Abstract
Human toxocariasis has been identified as an under-diagnosed parasitic zoonosis and health disparity of significant public health importance in the United States due to its high seropositivity among socioeconomically disadvantaged groups, and possible links to cognitive and developmental delays. Through microscopy and quantitative PCR, we detected that Toxocara eggs are widespread in New York City public spaces, with evidence of significant levels of contamination in all five boroughs. The Bronx had the highest contamination rate (66.7%), while Manhattan had the lowest contamination rate (29.6%). Moreover, infective eggs were only found in the Bronx playgrounds, with over 70% of eggs recovered in embryonic form and the highest egg burden (p = 0.0365). All other boroughs had eggs in the pre-infectious, unembronyated form. Toxocara cati, the cat roundworm, was the predominant species. These results suggest that feral or untreated cats in New York City represent a significant source of environmental contamination. These findings indicate that human toxocariasis has emerged as an important health disparity in New York City, with ongoing risk of acquiring Toxocara infection in public spaces, especially in poorer neighborhoods. There is a need for reducing environmental Toxocara contamination. Additional rigorous public health interventions should explore further approaches to interrupt transmission to humans.
Author summary
Toxocara canis and Toxocara cati are helminth worms that infect dogs and cats, respectively. Infected dogs and cats will defecate thousands of Toxocara eggs into the environment. Humans are incidental hosts and are exposed when consuming contaminated soils via the fecal-oral route. After leaving the gastrointestinal tract, the Toxocara larvae will enter the vasculature and can migrate to any major organ system, including lungs, ocular, and central nervous system. Symptoms can range from mild muscle aches to severe asthma, blindness, and encephalitis. Humans are not definitive hosts of the parasite and cannot transmit Toxocara eggs to the environment or other humans. There is a need for research on the sanitary impact of Toxocara for both humans and animals, especially in large urban cities such as New York City. Poverty is also associated with higher rates of toxocariasis, with more contamination in poorer neighborhoods where animal control, deworming of pets, and less sanitary conditions exist. This study aims to understand further the disparity of lower socioeconomic areas having higher rates of contaminated parks and playgrounds, comparing the five boroughs of New York City.
Introduction
Toxocara canis and Toxocara cati are ascarid nematodes that ubiquitously infect dogs and cats and can result in environmental contamination if the feces of infected animals contaminate community spaces. Eggs deposited in the soil can exhibit cryptobiosis when environmental conditions are not ideal and may survive for many years. [1] Human ingestion of embryonated eggs through contaminated soil, poor hygiene practices, or uncleaned vegetables, can result in paratenic zoonotic toxocariasis. [2–4] As Toxocara eggs develop in the soil, it is possible to detect the developmental progression of the helminth from germinal cells to the presence of viable infective larvae via microscopy. Eggs containing fully developed larvae are infectious to humans, whereas Toxocara at earlier stages of development are pre-infectious and cannot lead to toxocariasis. Following the ingestion of an embryonated egg, the third stage larva enters the bloodstream. It burrows through body tissues, where the worms can accumulate in the eye, brain, liver, or skin, leading to visceral or ocular larva migrans, blindness, subclinical cerebral infection, or covert infection which can diminish neurological cognition or result in developmental delays. [5–9]
Toxocariasis is an underreported and understudied disease in the United States. Both the parasite and human toxocariasis have been described as ‘enigmatic’ due to numerous deficiencies in the understanding of the organism, including the role of cerebral toxocariasis and the possible link to neurocognitive deficits and blindness in children. [2, 5] The US Centers for Disease Control and Prevention (CDC) identifies human toxocariasis as one of five neglected parasitic infections in the U.S., and it is possibly the most common helminthiasis in the United States after pinworm (Enterobius vermicularis). [8, 10] Toxocariasis is a neglected disease of poverty due to its disproportionately high seroprevalence in areas of community poverty, especially among underrepresented minority populations living in poor areas. [10–12]
Among the most enigmatic features of toxocariasis is its covert form, which generally is not associated with visceral larva migrans. Covert toxocariasis is subclinical, with only eosinophilia as a biomarker for suspicion of infection. [4] Covert toxocariasis has been linked to cognitive and developmental delays, lung dysfunction, and asthma. However, research is still in the nascent stages of understanding the full clinical spectrum of illness caused by T. canis and T. cati. [5] Overall, toxocariasis may be severely underdiagnosed due to the covert nature of the illness and gaps in medical knowledge; it is a disease that should not be overlooked as a cause of neurocognitive delay in children. [2, 4, 5, 7, 13, 14] A 1987 study in New York City (NYC) correlated T. canis seroprevalence to neurocognitive deficits in children. [5, 15, 16] Prior research has also shown that simple incubation of a Toxocara embryo allows it to become infective, and after infection, living larvae have been found in the brains of mice. [1] Mice with infected brains have significant impairment, including cellular damage, and may have different areas of brain involvement depending on the infecting Toxocara species. [17, 18] Covert toxocariasis may also represent an important environmental cause of asthma among disadvantaged American children. [19, 20]
Throughout the history of the National Health and Nutrition Examination Survey (NHANES), the prevalence of Toxocara within the United States population has varied, ranging from 5%-14% during analyses from 1988–1994 and 2011–2014. [11, 21] In both studies, Toxocara seropositivity was disproportionately higher in persons from lower socioeconomic status communities, particularly in Hispanic, non-Hispanic black communities, and those with less than a high-school education. [11, 21]
Few studies have been conducted in the United States, and most were published over two decades ago. [22–28] In the few US studies, contamination rates ranged from 0.3%-27.5%, with the contamination rate defined as the number of eggs found per park or space evaluated. [22–28]
Walsh et al. created a predicted probability model to estimate the seroprevalence of toxocariasis in NYC using estimates from the NHANES III and correlated findings with available sociodemographic information, which suggested higher levels of exposure to Toxocara species in low income, minority, and immigrant communities. [29] This study is the first geo-surveillance of Toxocara species in all five boroughs of NYC attempting to address the potential risk of infection within NYC communities by first examining soil from around the city.
Methods
Sample collection
Samples were collected from 3–5 cm below the surface after the top layer of soil was removed. Five samples were collected from each location on the same day, and each sample was from a different geographic area of the sample site, attempts were made to sample the entire site equally. These were combined before laboratory analysis into one larger sample weighing 100-150g. The samples were collected randomly during October 2015-July 2016. A sample size calculation was used to determine the level of precision using a metanalysis (Fakhri et al.) confidence interval of 16 to 27% soil contamination worldwide from 42,797 samples, 79 samples were needed for statistical significance, and a total of 91 samples were collected for this study.[30] A total of 91 sites were surveyed in New York City from October 2015-July 2016, and samples were collected from different sites at different times during this period. Of these, 27 (29.6%) were in Manhattan, 15 (16.5%) were in the Bronx, 13 (14.3) were in Brooklyn, 18 (19.8%) were in Queens, and 18 (19.8%) were in Staten Island. Sample sites were selected at random to maximize coverage of each borough, due to limited access, parts of Brooklyn and Staten Island were not tested.
Microscopic analysis
Samples were analyzed using a modified soil flotation technique derived from previously published floatation methods of helminth egg recovery from the soil and human stool. [31, 32] Sodium nitrate was chosen as the optimal flotation solution due to elevated specific gravity (1.25–1.35), best-published egg recovery results, and low laboratory cost. All specimens were sifted to remove large debris before being washed in 0.1% Tween 20 three times. Samples were subsequently mixed with approximately 10 to 14 ml sodium nitrate, specific gravity 1.30 (Vedco Feca Med, St Joseph, MO) enough to form a convex meniscus at the opening of the tube. With a coverslip in place, samples were centrifuged at 500 g for 5 minutes and allowed to stand for 5 minutes before viewing using a standard light microscope. Eggs, if present, were easily visualized and counted using this technique. Parasites were identified by structural characteristics, and embryonated forms were described if larvae were noted within the egg. Other incidentally discovered parasites were recorded and categorized using a subjective semiquantitative “+” system (Table 1). Coverslips were then washed into 2mL Eppendorf tubes with DNase-free water and saved at -20 degrees Celsius for later real-time PCR testing.
Table 1. Prevalence of Toxocara sp. including the presence of larval forms in specimens collected from sites in each NYC borough.
Larvated, infectious Toxocara sp. were only found in the Bronx, the borough that had the highest prevalence of Toxocara contamination.
| Borough | Positives/Total | % Toxocara positive (95% CI) | Eggs (mean and range) | Larvated forms |
|---|---|---|---|---|
| Bronx | 10/15 | 66.7 (41.7–84.8) | 15.7 (1–102) | +++ |
| Staten Island | 7/18 | 38.9 (20.3–61.4) | 3.4 (1–5) | - |
| Queens | 6/18 | 33.3 (16.3–56.3) | 1.8 (1–2) | - |
| Brooklyn | 4/13 | 30.8 (12.7–57.6) | 2.7 (1–7) | - |
| Manhattan | 8/27 | 29.6 (15.9–48.5) | 6.3 (4–12) | - |
Molecular methods
Parasite DNA was extracted from soil samples with MP FastDNA spin kits for soil (MP Biomedical, Santa Ana, CA) using supernatant from the coverslips from the previous centrifugation step. We processed this supernatant using bead beating for five minutes at 3,000 RPM, followed by a ten-minute heating step at 90°C to promote egg disruption. This same technique was utilized to extract DNA from T. canis and T. cati eggs for the standard controls. [33] The T. canis and T. cati DNA from these samples were quantified using a multi-parallel real-time quantitative polymerase chain reaction (qPCR) protocol. [33] All samples underwent qPCR testing using previously published probes with the following modifications [T. canis (5’-FAM-CCATTACCACACCAGCATAGCTCACCGA-3’-NFQ-MGB) and T. cati (5-FAM-TCTTTCGCAACGTGCATTCGGTGA-3’-NFQ-MGB)] and forward primers [T. canis (5’-GCGCCAATTTATGGAATGTGAT-3’) and T. cati (5’-ACGCGTACGTATGGAATGTGCT-3’)] and shared reverse primer 5’-GAGCAAACGACAGCSATTTCTT-3’) to both Toxocara species. [34] PCR was performed using an ABI ViiA 7 (Applied Biosystems, Foster City, CA) as previously described [33]. A sample was considered positive if there was detectable DNA at or before a cycle threshold of 38. The threshold of positivity was determined by T. canis/cati genomic DNA dilutions for the dynamic range. T. canis and T. cati eggs were isolated and washed with distilled water three times. Eggs were counted using a McMasters microscope slide (Advanced Equine Products, Issaquah, WA), and serial dilutions were used as standards. All samples were performed in duplicate and were repeated for discordant results. A standard curve was constructed using serial dilutions of 10,000 T. cani and T. cati eggs. Standards were used as positive controls with no-template for negative controls. A known concentration of plasmid internal control was added to all samples prior to bead beating to validate successful DNA extraction [35].
Demographic data
Socioeconomic data were obtained from the websites of the US Census and the New York State Department of health. [36, 37]. Zipcodes were recorded from each sampling area, and New York City income data per zip code was obtained from a website of current census bureau income statistics for United States zip codes. [38]
Statistical methods
Statistical analysis was carried out using GraphPad Prism version 7.0d (GraphPad, La Jolla, CA). The number of Toxocara eggs was compared by zip code, and Toxocara eggs per sample site were compared to individual boroughs using the Kruskal-Wallis test. Comparing the prevalence of Toxocara species by zip code or borough, a spearman rank was conducted comparing the prevalence of positive parks per borough and the median income per resident living in those zip codes.
Results
Environmental survey results (Microscopy results)
Of the 27 Manhattan sample sites, Toxocara eggs were found in 29.6% of playgrounds with at least one egg being identified by microscopy. Of the Staten Island sites tested, 38.9% had Toxocara eggs found, of the 13 Brooklyn sites tested 30.8% had eggs found, Queens had 33.3% of sites contaminated, and the Bronx had 66.7% sites contaminated (Table 1). The overall prevalence of 38.5% (35/91) contaminated playgrounds in NYC.
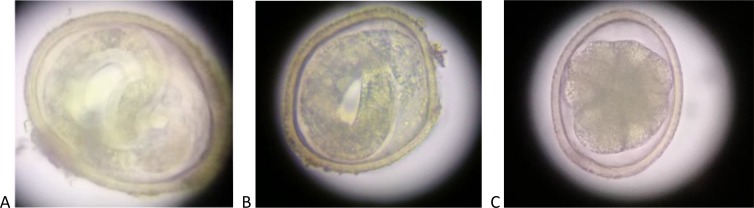
Infective embryonated eggs (Fig 1A and 1B, Fig 2) were only found in the Bronx playgrounds, with over 70% of eggs recovered being in their larvated state. All other boroughs had eggs in the pre-infectious, unembryonated (unlarvated) form (Fig 1C, Fig 2).
Fig 1.
Toxocara forms isolated in NYC specimens: a and b: Larvated ‘infective’ Toxocara eggs at 40x magnification, c: Unlarvated Toxocara egg at 40 x magnification, obtained in NYC study.
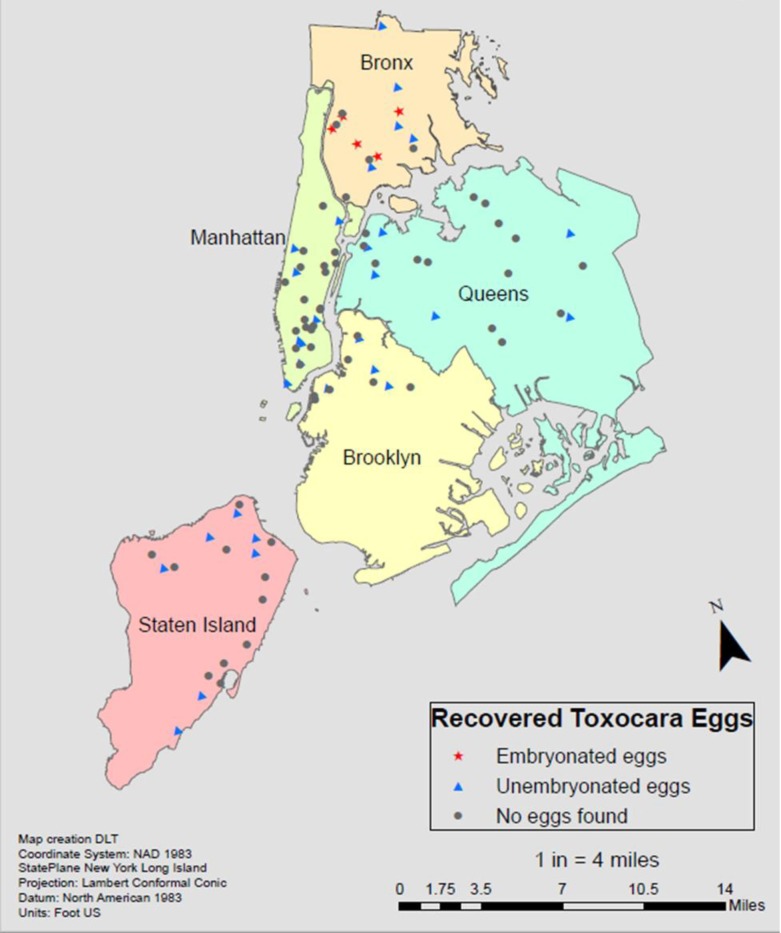
Fig 2. Geospatial results of Toxocara sampling across NYC.
Toxocara was found in those sites represented by either blue triangles (unembryonated egg(s)) red stars (embryonated egg(s)). Toxocara was not found at sites marked by black circles. The highest density of Toxocara geo-contamination was found in the Bronx, which was the only borough where infected larvae were found. Sampling ArcMap of NYC, created using ArcGIS 10.6.1.
Toxocara species results (qPCR results)
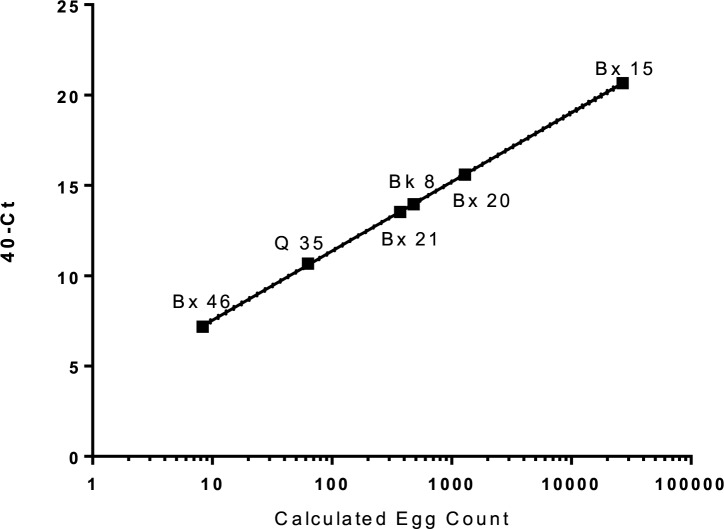
Of all the positive samples, six were found to have T. cati DNA present (Fig 3, Table 1). None of the parks were found to have T. canis DNA present. More than 4 of 6 parks that tested positive were from samples with the highest number of larvated eggs, accounting for the higher copy numbers of genomic DNA present in many of the samples (mean 19.5, 0 to 102 eggs). (Fig 3) The six positive samples had calculated egg counts from the standard dilutions using linear regression that correlated to 40 –Cycle threshold (Ct) (Spearman r = 0.999, p < 0.0001). One sample (Bk8) was negative by microscopy but positive by qPCR (13.958, 40-Ct). One egg of either T. canis or T. cati in 500 μl of water was consistently detected by qPCR with Ct values of 37 to 38.
Fig 3. qPCR results, parks that were qPCR-positive were contaminated with T. cati.
Calculated egg count was derived from dilutions of a known amount of Toxocara cati eggs and correlated to 40 –Ct (Spearman r = 0.999, p < 0.0001).
Association of geo-contamination and socioeconomic data
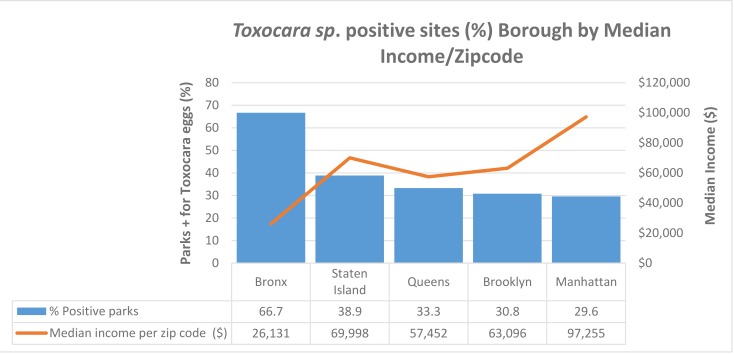
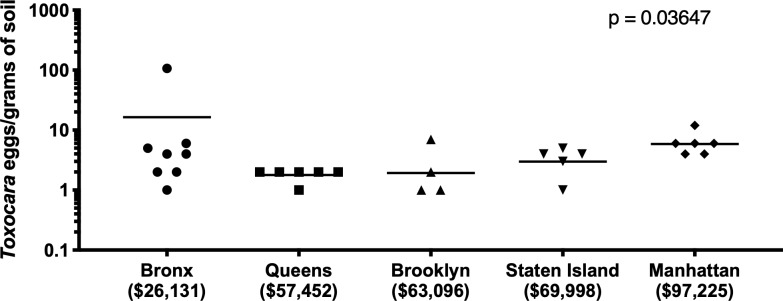
Contamination was more prevalent in lower socioeconomic neighborhoods. Manhattan, the borough with the highest median income ($97,255) of zip codes where sampling was performed, had the lowest rate of contamination (29.6%) compared to a contamination rate of 66.7% in the Bronx, which had the lowest median income ($26,131) (Fig 4). Queens, Brooklyn, and Staten Island had contamination rates from 30.8–38.9% and median incomes between $57,452-$69,998, Spearman r = -0.7, p = 0.233 (Fig 4). The Bronx sample sites had the highest mean egg burden (Bronx: 16.4, Queens: 1.8, Brooklyn: 1.9, Staten Island: 3.0, Manhattan: 5.9, p = 0.0365) and prevalence. (Fig 5) Overall, there was an inverse relationship between median income and the likelihood of Toxocara species contamination (Fig 5).
Fig 4. Association of positive Toxocara results with the median income of residents living in the ZIP codes of sampled sites.
The line represents the median income of families living in the zip code where Toxocara testing was performed. Results aggregated by borough. (Spearman r = -0.7, p = 0.233).
Fig 5. Toxocara eggs/park by borough.
Kruskal-Wallis P = 0.0365. Eggs were not equally distributed in the parks by borough. The Bronx* has the highest egg burden compared to the other boroughs.
Discussion
In this first environmental study of Toxocara prevalence in NYC, approximately one-third of sites sampled throughout the five boroughs contained Toxocara eggs (Table 1). The distribution of contamination was not equal across the city. The Bronx had the highest Toxocara burden and prevalence, compared to the other boroughs (Figs 4 and 5). All boroughs, except the Bronx, had eggs in their unlarvated, pre-infectious form (Fig 1).
From the qPCR data, T. cati was the only species identified. This suggests that cats are the predominant reservoir causing contamination in New York City play areas, although dogs can transiently carry T. cati from eating cat feces but are not part of the parasites’ life cycle. [39] In fact, the finding of cat contamination is not entirely unprecedented, as NHANES data have shown high co-infection rates with toxocariasis and toxoplasmosis nationally, suggesting that cats may represent an important but often underappreciated source of Toxocara eggs. [40] There are several reasons for predominant feline contamination. Park fencing consists of vertical metal bars, which will effectively prevent dog entry into parks (unless pet owners allow access) but are unlikely to restrict stray cat access. Furthermore, people are more likely to remove feces from their dogs than cats. Cats can be infected with T. cati through all stages of life, whereas dogs are most severely infected as puppies. [41] Veterinary data in the New York City area shows that cats test positive for roundworm infections at least four times more frequently than dogs, regardless of the borough. [42] Furthermore, pet cats shed Toxocara more commonly than pet dogs. [43] Although the samples were not optimally processed for qPCR and the lack of T. cani DNA may be attributed to the small sample size.
Similar results have been recently reported in several cities in Europe [44, 45] and have important implications in determining strategies to reduce contamination and risk for human infection. [39] In a recent metanalysis by Fakhri et al., the average soil contamination with Toxocara in the USA was 4 to 23% playgrounds in NYC likely are have concentrations of animal exposure and results in higher egg counts.[30] Several considerations exist regarding why Toxocara qPCR was discordant for samples that were positive or negative by microscopy. One may be due to the way the specimens were saved and prepared for qPCR. The qPCR is not a direct comparison to microscopy as samples were first processed for microscopic analysis, and qPCR was performed on the supernatant obtained from the coverslips of the slides. The specific transfer method after microscopy could account for a loss of eggs. Quantitative PCR was performed on the supernatants removed from the slide subsequent to the microscopy examination and not directly from the soil specimen. Given the low number of eggs (1–2) in many specimens, any loss could result in a negative qPCR. Specimens with embryonated eggs have an abundance of Toxocara DNA, often with very high calculated genomic equivalents, while non-larvated cells have only a single copy of DNA. Thus, PCR may better be able to identify samples with larvated cells present. Sample Bx15 was outside the dynamic range of the standards, and this is likely because several eggs were in the larvated stage and would have many copies of the target sequence, giving an exaggerated calculated egg count (Fig 3). Sample Bk8 was negative by microscopy, but positive by qPCR with significant 40-Ct value (13.958), showing that microscopy is subjective and can miss visualizing eggs. Prior literature studies [46, 47] suggest that these eggs can adhere to plastic and may have been lost during the rinsing of the coverslip and slide when the eggs were transferred into the Eppendorf tube in preparation for freezing. Other limitations are samples were collected in different seasons, with changes in environmental temperatures influencing embryonated eggs. These temperature changes could have influenced the larvated egg results found throughout the boroughs. Geography can also influence the prevalence, burden, and stages of embryonated eggs in the environment. Staten Island and Manhattan are not contiguous with the mainland and can have decreased migrating dogs or cats, therefore impacting the results.
The distribution of Toxocara contaminated parks was not homogenous across the city but was more prevalent in areas of lower socioeconomic status. Of the five boroughs, the Bronx has the lowest median income but the highest level of soil contamination with the highest number of infectious, larvated parasites. Based on embryonated/larvated eggs, the risk of Toxocariasis from soil ingestion was highest in the Bronx. It is unknown whether these areas have more pets or stray cats, but given the association with the lower-income, it is likely that the higher contamination rate and the higher infectious potential may be related to the ability to pay for regular veterinary check-ups and deworming of pets. Animals that frequent contaminated areas are likely to become re-infected even after deworming if neighboring animals have not been dewormed. Brooklyn, Queens, and Staten Island have higher median incomes with 30–38% geo-contamination. Manhattan has the highest median income but the lower percentage of contamination at 29.6%, but still indicating that over one-quarter of Manhattan sites tested have Toxocara present. Because there was variability in the soil weights between playgrounds (100 gm to 150 gm), there is a potential for sampling bias, since increased grams of soil can mean more eggs available to be seen by microscopy or detected by qPCR. This discrepancy may have increased the number of eggs in the Bronx (Fig 5). The overall high level of parasites throughout all boroughs may be a result of the ‘pet boom’; the number of household U.S. cats and dogs has more than doubled in the past four decades, contributing to an increasing problem of stray cats in poorer neighborhoods. [48]
A disparity of higher Toxocara environmental contamination distribution in poorer neighborhoods is present and likely translates into an actual health disparity, but this remains to be evaluated as there are other ways that Toxocara can be transmitted to humans. Recent definitive data on the seroprevalence of Toxocara infection in children and adults living in those areas are lacking. However, results from a 1987 study did find higher seroprevalence in children and adolescents from poorer neighborhoods in NYC. [15] Furthermore, both Toxocara NHANES studies suggest minority individuals and those with lower socioeconomic status are at greater risk of Toxocariasis. [11, 21] Therefore, the prudent approach, given the potentially serious health consequences of Toxocara infection, especially for young children and adolescents, is to assume that transmission to humans will occur and to make every effort to reduce environmental contamination by Toxocara as much as possible and as soon as possible.
Beyond its importance as a human health disparity, Toxocara soil contamination is a One Health issue; it impacts the cleanliness of the environment and may impact the health of domestic pets and wildlife. Infective eggs represent an environmental risk and potential health hazard to children with pica. Other paratenic hosts of this parasite include rodents, birds, or rabbits, which, when infected, continue to contribute to the lifecycle of the parasite. Finally, other larvated parasites were seen by microscopy but unable to be definitively identified in these samples, posing a potential infectious risk to visiting animals or children.
Conclusions
Toxocara species are common pet parasites that are found in the sand and soil of almost one-third of sample sites in NYC, especially in poorer neighborhoods. The predominant species in NYC appears to be T. cati. Preventive measures should be taken. These include improved fencing of play areas to prevent feral cat entry, deworming of domestic pets according to national veterinarian guidelines, better control of stray cats and dogs, picking up feces of pets, avoiding consumption of food that may have become contaminated, restriction of children with pica from play areas, and frequent handwashing after visiting play areas before ingestion of food or snacks.
Acknowledgments
The authors thank all current and past collaborators for their contributions to Toxocara research and acknowledge the inability to site all peer-reviewed publications. DLT is grateful to those who have helped with advice and education in regard to this research, especially Susan Little DVM Ph.D. DACVM, Yonghua Li MD, William Borkowski MD, Mona Rigaud MD, Celia Holland BSc Ph.D., and Gloria Heresi MD. Special thanks to Dwight Bowman Ph.D. for providing Toxocara cani eggs for qPCR validation, and Anne Zajac DVM MS Ph.D. for providing feline samples infected with Toxocara cati eggs for qPCR validation.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Acknowledgemets: This research was supported in part by the Committee of Interns and Residents Patient Care Trust Fund Research Project Grant funded from 2015-2017. Research funding support for RM was provided by the U.S. Department of Health and Human Services, Health Resources and Services Administration for Baylor College of Medicine Center of Excellence in Health Equity, Training and Research (Grant No: D34HP31024). Funding also provided by the Texas Children’s Hospital Center for Vaccine Development, and the National School of Tropical Medicine, Baylor College of Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Borg OA, Woodruff AW. Prevalence of infective ova of Toxocara species in public places. British medical journal. 1973;4(5890):470–2. 10.1136/bmj.4.5890.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland CV. Knowledge gaps in the epidemiology of Toxocara: the enigma remains. Parasitology. 2015:1–14. Epub 2015/12/17. 10.1017/s0031182015001407 . [DOI] [PubMed] [Google Scholar]
- 3.Strube C, Heuer L, Janecek E. Toxocara spp. infections in paratenic hosts. Veterinary parasitology. 2013;193(4):375–89. 10.1016/j.vetpar.2012.12.033 . [DOI] [PubMed] [Google Scholar]
- 4.Ma G, Holland CV, Wang T, Hofmann A, Fan CK, Maizels RM, et al. Human toxocariasis. The Lancet Infectious diseases. 2018;18(1):e14–e24. 10.1016/S1473-3099(17)30331-6 . [DOI] [PubMed] [Google Scholar]
- 5.Walsh MG, Haseeb MA. Reduced cognitive function in children with toxocariasis in a nationally representative sample of the United States. Int J Parasitol. 2012;42(13–14):1159–63. Epub 2012/11/06. 10.1016/j.ijpara.2012.10.002 . [DOI] [PubMed] [Google Scholar]
- 6.Fan CK, Holland CV, Loxton K, Barghouth U. Cerebral Toxocariasis: Silent Progression to Neurodegenerative Disorders? Clinical microbiology reviews. 2015;28(3):663–86. Epub 2015/06/13. 10.1128/CMR.00106-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland CV, Hamilton CM. The significance of cerebral toxocariasis: a model system for exploring the link between brain involvement, behaviour and the immune response. The Journal of experimental biology. 2013;216(Pt 1):78–83. Epub 2012/12/12. 10.1242/jeb.074120 . [DOI] [PubMed] [Google Scholar]
- 8.CDC. Neglected Parasitic Infections (NPIs) in the United States: Centers for Disease Control and Prevention; 2014. [updated 7/10/14; cited 2016 6/7/2016]. Available from: http://www.cdc.gov/parasites/npi/. [Google Scholar]
- 9.Brunaska M, Dubinsky P, Reiterova K. Toxocara canis: ultrastructural aspects of larval moulting in the maturing eggs. Int J Parasitol. 1995;25(6):683–90. Epub 1995/06/01. 10.1016/0020-7519(94)00183-o . [DOI] [PubMed] [Google Scholar]
- 10.Hotez PJ, Wilkins PP. Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl Trop Dis. 2009;3(3):e400 10.1371/journal.pntd.0000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodhall DM, Eberhard ML, Parise ME. Neglected parasitic infections in the United States: toxocariasis. The American journal of tropical medicine and hygiene. 2014;90(5):810–3. 10.4269/ajtmh.13-0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2(6):e256 10.1371/journal.pntd.0000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won KY, Kruszon-Moran D, Schantz PM, Jones JL. National seroprevalence and risk factors for Zoonotic Toxocara spp. infection. The American journal of tropical medicine and hygiene. 2008;79(4):552–7. . [PubMed] [Google Scholar]
- 14.Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends in parasitology. 2009;25(4):182–8. 10.1016/j.pt.2009.01.006 . [DOI] [PubMed] [Google Scholar]
- 15.Marmor M, Glickman L, Shofer F, Faich LA, Rosenberg C, Cornblatt B, et al. Toxocara canis infection of children: epidemiologic and neuropsychologic findings. American journal of public health. 1987;77(5):554–9. 10.2105/ajph.77.5.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson LD, Gale SD, Berrett A, Brown BL, Hedges DW. Association between toxocariasis and cognitive function in young to middle-aged adults. Folia Parasitol (Praha). 2015;62 Epub 2015/09/17. 10.14411/fp.2015.048 . [DOI] [PubMed] [Google Scholar]
- 17.Janecek E, Beineke A, Schnieder T, Strube C. Neurotoxocarosis: marked preference of Toxocara canis for the cerebrum and T. cati for the cerebellum in the paratenic model host mouse. Parasites & vectors. 2014;7:194 10.1186/1756-3305-7-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuer L, Haendel S, Beineke A, Strube C. Effects of Toxocara larvae on brain cell survival by in vitro model assessment. Parasitology. 2015;142(10):1326–34. 10.1017/S0031182015000694 . [DOI] [PubMed] [Google Scholar]
- 19.Walsh MG, Haseeb MA. Toxocariasis and lung function: relevance of a neglected infection in an urban landscape. Acta parasitologica / Witold Stefanski Institute of Parasitology, Warszawa, Poland. 2014;59(1):126–31. 10.2478/s11686-014-0221-7 . [DOI] [PubMed] [Google Scholar]
- 20.Sharghi N, Schantz PM, Caramico L, Ballas K, Teague BA, Hotez PJ. Environmental exposure to Toxocara as a possible risk factor for asthma: a clinic-based case-control study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001;32(7):E111–6. 10.1086/319593 . [DOI] [PubMed] [Google Scholar]
- 21.Farmer A, Beltran T, Choi YS. Prevalence of Toxocara species infection in the U.S.: Results from the National Health and Nutrition Examination Survey, 2011–2014. PLoS Negl Trop Dis. 2017;11(7):e0005818 10.1371/journal.pntd.0005818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dada BJ, Lindquist WD. Prevalence of Toxocara spp. eggs in some public grounds and highway rest areas in Kansas. Journal of helminthology. 1979;53(2):145–6. 10.1017/s0022149x00005885 . [DOI] [PubMed] [Google Scholar]
- 23.Surgan MH, Colgan KB, Kennett SI, Paffmann JV. A survey of canine toxocariasis and toxocaral soil contamination in Essex County, New Jersey. American journal of public health. 1980;70(11):1207–8. 10.2105/ajph.70.11.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chorazy ML, Richardson DJ. A survey of environmental contamination with ascarid ova, Wallingford, Connecticut. Vector borne and zoonotic diseases (Larchmont, NY). 2005;5(1):33–9. 10.1089/vbz.2005.5.33 . [DOI] [PubMed] [Google Scholar]
- 25.Paul AJ, Todd KS Jr., DiPietro JA. Environmental contamination by eggs of Toxocara species. Veterinary parasitology. 1988;26(3–4):339–42. 10.1016/0304-4017(88)90102-1 . [DOI] [PubMed] [Google Scholar]
- 26.Dubin S, Segall S, Martindale J. Contamination of soil in two city parks with canine nematode ova including Toxocara canis: a preliminary study. American journal of public health. 1975;65(11):1242–5. 10.2105/ajph.65.11.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludlam KE, Platt TR. The relationship of park maintenance and accessibility to dogs to the presence of Toxocara spp. ova in the soil. American journal of public health. 1989;79(5):633–4. 10.2105/ajph.79.5.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Childs JE. The prevalence of Toxocara species ova in backyards and gardens of Baltimore, Maryland. American journal of public health. 1985;75(9):1092–4. 10.2105/ajph.75.9.1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh MG, Haseeb MA. Small-area estimation of the probability of toxocariasis in New York City based on sociodemographic neighborhood composition. PloS one. 2014;9(6):e99303 10.1371/journal.pone.0099303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakhri Y, Gasser RB, Rostami A, Fan CK, Ghasemi SM, Javanian M, et al. Toxocara eggs in public places worldwide—A systematic review and meta-analysis. Environ Pollut. 2018;242(Pt B):1467–75. Epub 2018/08/25. 10.1016/j.envpol.2018.07.087 . [DOI] [PubMed] [Google Scholar]
- 31.Dryden MW, Payne PA, Ridley R, Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Veterinary therapeutics: research in applied veterinary medicine. 2005;6(1):15–28. . [PubMed] [Google Scholar]
- 32.Kazacos KR. Improved method for recovering ascarid and other helminth eggs from soil associated with epizootics and during survey studies. American journal of veterinary research. 1983;44(5):896–900. . [PubMed] [Google Scholar]
- 33.Mejia R, Vicuna Y, Broncano N, Sandoval C, Vaca M, Chico M, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. The American journal of tropical medicine and hygiene. 2013;88(6):1041–7. 10.4269/ajtmh.12-0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durant JF, Irenge LM, Fogt-Wyrwas R, Dumont C, Doucet JP, Mignon B, et al. Duplex quantitative real-time PCR assay for the detection and discrimination of the eggs of Toxocara canis and Toxocara cati (Nematoda, Ascaridoidea) in soil and fecal samples. Parasites & vectors. 2012;5:288 10.1186/1756-3305-5-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deer DM, Lampel KA, Gonzalez-Escalona N. A versatile internal control for use as DNA in real-time PCR and as RNA in real-time reverse transcription PCR assays. Lett Appl Microbiol. 2010;50(4):366–72. 10.1111/j.1472-765X.2010.02804.x . [DOI] [PubMed] [Google Scholar]
- 36.Hygiene NYCDoHaM. Epiquery: NYC Interactive Health Data System—[NYC Population Data 2010] 2010. Available from: https://a816-healthpsi.nyc.gov/epiquery/.
- 37.Bureau USC. Community Facts -New York city, New York 2016. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk.
- 38.Carney K, Morales, A,. Income by Zip Code, 2016 [cited 2016 Aug 10]. Available from: https://www.incomebyzipcode.com.
- 39.Overgaauw PA, van Knapen F. Veterinary and public health aspects of Toxocara spp. Veterinary parasitology. 2013;193(4):398–403. Epub 2013/01/12. 10.1016/j.vetpar.2012.12.035 . [DOI] [PubMed] [Google Scholar]
- 40.Jones JL, Kruszon-Moran D, Won K, Wilson M, Schantz PM. Toxoplasma gondii and Toxocara spp. co-infection. The American journal of tropical medicine and hygiene. 2008;78(1):35–9. . [PubMed] [Google Scholar]
- 41.Little SE. Toxocara cati (Proceedings). In: Care UA, editor. CVC in Washington, DC Proceedings; April 1, 2010; Washington DC: DVM360.com; 2010. p. Toxocara cati (Proceedings) CVC in Washington, D.C. Proceedings.
- 42.Companion Animal Parasite Council. CAPC Vet parasite prevalence maps 2017 [cited 2017 July 5]. Available from: https://www.capcvet.org/maps/#2017/all/roundworm/dog/united-states/.
- 43.Lucio-Forster A, Mizhquiri Barbecho JS, Mohammed HO, Kornreich BG, Bowman DD. Comparison of the prevalence of Toxocara egg shedding by pet cats and dogs in the U.S.A., 2011–2014. Veterinary Parasitology: Regional Studies and Reports. 2016;5:1–13. 10.1016/j.vprsr.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Vanhee M, Dalemans A-C, Viaene J, Depuydt L, Claerebout E. Toxocara in sandpits of public playgrounds and kindergartens in Flanders (Belgium). Veterinary Parasitology: Regional Studies and Reports. 2015;1–2:51–4. 10.1016/j.vprsr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Otero D, Alho AM, Nijsse R, Roelfsema J, Overgaauw P, Madeira de Carvalho L. Environmental contamination with Toxocara spp. eggs in public parks and playground sandpits of Greater Lisbon, Portugal. Journal of infection and public health. 2018;11(1):94–8. 10.1016/j.jiph.2017.05.002 . [DOI] [PubMed] [Google Scholar]
- 46.Kleine A, Springer A, Strube C. Seasonal variation in the prevalence of Toxocara eggs on children's playgrounds in the city of Hanover, Germany. Parasites & vectors. 2017;10(1):248 10.1186/s13071-017-2193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleine A, Janecek E, Waindok P, Strube C. Flotation and adherence characteristics of Toxocara canis and T. cati and a reliable method for recovering Toxocara eggs from soil. Veterinary parasitology. 2016;227:35–41. 10.1016/j.vetpar.2016.07.023 . [DOI] [PubMed] [Google Scholar]
- 48.Andrew N. Rowan Ph.D. THSotUS. Animal Sheltering Trends in the U.S. A historical lesson from—and for—U.S. animal shelters. 2017 Jan 21, 2009.