Abstract
Vulnerability to relapse during periods of attempted abstinence from cocaine use is hypothesized to result from the rewiring of brain reward circuitries, particularly ventral tegmental area (VTA) dopamine neurons. How cocaine exposures act on midbrain dopamine neurons to precipitate addiction-relevant changes in gene expression is unclear. We found that histone H3 glutamine 5 dopaminylation (H3Q5dop) plays a critical role in cocaine-induced transcriptional plasticity in the midbrain. Rats undergoing withdrawal from cocaine showed an accumulation of H3Q5dop in the VTA. By reducing H3Q5dop in the VTA during withdrawal, we reversed cocaine-mediated gene expression changes, attenuated dopamine release in the nucleus accumbens, and reduced cocaine-seeking behavior. These findings establish a neurotransmission-independent role for nuclear dopamine in relapse-related transcriptional plasticity in the VTA.
Cocaine increases dopamine neurotrans-mission from the ventral tegmental area (VTA) to reward-relevant brain regions. This action is central to its addictive properties. Non-neurotransmission roles for dopamine in cocaine dependency have not been considered. Our laboratory has recently described a role for serotonin (5-HT) in developing 5-HTergic neurons, whereby 5-HT located in the nucleus of these neurons was shown to covalently attach to histone proteins—specifically on H3 glutamine 5 (H3Q5)—to regulate gene expression through a process called serotonylation (1, 2). We had hypothesized that this mechanism may generalize to other monoamines in brain, such as dopamine. If true, this process could potentially play a role in the addiction-relevant actions of drugs that stimulate dopaminergic transmission.
Drug addictions are defined by pathological drug-seeking behavior that persists despite adverse consequences. Prolonged vulnerability to relapse is hypothesized to reflect the functional rewiring of brain reward circuitries (3, 4). This is precipitated, at least in part, by drug-induced transcriptional plasticity in midbrain dopamine neurons (5, 6). Histone mechanisms that control chromatin structures, and consequently gene expression, regulate addiction-relevant behaviors (7, 8). Given that histone H3 can be modified by monoamines in response to fluctuations in intracellular availability, we assessed whether dopamine, like 5-HT, can be transferred to the H3 N-terminal tail. We performed targeted, peptide-based liquid chromatography–tandem mass spectrometry (LC-MS/MS) after in vitro transglutaminase 2 (TGM2) (1, 2, 9) enzymatic assays with dopamine. Peptide LC-MS/MS analyses (fig. S1, A to D) revealed Q5 as a reactive substrate for the dopaminyl mark [H3 glutamine 5 dopaminylation (H3Q5dop)]. Given that the serotonyl modification can exist both in isolation (H3Q5ser) and in combination with H3 lysine 4 trimethylation (H3K4me3Q5ser), we examined the effect of K4me3 on TGM2-mediated dopaminylation in vitro. Unmodified versus methylated mononucleosomes were subjected to TGM2 dopaminylation assays. Using an antibody against H3Q5dop, vide infra, we found that TGM2 equally dopaminylates unmodified and K4me3 substrates (fig. S2), which suggests that both modifications may occur in vivo.
To assess roles for H3 dopaminylation in the context of adult neuronal plasticity, we raised and fully validated single (H3Q5dop) and dual (H3K4me3Q5dop) modification-specific antibodies (fig. S3, A to J). We examined whether dopaminyl modifications in the adult brain are modulated by clinically relevant levels of drug exposure. We assessed the expression of these modifications in postmortem human brain tissues obtained from cocaine-dependent individuals compared with matched controls. We focused our investigations on the VTA, the origin of many of the dopaminergic projection neurons that compose the mesocorticolimbic dopamine system (10, 11). H3Q5dop, but not H3K4me3Q5dop, was significantly reduced in its expression in the VTAs of cocaine users; H3K4me3, total H3, and Tgm2 were unchanged in their relative levels of expression (Fig. 1A and fig. S5A). However, nearly all of the cocaine users examined in this study displayed pronounced peripheral concentrations of cocaine metabolites at time of death, which may conflate the acute pharmacological actions of cocaine with long-term adaptive responses to the drug.
Fig. 1. Histone H3 dopaminylation in the VTA is dysregulated by cocaine.
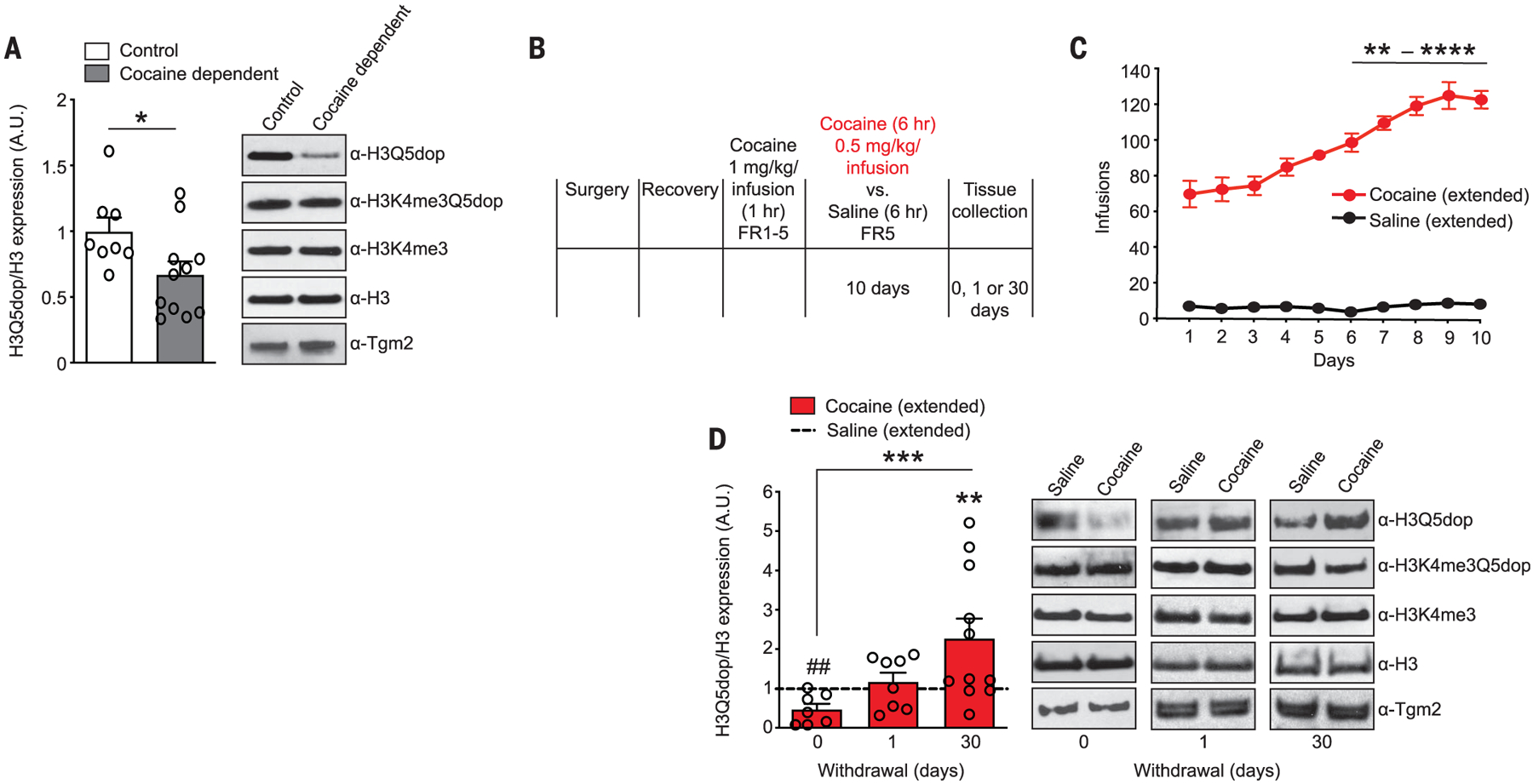
(A) H3 dopaminylation in human postmortem VTAs from cocaine-dependent subjects versus controls. No changes were observed in H3K4me3Q5dop, H3K4me3, H3, or Tgm2 expression (fig. S5A). A.U., arbitrary units. (B) Experimental timeline of cocaine self-administration (SA) followed by tissue collection time points during withdrawal (WD). FR1–5, fixed-ratio 1 to 5. (C) Number of infusions earned in daily 6-hour test sessions in rats self-administering cocaine or saline. (D) Analysis of H3 dopaminylation in the VTAs (0 versus 1 versus 30 days of WD) from rats with extended access to cocaine versus saline (see fig. S5B for full scatter plots). No changes were observed in H3K4me3Q5dop, H3K4me3, H3, or Tgm2 expression (fig. S5, C to E). Data presented as averages ± SEM. See supplementary materials for full figure legends with statistical comparisons.
Therefore, we employed intravenous cocaine self-administration in rats, a well-established procedure to study drug abuse (12, 13), to further explore potential contributions of H3Q5dop to addiction-relevant behaviors. Animals were trained to self-administer cocaine (or saline) under a fixed-ratio 5 schedule of reinforcement (see materials and methods for details). After training, independent cohorts of animals were allocated to two drug treatment groups (as were the respective saline controls): extended access (6-hour sessions) or restricted access (1-hour sessions) (Fig. 1B and fig. S4A). Animals with extended access to self-administration, but not those with restricted access, demonstrate a gradual escalation of intake across sessions (Fig. 1C and fig. S4A) (12, 14, 15). After 10 days of self-administration, VTA tissues were collected at three different time points: 0, 1, or 30 days. Global levels of H3Q5dop were significantly down-regulated in the VTAs of animals with extended access to cocaine at day 0 (Fig. 1D and fig. S5B), a time point that most appropriately mimics our human subject conditions, in that cocaine is still present at time of death. This reduction was transient, as H3Q5dop levels steadily increased over the course of the next 30 days (Fig. 1D and fig. S5B). By contrast, no changes in VTA H3Q5dop were observed in rats with restricted access to cocaine after 30 days of withdrawal (fig. S4B). Alterations in H3K4me3Q5dop, H3K4me3, total H3, or Tgm2 expression were not observed in animals with either extended or restricted access to cocaine compared with their controls (Fig. 1D, fig. S4B, and fig. S5, C to F). Finally, we assessed levels of the marks in VTA tissues at 30 days of withdrawal in animals receiving cocaine passively—either by being yoked to extended-access rats (fig. S4C and fig. S5G) or through experimenter-administered (intraperitoneal) injections (fig. S4D and fig. S5H)— and in animals trained to self-administer food under extended-access conditions (fig. S4E and fig. S5I). In all three cases, levels of H3Q5dop, along with expression of H3K4me3Q5dop, H3K4me3, total H3, and Tgm2, remained unaffected.
To explore the functional consequences of H3Q5dop during withdrawal, we delivered a virus vector into the VTA that expresses the H3 variant, H3.3—containing a glutamineto-alanine substitution at position 5 on its N-terminal tail—which let us compare H3.3Q5A with H3.3 wild-type (WT) and empty vector controls (1). The H3.3Q5A vector expressed effectively in a nuclear-specific manner in adult neurons (Fig. 2A), leading to significant down-regulation of H3Q5dop (Fig. 2B). This reduced the expression of the mark in a dominant-negative fashion without our needing to manipulate the activity of Tgm2, an enzyme with diverse functions in the brain that are independent from its histone transamidase activity. H3K4me3Q5dop expression was unaffected by H3.3Q5A delivery. We explored the impact of attenuating H3Q5dop accumulation in the VTA on drug-induced transcriptional programs that may be responsible for cocaine seeking after prolonged withdrawal. After chronic cocaine versus saline self-administration, rats were infected, intra-VTA, with one of the three viruses, followed by a 30-day period of enforced abstinence (Fig. 2C). After withdrawal, infected VTA tissues were microdissected and processed for RNA sequencing (RNA-seq) (fig. S6, A and B). We first used a threshold-free rank-rank hypergeometric overlap test (RRHO) to assess the patterns and significance of the overlap (Fig. 2D) between differential (cocaine versus saline) gene expression profiles observed after infection with our viral control vectors (H3.3 WT versus empty). The effect of cocaine self-administration on gene expression significantly overlapped between the two groups, which indicates that viral H3.3 WT expression does not affect cocaine-mediated gene expression profiles (Fig. 2E, left panel). We then compared differential gene expression profiles after infection—with either empty (Fig. 2E, middle panel) or H3.3 WT viruses (Fig. 2E, right panel)—with H3.3Q5A-infected VTA after cocaine self-administration. In both cases, attenuating H3Q5dop expression resulted in significant reversals of cocaine-mediated gene expression. Pairwise comparisons were used to identify specific genes displaying dysregulated expression between cocaine versus saline animals (empty or H3.3 WT). Then, overlay assessments were performed to align these sets of genes with those that are also regulated in cocaine self-administering animals expressing H3.3Q5A versus empty [Fig. 2F and tables S1 and S2; 720 differentially expressed protein coding genes (PCGs) were found to overlap] or H3.3 WT (Fig. 2G and tables S3 and S4; 211 differentially expressed PCGs were found to overlap) viruses. Further evaluation of these overlapping PCGs found that ~95 to 87% were significantly rescued in their expression with H3.3Q5A delivery; H3.3Q5A expression in saline self-administering animals had little to no impact on the expression of overlapping genes (Fig. 2, H and I). Gene enrichment analysis [Human Kyoto Encyclopedia of Genes and Genomes (KEGG) 2019] indicated strong associations with pathways involved in the regulation of synaptic function and drug addiction (Fig. 2J and table S5). These findings suggest that H3Q5 dopaminylation plays an important role in cocaine-induced transcriptional plasticity in the VTA.
Fig. 2. H3Q5dop in the VTA contributes to cocaine-mediated gene expression.
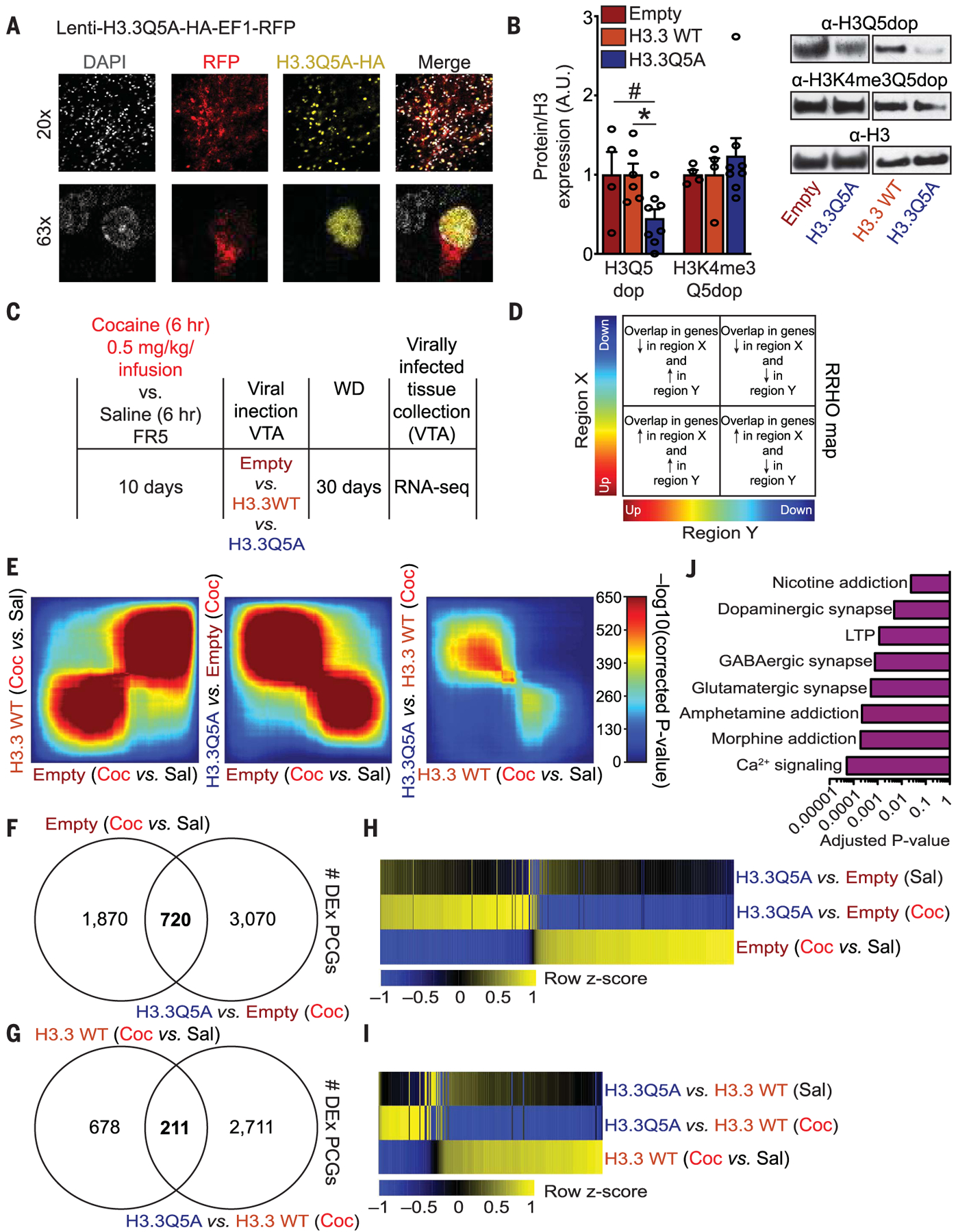
(A) VTA transduced with a lentivirus expressing H3.3Q5A-HA-EF1-RFP (red fluorescent protein) overlayed with a nuclear co-stain [4′,6-diamidino-2-phenylindole (DAPI)]. (B) H3 dopaminylation in VTAs infected with lentiviral vectors. (C) Experimental timeline of self-administration RNA-seq experiment after viral transduction. (D) RRHO map key describing the extent and directionality of overlap between differential gene expression. (E) RRHO comparing differential expression between cocaine-regulated genes. Each pixel represents the overlap between differential transcriptomes, with the significance of overlap of a hypergeometric test color-coded. Coc, cocaine; Sal, saline. (F and G) Overlap of differentially expressed (DEx) PCGs in VTA tissues comparing cocaine versus saline (empty) (F) or cocaine versus saline (H3.3 WT) (G) to H3.3Q5A versus empty or H3.3 WT (cocaine) animals, respectively. (H and I) Heat maps of overlapping genes obtained from RNA-seq data comparing cocaine versus saline (empty) and H3.3Q5A versus empty (cocaine) animals (H) or cocaine versus saline (H3.3 WT) and H3.3Q5A versus H3.3 WT (cocaine) animals (I) using normalized RNA expression values. (J) KEGG 2019 pathway enrichment analysis for the 211 overlapping PCGs displaying reversals in cocaine-mediated gene expression from (G) and (I). LTP, long-term potentiation. Data presented as averages ± SEM.
Next, we examined the effect of H3Q5dop on dopaminergic neuronal activity. Cocaine-naïve rats were injected intra-VTA with viruses expressing empty vector or H3.3 WT controls versus H3.3Q5A. We recorded spontaneous action potentials (sAPs) from infected dopaminergic neurons expressing hyperpolarization-activated currents (Fig. 3A). Attenuation of H3Q5dop significantly reduced the frequency of sAPs arising from dopaminergic neurons.To more directly assess the impact that blocking H3Q5dop during cocaine withdrawal has on dopamine release into the nucleus accumbens (NAc), rats were allowed to self-administer cocaine under extended-access conditions and were infected intra-VTA with one of the three viruses. After 30 days of withdrawal and cue-induced cocaine seeking, ex vivo assessments of dopamine release using fast-scanning cyclic voltammetry (FSCV) were performed (Fig. 3B). Stimulation-induced dopamine release into the NAc was significantly attenuated (Fig. 3, C to F) in response to reductions in H3Q5dop accumulation.
Fig. 3. Attenuating H3Q5dop expression in the VTA after extended access to cocaine reduces dopamine release in the ventral striatum.
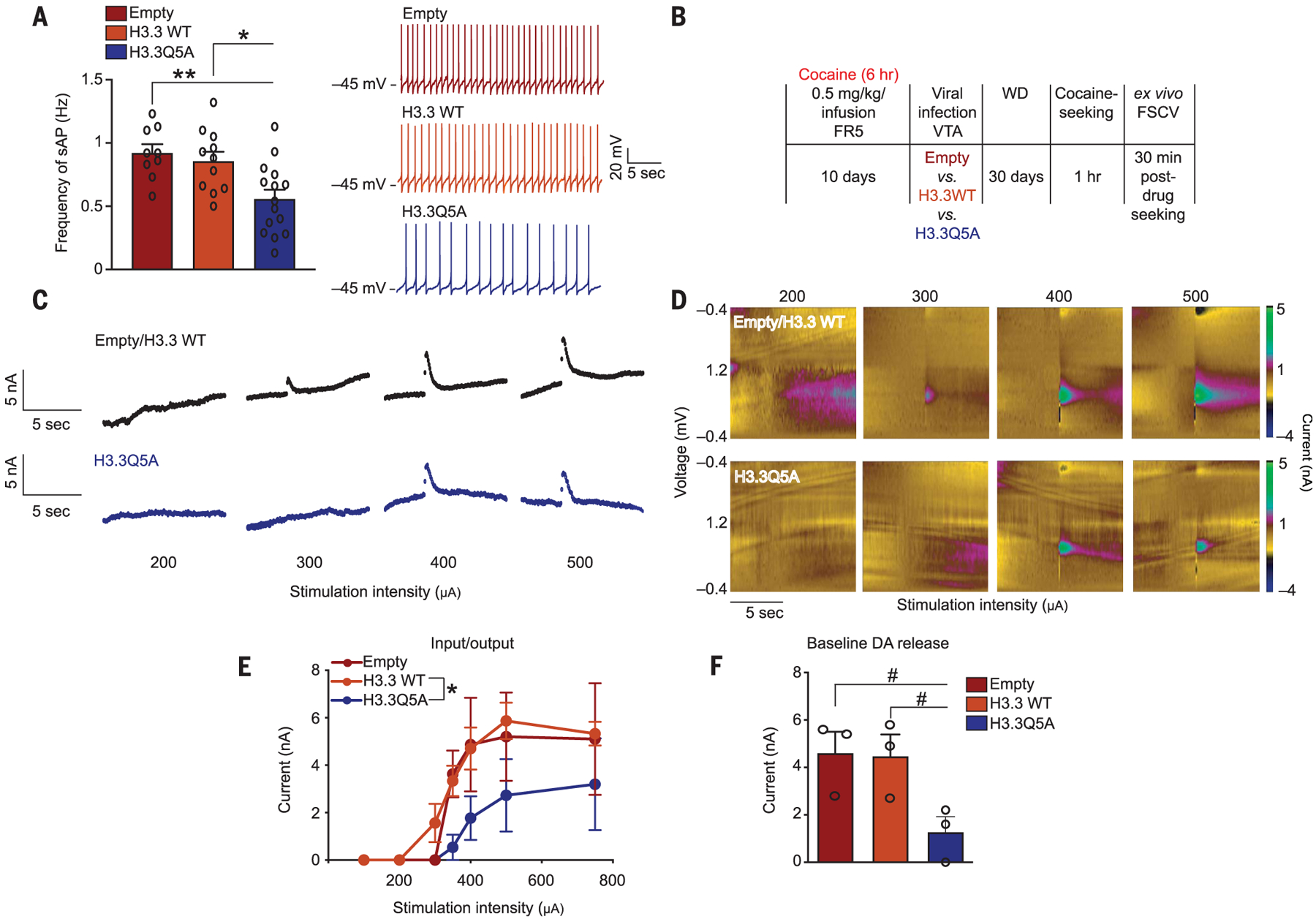
(A) Frequency of sAPs in VTA dopaminergic neurons from cocaine-naïve rats infected with empty vector, H3.3 WT, or H3.3Q5A. Representative sAP traces are provided. (B) Experimental timeline of cocaine SA FSCV experiments after viral transduction and 1 hour of cocaine seeking. (C to E) Current versus time traces (C) and color plots (D) demonstrating reduced dopamine release (evoked) in the NAc of H3.3Q5A versus empty and H3.3 WT (VTA) expressing animals. Quantified as input-output curves (E) (current versus stimulation intensity). (F) Baseline dopamine (DA) release into the NAc was similarly reduced by H3.3Q5A. Data presented as averages ± SEM.
To investigate what consequences modifying H3Q5dop levels has on relapse-relevant behaviors, we allowed an independent cohort of animals to self-administer cocaine under extended-access conditions followed by intra-VTA viral manipulations. After withdrawal, rats were returned to a drug-paired context, and cocaine seeking was assessed (Fig. 4A). Preventing H3Q5dop accumulation in animals with extended access significantly reduced their drug-seeking behavior (Fig. 4, B and C). Reducing H3Q5dop levels in the VTAs of a separate cohort of animals with restricted access did not affect their drug-seeking behavior (fig. S7, A to C). To ensure that such manipulations do not impair motivation to seek natural rewards, additional rats were trained to respond for food rewards under the same extended-access schedule of reinforcement, followed by viral manipulations, 30 days of ad libitum feeding, and subsequent reward seeking (fig. S8A). Food-seeking responses remained unaffected by manipulations of H3Q5dop (fig. S8, B and C). Given the fact that chronic cocaine use can elicit numerous additional behavioral abnormalities, we performed intra-VTA viral manipulations in cocaine-naïve rats, followed by assessments of psychomotor sensitization in response to repeated experimenter-administered cocaine. Both control (H3.3 WT) and knockdown (H3.3Q5A) animals displayed similar levels of cocaine-induced locomotor sensitization (fig. S8, D and E).
Fig. 4. Reducing H3Q5dop in the VTA attenuates cocaine seeking.
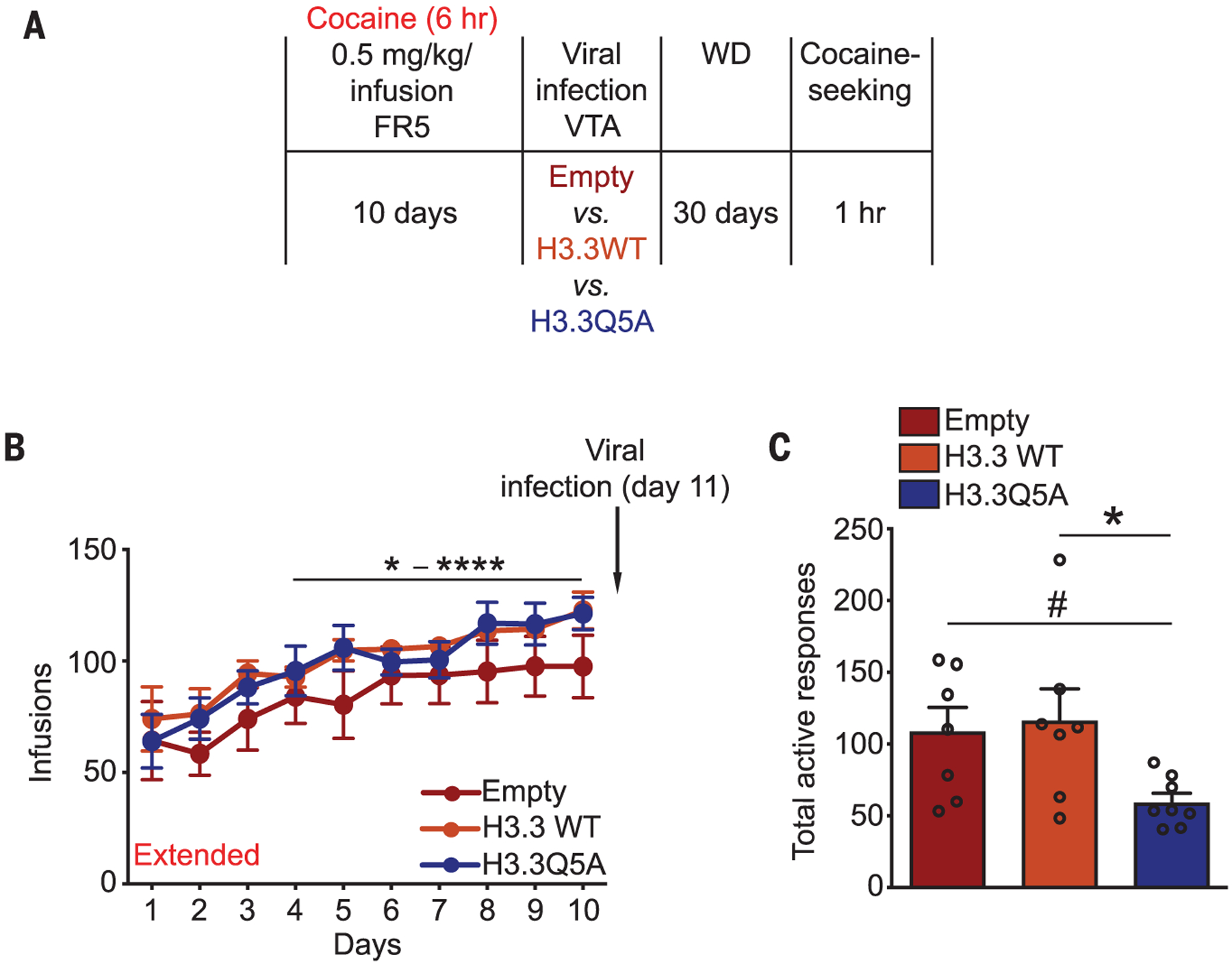
(A) Experimental timeline of cocaine SA drug-seeking experiments after viral transduction. (B and C) After 10 days of extended access to cocaine, rats were infected intra-VTA with one of the three viral vectors (on day 11) (B), followed by 30 days of WD and 1 hour of cocaine seeking (C). Data presented as averages ± SEM.
We demonstrated that a previously un-characterized chromatin modification, histone dopaminylation, is critically involved in regulating aberrant neuronal gene expression patterns in the VTA in response to cocaine consumption. The increased expression of H3Q5dop that follows prolonged withdrawal from extended, but not restricted, access to cocaine self-administration regulates relapse-like behaviors. It does so, in part, through the dysregulated transcription of addiction- and synaptic plasticity–related genes in the VTA, as well as through aberrant dopamine release dynamics in the NAc after cocaine withdrawal (fig. S9, model). Gaining a better understanding of the mechanistic roles for H3Q5dop in mediating permissive compared with repressive transcription, as well as the genes regulated through this mechanism, will greatly improve our knowledge of the molecular underpinnings of drug addiction.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank T. Muir and R. Thompson (Princeton Univ.) for generating dopaminylated peptides for use in antibody generation. We would also like to thank A. G. DiFeliceantonio (Virginia Tech) for providing supporting code for yoked cocaine experiments, K. Brennand and S. Powell (ISMMS) for providing hPSC-derived dopaminergic neurons for use in antibody control experiments, and A. Soshnev (Rockefeller Univ.) for help with illustrations. The NIDA Drug Supply Program generously gifted the cocaine used in these studies.
Funding: This work was supported by grants from the National Institutes of Health [DP1 DA042078 (I.M.), R21 DA044767 (I.M.), P50 MH096890 (I.M.), R01 DA025983 (P.J.K.), R01 MH108842 (Z.Y.), R01 DA037257 (D.M.D.), R21 DA044486 (D.M.D.), F99 NS108543 (J.A.M.), R00 DA042111 (E.S.C.), and F31 DA045428 (A.E.L.)] as well as the MQ Mental Health Research Charity [MQ15FIP100011 (I.M.)], Alfred P. Sloan Foundation Fellowship in Neuroscience (I.M.), Brain Research Foundation’s Fay/Frank Seed Grant Award (I.M.), Brain and Behavior Research Foundation (E.S.C. and A.C.W.S.), Whitehall Foundation (E.S.C.), and Edward Mallinckrodt, Jr. Foundation (E.S.C.).
Footnotes
Competing interests: P.J.K. is a cofounder of Eolas Therapeutics, Inc. The remaining authors declare no competing interests, financial or otherwise.
Data and materials availability: Data from RNA-seq experiments have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database under accession number GSE124055. We declare that the data supporting findings for this study are available within the article and supplementary materials. No restrictions on data availability apply.
SUPPLEMENTARY MATERIALS
science.sciencemag.org/content/368/6487/197/suppl/DC1
Materials and Methods
Extended Captions for Figs. 1 to 4
Figs. S1 to S9
Tables S1 to S5
References (16–30)
REFERENCES AND NOTES
- 1.Farrelly LA et al. , Nature 567, 535–539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther DJ et al. , Cell 115, 851–862 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Grimm JW, Hope BT, Wise RA, Shaham Y, Nature 412, 141–142 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrad KL et al. , Nature 454, 118–121 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickens CL et al. , Trends Neurosci. 34, 411–420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm JW et al. , J. Neurosci 23, 742–747 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maze I, Nestler EJ, Ann. N. Y. Acad. Sci 1216, 99–113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt HD, McGinty JF, West AE, Sadri-Vakili G, Cold Spring Harb. Perspect. Med 3, a012047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummerich R, Thumfart JO, Findeisen P, Bartsch D, Schloss P, FEBS Lett. 586, 3421–3428 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Cooper S, Robison AJ, Mazei-Robison MS, Neurotherapeutics 14, 687–697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler RA, Carelli RM, Neuropharmacology 56, 149–159 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed SH, Koob GF, Science 282, 298–300 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Shalev U, Grimm JW, Shaham Y, Pharmacol. Rev 54, 1–42 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Lesscher HM, Vanderschuren LJ Rev. Neurosci 23, 731–745 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Deroche-Gamonet V, Piazza PV, Neuropharmacology 76, 437–449 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


