figure 8E,F). Directly replicating the hyperactivity pattern observed in Study 1 (Figure 3A,B), developmental exposure to PFOS caused hyperactivity in the L1 (), L2 (), and D1 () periods but no effect on the D2 period (Figure 8I,J; see also Figures S8 and S9). Similarly, exposure to PFHpS also triggered L1 (), L2 (), and D1 () hyperactivity but no effect on the D2 period (Figure 8G,H; see also Figures S8 and S9).
Figure 7.
Measures of developmental toxicity in zebrafish exposed to alkyl sulfonic acid PFAS. Zebrafish were semi-statically exposed to PFBS, PFPeS, PFHxS, PFHpS, or PFOS or 0.4% DMSO daily from dpf. At 6 dpf, larvae were assessed for developmental toxicity. DevTox assay scores for (A) PFBS, (B) PFPeS, (C) PFHxS, (D) PFHpS, or (E) PFOS are shown. Significance relative to the 0.4% DMSO control was determined by a Kruskal-Wallis ANOVA with a Dunn’s multiple comparison test (**). If a test for linear trend was significant (), with developmental toxicity observed at the highest concentration tested, nonlinear regression was performed with Hill slope curve fitting for half-maximal value determinations. larvae per concentration per chemical tested. Note: ANOVA, analysis of variance; DevTox, developmental toxicity; DMSO, dimethyl sulfoxide; dpf, days post fertilization; , half maximal effective concentration; PFAS, per- and polyfluoroalkyl substance; PFBS, perfluorobutanesulfonic acid; PFHpS, perfluoroheptanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentanesulfonic acid.
Comparison of Toxicity Phenotypes in Zebrafish Developmentally Exposed to PFAS
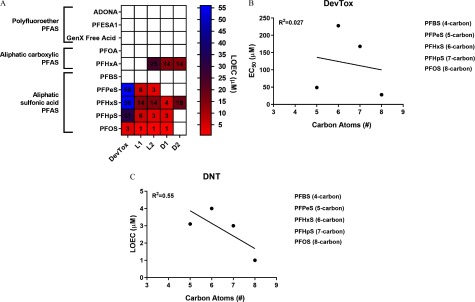
Collective analysis of developmental toxicity and developmental neurotoxicity data sets revealed a shared toxicity phenotype for sulfonic acid PFAS that contain five or more fluorinated carbons (e.g., PFPeS, PFHxS, PFHpS, and PFOS) that was generally characterized by abnormal ventroflexion of the tail and failed swim bladder inflation and, at nonteratogenic concentrations, hyperactivity in the L1, L2, and D1 periods (Figure 9A). Because PFPeS was more potent for developmental toxicity (; ), relative to PFHxS (; Study 2 ) and PFHpS (; ), we did not identify a linear relationship between sulfonic acid carbon chain length and values for developmental toxicity () (Figure 9B). In the DNT assay however, sulfonic acid carbon chain length was correlated with Study 2 LOEC values for hyperactivity () (Figure 9C). These data also show that PFHxA has a unique toxicity phenotype consisting of hyperactivity in the L2, D1, and D2 periods with no observed developmental toxicity identified at the highest concentration tested (Figure 9A). Last, exposure to fluoroether PFAS (i.e., ADONA, GenX Free Acid, or PFESA1) failed to provoke developmental toxicity or developmental neurotoxicity in zebrafish.
Figure 9.
Identification of shared phenotypes between structurally similar PFAS. (A) Heatmap depicting LOEC values for the DevTox assay and significant hyperactivity in the L1, L2, D1, and/or D2 periods of the DNT assay (Studies 1, 2, and 3). If chemicals were replicated in Study 1 and Study 2, the lowest observed LOEC value was used. Linear regression of (B) Study 3 DevTox assay or (C) Study 3 DNT assay LOEC values for aliphatic sulfonic acid PFAS. Note: ADONA, 4,8-dioxa-3H-perfluorononanoate; D, dark period; DevTox, developmental toxicity; DNT, developmental neurotoxicity; , half maximal effective concentration; GenX Free Acid, perfluoro-2-propoxypropanoic acid; L, light period; LOEC, lowest observed effect concentration; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHpS, perfluoroheptanesulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentanesulfonic acid.
Discussion
PFAS are a class of ubiquitous environmental contaminants. There is insufficient toxicity data for the majority of PFAS used in industry and consumer products (OECD 2018). The initial goal of this study was to evaluate the developmental toxicity and developmental neurotoxicity of seven PFAS, including compounds that have been phased out of use but are still widely detected in human serum (i.e., PFOA, PFOS, PFHxS, and PFHxA). In addition, the study included several emerging fluoroether compounds (e.g., ADONA) for which there are limited developmental toxicity data (Gordon 2011; Rushing et al. 2017). Last, we tested PFESA1, a by-product associated with the synthesis of polymer products.
One major finding of this work is that exposure to several PFAS resulted in developmental neurotoxicity characterized by hyperactivity. Epidemiological studies report both positive (Ghassabian et al. 2018; Hoffman et al. 2010; Hoyer et al. 2015; Rappazzo et al. 2017) and negative (Lyall et al. 2018; Rappazzo et al. 2017; Stein and Savitz 2011; Stein et al. 2013) associations between PFAS exposures and neurodevelopmental outcomes. Similarly, exposure to PFAS has been reported to cause behavioral toxicity in some (Goulding et al. 2017; Johansson et al. 2008; Long et al. 2013; Sato et al. 2009; Wang et al. 2015), but not all (Butenhoff et al. 2009), animal studies. In those studies where a positive relationship was revealed, affected behavioral end points included hyperactivity (Goulding et al. 2017; Johansson et al. 2009), reduced habituation (Johansson et al. 2008), impairments in spatial learning and memory resulting from adult (Long et al. 2013) and prenatal exposures (Wang et al. 2015), and tonic convulsions in response to an ultrasonic stimulus (Sato et al. 2009). Molecular results obtained in animal studies suggest that PFAS exposures may disrupt dopaminergic and/or calcium signaling pathways during neurogenesis (Hallgren and Viberg 2016; Johansson et al. 2009; Lee and Viberg 2013; Liu et al. 2010a, 2010b; Zeng et al. 2011). Overall, because of contradictory evidence in human epidemiological and animal behavior studies, it remains unclear whether PFAS exposure is associated with adverse neurophysiological effects. To gain insight into this critical question, concentration-dependent automated behavioral data are needed to evaluate a variety of related and dissimilar PFAS structures. The zebrafish model represents an excellent alternative experimental system that can be used to address this growing research need because multiple chemicals can be evaluated in parallel using automated behavioral tests coupled with a powerful concentration–response design.
Legacy PFAS such as PFOA and PFOS have been extensively evaluated in vitro and in animal and epidemiological studies. However, PFAS are by no means a monolithic class of chemicals. They can be per- or polyfluorinated, straight or branched chained, and contain alkyl chains of varying lengths. PFAS may also contain ether linkages and either sulfonic acid or carboxylic acid R-group moieties. In the United States, there are 602 PFAS in active commercial use (U.S. EPA 2019) and the OECD has identified 4,730 PFAS structures included in various publicly accessible databases (OECD 2018), most of which lack adequate toxicity data. The Zürich Statement on PFAS lays out a strategy for tackling the huge gap in our understanding of PFAS toxicity (Ritscher et al. 2018). Given the large number of PFAS in commerce and rather than cataloging the effects of individual chemicals, the statement calls for action on grouping PFAS (Ritscher et al. 2018). One obvious way to achieve this is to group chemicals by their toxicological activities and, perhaps in doing so, identify structural features that provoke the same toxicity phenotypes in vivo. In the current study, we identified three groups of PFAS toxicity outcomes in zebrafish. Aliphatic sulfonic acid PFAS with greater than four fluorinated carbons resulted in similar morphological and behavioral phenotypes, characterized by failed swim bladder inflation, abnormal ventroflexion of the tail, and, at nonteratogenic concentrations, hyperactivity in the L1, L2, and D1 periods. The second phenotype was unique to PFHxA, an aliphatic carboxylic acid PFAS. Exposure to PFHxA was negative for developmental toxicity but caused pronounced hyperactivity in the L2, D1, and D2 periods. The third group of chemicals consisted of three fluoroether PFAS (i.e., GenX Free Acid, ADONA, and PFESA1), all of which were negative in both toxicity assays.
In addition to grouping PFAS based on attributes such as structure or biological activity, the Zürich statement also recommends amassing data on the toxicokinetics and toxicodynamics of PFAS exposures, particularly those for which little toxicity data exist, with the goal of identifying safer PFAS that can be prioritized for commercial use (Ritscher et al. 2018). Interestingly, we observed a trend of reduced BCFs with increasing nominal concentrations of several PFAS (i.e., ADONA, PFESA1, PFOA, and PFOS). These data replicate a recent study conducted in larval zebrafish that showed an inverse relationship between BCF values and increasing concentrations of perfluorobutanoic acid (PFBA), PFHxS, PFOA, and PFOS, which was suggested to result from the saturation of substrate binding sites (Vogs et al. 2019). Targeted analytical chemistry also revealed detectable levels of ADONA, PFESA1, and PFOA in fish tissue at 6 dpf, indicating that these chemicals are negative for developmental toxicity and developmental neurotoxicity in zebrafish (up to ).
To our knowledge, this is the first published report showing that GenX Free Acid, a branched fluoroether PFAS with a carboxylic acid group directly adjacent to an ether linkage, is not stable in DMSO. This is significant because DMSO is a commonly used solvent for zebrafish and high-throughput in vitro and biochemical toxicity screening studies. Assessment of GenX Free Acid diluted in DI water showed that, although this compound was detectable in zebrafish tissue at the end of the 6-d study period, it was negative for developmental toxicity and developmental neurotoxicity. Collectively, these results suggest that, at least for the types and concentrations tested in the current study, larger fluoroether compounds (i.e., ADONA, GenX Free Acid, and PFESA1) were nontoxic in zebrafish. More work should be performed to explore whether this finding can be extended to other large fluoroether replacement PFAS (Wang et al. 2013).
Compared with previously reported morphological and behavioral effects following exposure to PFBS, PFHxS, PFOS, or PFHxA (summarized in Table 5) (Hagenaars et al. 2011; Huang et al. 2010; Jantzen et al. 2016; Khezri et al. 2017; Padilla et al. 2012; Spulber et al. 2014; Truong et al. 2014; Ulhaq et al. 2013a, 2013b), this study expanded our understanding of aliphatic PFAS toxicity to include data on PFPeS and PFHpS for the first time and reported novel results for PFHxA and PFHxS. Although not systematically designed to test a specific PFAS R-group (i.e., sulfonic or carboxylic acids), Ulhaq et al. (2013a) tested 4-, 8-, 9-, and 10-carbon carboxylic acid aliphatic PFAS and 4- and 8-carbon sulfonic acid aliphatic PFAS in a zebrafish developmental toxicity assay and proposed the idea that carbon chain length may be a determinant of PFAS toxicity in zebrafish. The work presented here systematically tested the effects of aliphatic sulfonic acid PFAS with 4, 5, 6, 7, or 8 fluorinated carbons. Because of the 5-carbon compound PFPeS, carbon chain length was not correlated with malformations in the DevTox assay (; PFBS was negative; ). PFPeS was nearly as potent as PFOS, the most potent chemical evaluated in our study. In comparison, in the DNT assay, increasing carbon chain length was associated with increasing potency for hyperactivity (; PFBS was negative; ). Collectively, these data raise two important points. First, sulfonic acid aliphatic PFAS can be grouped based on their ability to cause the same morphological and behavioral toxicity phenotypes in zebrafish (i.e., failed swim bladder inflation, abnormal ventroflexion of the tail, and, at nonteratogenic concentrations, hyperactivity). Second, although carbon chain length generally increases PFAS potency, this dogma cannot be universally applied to all structurally similar PFAS, as exceptions to the rule exist (i.e., PFPeS).
Table 5.
Summary of key zebrafish toxicity data.
| Compound | Class | DevTox assay phenotype | Ref | DNT assay phenotype | Ref |
|---|---|---|---|---|---|
| ADONA | Polyfluoroether | Negativea | This study | Negativea | This study |
| GenX Free Acid | Branched polyfluoroether | Negativea | This study | Negativea | This study |
| PFESA1 | Branched polyfluoroether | Negativea | This study | Negativea | This study |
| PFHxA | Aliphatic carboxylic acid | Negative | This study; Truong et al. 2014 | Hyperactivitya | This study |
| PFOA | Aliphatic carboxylic acid | Negative | This study; Padilla et al. 2012; Truong et al. 2014 | Negative | This study; Khezri et al. 2017 |
| Positive | Hagenaars et al. 2011; Jantzen et al. 2016; Ulhaq et al. 2013a | Hyperactivity | Ulhaq et al. 2013b | ||
| PFBS | Aliphatic sulfonic acid | Negative | This study; Truong et al. 2014 | Negativea | This study |
| Positive | Hagenaars et al. 2011; Ulhaq et al. 2013a | Hyperactivity | Ulhaq et al. 2013b | ||
| PFPeS | Aliphatic sulfonic acid | Positivea | This study | Hyperactivitya | This study |
| PFHxS | Aliphatic sulfonic acid | Positive | This study; Truong et al. 2014 | Hyperactivitya | This study |
| Negative | Khezri et al. 2017 | ||||
| PFHpS | Aliphatic sulfonic acid | Positivea | This study | Hyperactivitya | This study |
| PFOS | Aliphatic sulfonic acid | Positive | This study; Hagenaars et al. 2011; Huang et al. 2010; Jantzen et al. 2016; Padilla et al. 2012; Truong et al. 2014; Ulhaq et al. 2013a, 2013b | Hyperactivity | This study; Huang et al. 2010; Khezri et al. 2017; Spulber et al. 2014 |
| Hypoactivity | Ulhaq et al. 2013b |
Note: Data on PFAS evaluated in the present study and previous work. ADONA, 4,8-dioxa-3H-perfluorononanoate; DevTox, developmental toxicity; DNT, developmental neurotoxicity; GenX Free Acid, perfluoro-2-propoxypropanoic acid; PFBS, perfluorobutanesulfonic acid; PFESA1, perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid; PFHpS, perfluoroheptanesulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluoro-n-octanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeS, perfluoropentaslufonic acid; Ref, reference.
Indicates previously unreported findings.
Here, we obtained DevTox and DNT Assay data for PFOS and PFHxS in two separate studies. This provided a unique opportunity to evaluate the consistency of observed toxicity effects across independent experiments. In the case of PFOS, values for developmental toxicity were similar, but not identical, with identified values of in Study 1 and in Study 3. Variability in calculated values was also observed in the PFHxS data set ( in Study 1; in Study 3). These discrepancies could reflect both inherent assay variability and the different concentrations ranges tested in Study 1 () relative to Study 3 (). In addition, the value calculated from Study 1 was based on a single positive concentration and, therefore, may not be as reliable as the value determined in Study 3. Last, the OECD Fish Embryo Acute Toxicity Test (No. 236) indicates that 20 animals should be tested across five concentrations of test chemicals to evaluate developmental toxicity (OECD 2013). Although this study assessed developmental toxicity across a six-point concentration–response curve with a minimum of 44 replicates for the 0.4% DMSO control group, only 6–10 biological replicates per exposure group were used. Collectively, these design choices may have contributed to differences in detected values described above. However, these data are in line with previously published DevTox assay data with reported PFOS values of (Padilla et al. 2012) and (Truong et al. 2014) and PFHxS values of (Padilla et al. 2012) and (Truong et al. 2014). Given that the strain, rearing temperature, chemical source, exposure regimen (i.e., static vs. semi-static), and end point evaluation protocol varied across studies, these data are generally consistent and show that exposure to PFOS or PFHxS causes developmental toxicity in zebrafish. In the DNT assay, highly consistent LOECs were observed for PFOS ( in Study 1; in Study 3) and PFHxS ( in Study 1; in Study 3). However, although the pattern of the hyperactivity was identical across studies for PFOS with observed hyperactivity in the L1, L2, and D1 periods, it varied following exposure to PFHxS, where elevated locomotor activity was observed in the L1, L2, and D1 periods in Study 1 and the L2, D1, and D2 periods in Study 3. Regardless, in line with previously published work (Huang et al. 2010; Khezri et al. 2017; Spulber et al. 2014), exposure to nonteratogenic concentrations of PFOS or PFHxS consistently triggered behavioral hyperactivity. Although more work is needed to understand the biological relevance of disparate xenobiotic-induced locomotor activity phenotypes, this represents a powerful approach for grouping chemicals based on shared toxicity phenotypes.
From a developmental toxicity perspective, we and others have showed that exposure to sulfonic acid alkyl PFAS with at least five carbon atoms can cause developmental lethality and elicit conserved malformations at nonteratogenic concentrations consisting of swim bladder inflation failure and dorsoflexion of the tail (Hagenaars et al. 2011; Huang et al. 2010; Jantzen et al. 2016; Padilla et al. 2012; Truong et al. 2014; Ulhaq et al. 2013a). Similar to zebrafish, neonatal rodents exposed to PFOS die in the postnatal period (Conley et al. 2019; Grasty et al. 2005). Although humans do not have a swim bladder, the organ shares functional, structural, ontological, and transcriptional similarities with the human lung (Winata et al. 2009). In zebrafish, swim bladder inflation is used for buoyancy and functions as a site for gas interchange at the air–mucus interface, with surfactant composition similar to the lung (Agier et al. 2019; Lapennas and Schmidt-Nielsen 1977; Robertson et al. 2007; Sullivan et al. 1998; Zeng et al. 2011). Toxicologically, neonatal rodents that died following prenatal exposure to PFOS presented with noted changes in lung histology (Grasty et al. 2005). In humans, epidemiologic evidence has shown that exposure to PFAS during pregnancy can result in decreased lung function in children (Agier et al. 2019). Although these similarities are intriguing, more work is needed to determine the relevance of zebrafish swim bladder defects for human health risk assessment.
Overall, this study rapidly assessed the developmental toxicity and developmental neurotoxicity of 10 PFAS, including some compounds that have never been previously tested in animal studies. However, limitations of the study warrant comment. In human NHANES data, mean PFAS levels are often higher in males relative to females (Calafat et al. 2007a, 2007b). We did not capture sex-specific outcomes in early life stage zebrafish. In addition, here we used locomotor activity in a light/dark behavior test as a functional readout of neurodevelopment. This is just one behavioral end point among many. Future studies should examine the effect of PFAS on other zebrafish behavior end points such as habituation to further support the developmental neurotoxicity effects reported here. Here, 0.4% DMSO was used for all exposure groups, except GenX Free Acid which was diluted in DI water. This concentration of DMSO is commonly used in zebrafish chemical screens for developmental toxicity (Padilla et al. 2012) and does not affect the larval zebrafish photomotor response (Kokel and Peterson 2011) or light/dark swimming response (Teixidó et al. 2019). However, it should be noted that effects on the transcriptome (Turner et al. 2012) and metabolome (Akhtar et al. 2016) have been reported in zebrafish exposed to 0.1% DMSO. There is also evidence that elevated DMSO concentrations can facilitate increased uptake of chemicals into the perivitaline space (Kais et al. 2013). Therefore, although all chemical exposure data were statistically compared with a DMSO control, it is possible that the concentration of solvent used in the current study affected chemical uptake and the assessed end points.
These data raise some interesting questions for future research. Namely, do behavioral hyperactivity effects persist in older animals? One intriguing study observed hyperactivity in 14 dpf zebrafish developmentally exposed to PFOA or PFOS from dpf (Jantzen et al. 2016), suggesting that early life perturbation of neuronal circuitry controlling the light/dark behavioral apparatus may persist at later life stages. Another key area that needs to be explored is the mechanism(s) by which PFAS cause locomotor hyperactivity in zebrafish. Last, humans and wildlife are exposed to a complex mixture of PFAS (Boiteux et al. 2016; Gebbink et al. 2017; Strynar et al. 2015), many of which may cause additive or synergistic disruption of neurodevelopmental signaling pathways (Khezri et al. 2017). Future research should consider testing groups of related PFAS in environmentally relevant mixtures. From a dosimetry perspective, we observed tissue doses of PFAS that have been measured in, for example, the Danish National Birth Cohort (Fei et al. 2007). To truly understand the relevance of zebrafish toxicity data for human risk assessment, there is an urgent need for physiologically based pharmacokinetic models that compare PFAS doses measured in whole-body zebrafish homogenates to maternal serum levels. Finally, analysis of larval behavior data is not standardized. Here, we opted to use a repeated measures ANOVA and parametric mixed model analysis to account for the complex data structure that contains multiple, repeated locomotor activity values for each animal over the 40-min testing period (Catron et al. 2019a; Irons et al. 2013; Phelps et al. 2017; Stevens et al. 2018). However, zebrafish behavior data are not normally distributed and may often be below the LOD. We therefore also analyzed the behavior data using a square root transformation and multiple value imputation strategy (Pleil 2016a, 2016b). Based on the LOD, a large percentage of individual values were imputed. Although the order of the raw data was preserved in the final corrected distributions, the large percentage of imputed values present in the light period data should be noted. However, the combined use of square root transformation and multiple value imputation allowed key regression assumptions (i.e., homoskedasticity and normality of residuals) to be met and the subsequent use of mixed-effects models further allowed the appropriate examination of repeated measures for individual zebrafish. Overall, the imputation-based analysis strategy was used to buttress findings generated from untransformed zebrafish behavior data while appropriately accounting for nonnormal distributions. Although both approaches revealed PFAS-dependent hyperactivity, standardized methods for the analysis of fish behavior data that account for repeat measurements and nonnormal data distributions are needed.
Taken together, and in keeping with recommendations by the Zürich Statement (Ritscher et al. 2018), we used zebrafish toxicity data to group PFAS based on their ability to cause developmental toxicity and/or developmental neurotoxicity. We specifically identified aliphatic sulfonic acid PFAS as a particularly bioactive class of PFAS, thereby identifying relationships between chemical structures and in vivo phenotypes that may arise from putative shared mechanisms of PFAS toxicity. We also used analytical chemistry to reveal that GenX Free Acid is unstable in DMSO, a solvent widely used for zebrafish and in vitro screening studies. These data show that this emerging PFAS, in addition to other branched and/or fluoroether PFAS examined here, is negative for developmental toxicity and developmental neurotoxicity in zebrafish, possibly identifying a less bioactive group of PFAS (at least in the context of fish toxicology). Finally, this study supports the use of in vivo developmental neurotoxicity testing when evaluating this class of widely occurring environmental contaminants.
Supplementary Material
Acknowledgments
S.G. participated in study design, performed zebrafish experiments, analyzed data, prepared figures, and assisted with manuscript preparation. A.S. performed targeted analytical chemistry experiments. J.R.S. participated in study design, data interpretation, and analyzed behavior and analytical chemistry data. X.M.H. performed zebrafish experiments. J.S. performed behavior statistical analyses. T.C., J.M., E.H., and M.S. participated in study design and data interpretation. T.T. designed the study, assisted with zebrafish experiments, analyzed and interpreted data, and wrote and rebutted the manuscript. All authors reviewed the manuscript.
We thank J. Hedge and the U.S. Environmental Protection Agency (EPA) zebrafish facility staff for fish husbandry and L. Adams for performing a data quality review. We are grateful to C. Lau, D. Villeneuve, and S. Hunter for their review of the manuscript. This manuscript has been reviewed by the U.S. EPA and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This work was supported by the U.S. EPA Office of Research and Development.
References
- Agier L, Basagaña X, Maitre L, Granum B, Bird PK, Casas M, et al. 2019. Early-life exposome and lung function in children in Europe: an analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet Health 3(2):e81–e92, PMID: 30737192, 10.1016/S2542-5196(19)30010-5. [DOI] [PubMed] [Google Scholar]
- Akhtar MT, Mushtaq MY, Verpoorte R, Richardson MK, Choi YH. 2016. Zebrafish as a model for systems medicine R&D: rethinking the metabolic effects of carrier solvents and culture buffers determined by 1H NMR metabolomics. OMICS 20(1):42–52, PMID: 26669610, 10.1089/omi.2015.0119. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. 2007. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115(11):1670–1676, PMID: 18008002, 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RE, Smart BE, Tatlow JC. 1994. Organofluorine Chemistry: Principles and Commercial Applications. 1st ed New York, NY: Springer US. [Google Scholar]
- Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, et al. 2015. The Madrid statement on poly- and perfluoroalkyl substances (PFAS). Environ Health Perspect 123(5):A107–A111, PMID: 25932614, 10.1289/ehp.1509934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux V, Bach C, Sagres V, Hemard J, Colin A, Rosin C, et al. 2016. Analysis of 29 per- and polyfluorinated compounds in water, sediment, soil and sludge by liquid chromatography–tandem mass spectrometry. Int J Environ Anal Chem 96(8):705–728, 10.1080/03067319.2016.1196683. [DOI] [Google Scholar]
- Borg D, Ivarsson J, Andersson A, Moore G. 2017. Nordic workshop on PFAS: outcomes. Publication No. 2017:913 Copenhagen, Denmark: Nordic Council of Ministers; https://www.norden.org/en/publication/nordic-workshop-pfass [accessed 3 March 2020]. [Google Scholar]
- Brox S, Ritter AP, Kuster E, Reemtsma T. 2014. A quantitative HPLC-MS/MS method for studying internal concentrations and toxicokinetics of 34 polar analytes in zebrafish (Danio rerio) embryos. Anal Bioanal Chem 406(20):4831–4840, PMID: 24948091, 10.1007/s00216-014-7929-y. [DOI] [PubMed] [Google Scholar]
- Brox S, Seiwert B, Küster E, Reemtsma T. 2016. Toxicokinetics of polar chemicals in zebrafish embryo (Danio rerio): influence of physicochemical properties and of biological processes. Environ Sci Technol 50(18):10264–10272, PMID: 27571242, 10.1021/acs.est.6b04325. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Ehresman DJ, Chang SC, Parker GA, Stump DG. 2009. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: developmental neurotoxicity. Reprod Toxicol 27(3–4):319–330, PMID: 19162172, 10.1016/j.reprotox.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. 2007a. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES). Environ Sci Technol 41(7):2237–2242, PMID: 17438769, 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007b. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: 18007991, 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron TR, Keely SP, Brinkman NE, Zurlinden TJ, Wood CE, Wright JR, et al. 2019a. Host developmental toxicity of BPA and BPA alternatives is inversely related to microbiota disruption in zebrafish. Toxicol Sci 167(2):468–483, PMID: 30321396, 10.1093/toxsci/kfy261. [DOI] [PubMed] [Google Scholar]
- Catron TR, Swank A, Wehmas LC, Phelps D, Keely SP, Brinkman NE, et al. 2019b. Microbiota alter metabolism and mediate neurodevelopmental toxicity of 17β-estradiol. Sci Rep 9(1):7064, PMID: 31068624, 10.1038/s41598-019-43346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2019. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. https://www.cdc.gov/exposurereport/ [accessed 3 March 2020].
- Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, et al. 2019. Adverse maternal, fetal, and postnatal effects of hexafluoropropylene oxide dimer acid (GenX) from oral gestational exposure in Sprague-Dawley rats. Environ Health Perspect 127(3):37008, PMID: 30920876, 10.1289/EHP4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Pan Y, Zhang H, Sheng N, Wang J, Guo Y, et al. 2018. Occurrence and tissue distribution of novel perfluoroether carboxylic and sulfonic acids and legacy per/polyfluoroalkyl substances in black-spotted frog (Pelophylax nigromaculatus). Environ Sci Technol 52(3):982–990, PMID: 29310433, 10.1021/acs.est.7b03662. [DOI] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, et al. 2018. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg Environ Health 221(3):569–577, PMID: 29514764, 10.1016/j.ijheh.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Dauchy X, Boiteux V, Colin A, Hémard J, Bach C, Rosin C, et al. 2019. Deep seepage of per- and polyfluoroalkyl substances through the soil of a firefighter training site and subsequent groundwater contamination. Chemosphere 214:729–737, PMID: 30293026, 10.1016/j.chemosphere.2018.10.003. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Spencer C, Scott BF, Backus S, Muir DC. 2011. Detection of a cyclic perfluorinated acid, perfluoroethylcyclohexane sulfonate, in the Great Lakes of North America. Environ Sci Technol 45(19):8060–8066, PMID: 21528907, 10.1021/es200135c. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, et al. 2009. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol 39(1):76–94, PMID: 18802816, 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- Dunnet D. 1980. Pairwise multiple comparisons in the unequal variance case. J Am Stat Assoc 75(372):796–800, 10.1080/01621459.1980.10477552. [DOI] [Google Scholar]
- Escoruela J, Garreta E, Ramos R, González-Solis J, Lacorte S. 2018. Occurrence of per- and polyfluoroalkyl substances in Calonectris shearwaters breeding along the Mediterranean and Atlantic colonies. Mar Pollut Bull 131(Pt A):335–340, PMID: 29886955, 10.1016/j.marpolbul.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682, PMID: 18008003, 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Wöckner M, Roscher E, Völkel W. 2017. ADONA and perfluoroalkylated substances in plasma samples of German blood donors living in South Germany. Int J Hyg Environ Health 220(2 Pt B):455–460, PMID: 28073630, 10.1016/j.ijheh.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, van Asseldonk L, van Leeuwen S. 2017. Presence of emerging per- and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in the Netherlands. Environ Sci Technol 51(19):11057–11065, PMID: 28853567, 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Bell EM, Ma WL, Sundaram R, Kannan K, Buck Louis GM, et al. 2018. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut 243(Pt B):1629–1636, PMID: 30296759, 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SC. 2011. Toxicological evaluation of ammonium 4,8-dioxa-3H-perfluorononanoate, a new emulsifier to replace ammonium perfluorooctanoate in fluoropolymer manufacturing. Regul Toxicol Pharmacol 59(1):64–80, PMID: 20875479, 10.1016/j.yrtph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Goulding DR, White SS, McBride SJ, Fenton SE, Harry GJ. 2017. Gestational exposure to perfluorooctanoic acid (PFOA): alterations in motor related behaviors. Neurotoxicology 58:110–119, PMID: 27888120, 10.1016/j.neuro.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasty RC, Bjork JA, Wallace KB, Wolf DC, Lau CS, Rogers JM. 2005. Effects of prenatal perfluorooctane sulfonate (PFOS) exposure on lung maturation in the perinatal rat. Birth Defects Res B Dev Reprod Toxicol 74(5):405–416, PMID: 16249997, 10.1002/bdrb.20059. [DOI] [PubMed] [Google Scholar]
- Guelfo JL, Adamson DT. 2018. Evaluation of a national data set for insights into sources, composition, and concentrations of per- and polyfluoroalkyl substances (PFASs) in U.S. drinking water. Environ Pollut 236:505–513, PMID: 29427949, 10.1016/j.envpol.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo JL, Marlow T, Klein DM, Savitz DA, Frickel S, Crimi M, et al. 2018. Evaluation and management strategies for per- and polyfluoroalkyl substances (PFASs) in drinking water aquifers: perspectives from impacted U.S. Northeast communities. Environ Health Perspect 126(6):065001, PMID: 29916808, 10.1289/EHP2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DG. 2008. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ Sci Technol 42(15):5807–5813, PMID: 18754513, 10.1021/es8005173. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Vergauwen L, De Coen W, Knapen D. 2011. Structure–activity relationship assessment of four perfluorinated chemicals using a prolonged zebrafish early life stage test. Chemosphere 82(5):764–772, PMID: 21111445, 10.1016/j.chemosphere.2010.10.076. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Viberg H. 2016. Postnatal exposure to PFOS, but not PBDE 99, disturb dopaminergic gene transcription in the mouse CNS. Environ Toxicol Pharmacol 41:121–126, PMID: 26686188, 10.1016/j.etap.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Hamm JT, Ceger P, Allen D, Stout M, Maull EA, Baker G, et al. 2019. Characterizing sources of variability in zebrafish embryo screening protocols. ALTEX 36(1):103–120, PMID: 30415271, 10.14573/altex.1804162s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. 2010. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect 118(12):1762–1767, PMID: 20551004, 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496(7446):498–503, PMID: 23594743, 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, et al. 2015. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years–a prospective study. Environ Health 14:2, PMID: 25567242, 10.1186/1476-069X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, et al. 2010. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat Toxicol 98(2):139–147, PMID: 20171748, 10.1016/j.aquatox.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L, et al. 2018. Time trends in per- and polyfluoroalkyl substances (PFASs) in California women: declining serum levels, 2011–2015. Environ Sci Technol 52(1):277–287, PMID: 29198103, 10.1021/acs.est.7b04650. [DOI] [PubMed] [Google Scholar]
- Irons TD, Kelly PE, Hunter DL, MacPhail RC, Padilla S. 2013. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol Biochem Behav 103(4):792–813, PMID: 23274813, 10.1016/j.pbb.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB. 2018. Time trends over 2003–2014 in the concentrations of selected perfluoroalkyl substances among US adults aged ≥20 years: interpretational issues. Sci Total Environ 645:946–957, PMID: 30248883, 10.1016/j.scitotenv.2018.07.198. [DOI] [PubMed] [Google Scholar]
- Jantzen CE, Annunziato KA, Bugel SM, Cooper KR. 2016. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat Toxicol 175:160–170, PMID: 27058923, 10.1016/j.aquatox.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen CE, Toor F, Annunziato KA, Cooper KR. 2017. Effects of chronic perfluorooctanoic acid (PFOA) at low concentration on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reprod Toxicol 69:34–42, PMID: 28143724, 10.1016/j.reprotox.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. 2015. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol 52(Pt B):194–209, PMID: 26348672, 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lust RM, Strynar MJ, Dagnino S, DeWitt JC. 2012. Perflurooctanoic acid induces developmental cardiotoxicity in chicken embryos and hatchlings. Toxicology 293(1–3):97–106, PMID: 22273728, 10.1016/j.tox.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Johansson N, Eriksson P, Viberg H. 2009. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci 108(2):412–418, PMID: 19211617, 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. 2008. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 29(1):160–169, PMID: 18063051, 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Kaboré HA, Vo Duy S, Munoz G, Méité L, Desrosiers M, Liu J, et al. 2018. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci Total Environ 616–617:1089–1100, PMID: 29100694, 10.1016/j.scitotenv.2017.10.210. [DOI] [PubMed] [Google Scholar]
- Kais B, Schneider KE, Keiter S, Henn K, Ackermann C, Braunbeck T. 2013. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET). Aquat Toxicol 140–141:229–238, PMID: 23831690, 10.1016/j.aquatox.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Khezri A, Fraser TW, Nourizadeh-Lillabadi R, Kamstra JH, Berg V, Zimmer KE, et al. 2017. A mixture of persistent organic pollutants and perfluorooctanesulfonic acid induces similar behavioural responses, but different gene expression profiles in zebrafish larvae. Int J Mol Sci 18(2):E291, PMID: 28146072, 10.3390/ijms18020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310, PMID: 8589427, 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kirla KT, Groh KJ, Steuer AE, Poetzsch M, Banote RK, Stadnicka-Michalak J, et al. 2016. From the cover: zebrafish larvae are insensitive to stimulation by cocaine: importance of exposure route and toxicokinetics. Toxicol Sci 154(1):183–193, PMID: 27521082, 10.1093/toxsci/kfw156. [DOI] [PubMed] [Google Scholar]
- Kissa E. 2001. Fluorinated Surfactants and Repellents. 2nd ed New York, NY: CRC Press. [Google Scholar]
- Kokel D, Peterson RT. 2011. Using the zebrafish photomotor response for psychotropic drug screening. Methods Cell Biol 105:517–524, PMID: 21951545, 10.1016/B978-0-12-381320-6.00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen J, Winkens K, Airaksinen R, Berger U, Vestergren R, Cousins IT, et al. 2018. Longitudinal trends of per- and polyfluoroalkyl substances in children’s serum. Environ Int 121(Pt 1):591–599, PMID: 30308470, 10.1016/j.envint.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Lapennas GN, Schmidt-Nielsen K. 1977. Swimbladder permeability to oxygen. J Exp Biol 67:175–196. https://jeb.biologists.org/content/jexbio/67/1/175.full.pdf [accessed 3 March 2020]. [Google Scholar]
- Lee I, Viberg H. 2013. A single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology 37:190–196, PMID: 23701969, 10.1016/j.neuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu W, Jin Y, Yu W, Liu L, Yu H. 2010a. Effects of subchronic perfluorooctane sulfonate exposure of rats on calcium-dependent signaling molecules in the brain tissue. Arch Toxicol 84(6):471–479, PMID: 20127074, 10.1007/s00204-010-0517-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu W, Jin Y, Yu W, Wang F, Liu L. 2010b. Effect of gestational and lactational exposure to perfluorooctanesulfonate on calcium-dependent signaling molecules gene expression in rats’ hippocampus. Arch Toxicol 84(1):71–79, PMID: 19756518, 10.1007/s00204-009-0467-2. [DOI] [PubMed] [Google Scholar]
- Long Y, Wang Y, Ji G, Yan L, Hu F, Gu A. 2013. Neurotoxicity of perfluorooctane sulfonate to hippocampal cells in adult mice. PLoS One 8(1):e54176, PMID: 23382877, 10.1371/journal.pone.0054176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, et al. 2018. Prenatal maternal serum concentrations of per- and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environ Health Perspect 126(1):017001, PMID: 29298162, 10.1289/EHP1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, et al. 2017. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int 108:278–284, PMID: 28917208, 10.1016/j.envint.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Massei R, Vogs C, Renner P, Altenburger R, Scholz S. 2015. Differential sensitivity in embryonic stages of the zebrafish (Danio rerio): the role of toxicokinetics for stage-specific susceptibility for azinphos-methyl lethal effects. Aquat Toxicol 166:36–41, PMID: 26210375, 10.1016/j.aquatox.2015.06.011. [DOI] [PubMed] [Google Scholar]
- McCord J, Newton S, Strynar M. 2018. Validation of quantitative measurements and semi-quantitative estimates of emerging perfluoroethercarboxylic acids (PFECAs) and hexfluoroprolyene oxide acids (HFPOAs). J Chromatogr A 1551:52–58, PMID: 29628221, 10.1016/j.chroma.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co-operation and Development). 2013. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals. https://www.oecd-ilibrary.org/docserver/9789264203709-en.pdf?expires=1583243117&id=id&accname=guest&checksum=F428A53FD38C4415FA1B9290D6677170 [accessed 3 March 2020].
- OECD. 2018. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs). Series on Risk Management No. 39. ENV/JM/MONO(2018)7. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en [accessed 3 March 2020].
- Padilla S, Corum D, Padnos B, Hunter DL, Beam A, Houck KA, et al. 2012. Zebrafish developmental screening of the ToxCast™ Phase I chemical library. Reprod Toxicol 33(2):174–187, PMID: 22182468, 10.1016/j.reprotox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Sun Y, et al. 2018. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ Sci Technol 52(14):7621–7629, PMID: 29749740, 10.1021/acs.est.8b00829. [DOI] [PubMed] [Google Scholar]
- Phelps D, Brinkman NE, Keely SP, Anneken EM, Catron TR, Betancourt D, et al. 2017. Microbial colonization is required for normal neurobehavioral development in zebrafish. Sci Rep 7(1):11244, PMID: 28894128, 10.1038/s41598-017-10517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD. 2016a. Imputing defensible values for left-censored ‘below level of quantitation’ (LoQ) biomarker measurements. J Breath Res 10(4):045001, PMID: 27753432, 10.1088/1752-7155/10/4/045001. [DOI] [PubMed] [Google Scholar]
- Pleil JD. 2016b. QQ-plots for assessing distributions of biomarker measurements and generating defensible summary statistics. J Breath Res 10(3):035001, PMID: 27491525, 10.1088/1752-7155/10/3/035001. [DOI] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E, Hines EP. 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health 14(7):E691, PMID: 28654008, 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritscher A, Wang Z, Scheringer M, Boucher JM, Ahrens L, Berger U, et al. 2018. Zürich statement on future actions on per- and polyfluoroalkyl substances (PFASs). Environ Health Perspect 126(8):84502, PMID: 30235423, 10.1289/EHP4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GN, McGee CAS, Dumbarton TC, Croll RP, Smith FM. 2007. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. J Morphol 268(11):967–985, PMID: 17702001, 10.1002/jmor.10558. [DOI] [PubMed] [Google Scholar]
- Route WT, Russell RE, Lindstrom AB, Strynar MJ, Key RL. 2014. Spatial and temporal patterns in concentrations of perfluorinated compounds in bald eagle nestlings in the upper Midwestern United States. Environ Sci Technol 48(12):6653–6660, PMID: 24898913, 10.1021/es501055d. [DOI] [PubMed] [Google Scholar]
- Rushing BR, Hu Q, Franklin JN, McMahen R, Dagnino S, Higgins CP, et al. 2017. Evaluation of the immunomodulatory effects of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in C57BL/6 mice. Toxicol Sci 156(1):179–189, PMID: 28115649, 10.1093/toxsci/kfw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato I, Kawamoto K, Nishikawa Y, Tsuda S, Yoshida M, Yaegashi K, et al. 2009. Neurotoxicity of perfluorooctane sulfonate (PFOS) in rats and mice after single oral exposure. J Toxicol Sci 34(5):569–574, PMID: 19797866, 10.2131/jts.34.569. [DOI] [PubMed] [Google Scholar]
- Scheringer M, Trier X, Cousins IT, de Voogt P, Fletcher T, Wang Z, et al. 2014. Helsingør statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 114:337–339, PMID: 24938172, 10.1016/j.chemosphere.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Barofsky DF, Field JA. 2003. Fluorinated alkyl surfactants. Environ Eng Sci 20(5):487–501, 10.1089/109287503768335959. [DOI] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. 2018. Prenatal exposure to perfluoroalkyl substances: infant birth weight and early life growth. Environ Epidemiol 2(2):e010, PMID: 30272047, 10.1097/EE9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souder JP, Gorelick DA. 2017. Quantification of estradiol uptake in zebrafish embryos and larvae. Toxicol Sci 158(2):465–474, PMID: 28535311, 10.1093/toxsci/kfx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber S, Kilian P, Wan Ibrahim WN, Onishchenko N, Ulhaq M, Norrgren L, et al. 2014. PFOS induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. PLoS One 9(4):e94227, PMID: 24740186, 10.1371/journal.pone.0094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA. 2011. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ Health Perspect 119(10):1466–1471, PMID: 21665566, 10.1289/ehp.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. 2013. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology 24(4):590–599, PMID: 23680941, 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Padilla S, DeMarini DM, Hunter DL, Martin WK, Thompson LC, et al. 2018. Zebrafish locomotor responses reveal irritant effects of fine particulate matter extracts and a role for TRPA1. Toxicol Sci 161(2):290–299, PMID: 29048608, 10.1093/toxsci/kfx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ Sci Technol 49(19):11622–11630, PMID: 26392038, 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Daniels CB, Phillips ID, Orgeig S, Whitsett JA. 1998. Conservation of surfactant protein A: evidence for a single origin for vertebrate pulmonary surfactant. J Mol Evol 46(2):131–138, PMID: 9452514, 10.1007/pl00006287. [DOI] [PubMed] [Google Scholar]
- Tal T, Kilty C, Smith A, LaLone C, Kennedy B, Tennant A, et al. 2017. Screening for angiogenic inhibitors in zebrafish to evaluate a predictive model for developmental vascular toxicity. Reprod Toxicol 70:70–81, PMID: 28007540, 10.1016/j.reprotox.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó E, Kießling TR, Krupp E, Quevedo C, Muriana A, Scholz S. 2019. Automated morphological feature assessment for zebrafish embryo developmental toxicity screens. Toxicol Sci 167(2):438–449, PMID: 30295906, 10.1093/toxsci/kfy250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. 2014. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci 137(1):212–233, PMID: 24136191, 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE. 2015. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol 54:26–36, PMID: 25499722, 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C, Sawle A, Fenske M, Cossins A. 2012. Implications of the solvent vehicles dimethylformamide and dimethylsulfoxide for establishing transcriptomic endpoints in the zebrafish embryo toxicity test. Environ Toxicol Chem 31(3):593–604, PMID: 22169935, 10.1002/etc.1718. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2019. EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan. EPA 823R18004. https://www.epa.gov/sites/production/files/2019-02/documents/pfas_action_plan_021319_508compliant_1.pdf [accessed 3 March 2020].
- Ulhaq M, Carlsson G, Örn S, Norrgren L. 2013a. Comparison of developmental toxicity of seven perfluoroalkyl acids to zebrafish embryos. Environ Toxicol Pharmacol 36(2):423–426, PMID: 23770452, 10.1016/j.etap.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Ulhaq M, Örn S, Carlsson G, Morrison DA, Norrgren L. 2013b. Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquat Toxicol 144–145:332–340, PMID: 24215719, 10.1016/j.aquatox.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Vogs C, Johanson G, Näslund M, Wulff S, Sjödin M, Hellstrandh M, et al. 2019. Toxicokinetics of perfluorinated alkyl acids influences their toxic potency in the zebrafish embryo (Danio rerio). Environ Sci Technol 53(7):3898–3907, PMID: 30844262, 10.1021/acs.est.8b07188. [DOI] [PubMed] [Google Scholar]
- Wang T, Vestergren R, Herzke D, Yu J, Cousins IT. 2016. Levels, isomer profiles, and estimated riverine mass discharges of perfluoroalkyl acids and fluorinated alternatives at the mouths of Chinese rivers. Environ Sci Technol 50(21):11584–11592, PMID: 27689437, 10.1021/acs.est.6b03752. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu W, Zhang Q, Zhao H, Quan X. 2015. Effects of developmental perfluorooctane sulfonate exposure on spatial learning and memory ability of rats and mechanism associated with synaptic plasticity. Food Chem Toxicol 76:70–76, PMID: 25524167, 10.1016/j.fct.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60:242–248, PMID: 24660230, 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Westerfield M. 2007. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). 5th ed Eugene, OR: University of Oregon Press. [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. 2012. Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol 175(12):1209–1216, PMID: 22517810, 10.1093/aje/kwr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Zheng W, Korzh V, Gong Z. 2009. Development of zebrafish swimbladder: the requirement of Hedgehog signaling in specification and organization of the three tissue layers. Dev Biol 331(2):222–236, PMID: 19422819, 10.1016/j.ydbio.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong LY, Jia T, Kalathil A, Latremouille J, et al. 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health 221(1):9–16, PMID: 28993126, 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HC, Li YY, Zhang L, Wang YJ, Chen J, Xia W, et al. 2011. Prenatal exposure to perfluorooctanesulfonate in rat resulted in long-lasting changes of expression of synapsins and synaptophysin. Synapse 65(3):225–233, PMID: 20687110, 10.1002/syn.20840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during the current study are available in Science Hub by searching for the manuscript title at https://sciencehub.epa.gov/sciencehub/.