Abstract
The advent of immune checkpoint inhibitors (ICIs) for cancer therapy has heralded increasing frequency of immune-related adverse events including endocrinopathies, hepatitis, colitis and rarely myocarditis and myasthenia gravis (MG). The heterogeneity in clinical presentations regardless of organ-specific involvement can lead to delayed recognition and management of these events and adverse health outcomes. We describe a case of ICI-induced subclinical focal myocarditis that was recognised and treated in the broader context of MG. It is essential that patients with ICI-induced MG should be screened and monitored for myocarditis, a potentially fatal complication.
Keywords: heart failure, radiology (diagnostics), malignant disease and immunosuppression, skin cancer, unwanted effects / adverse reactions
Background
Immunotherapy has transformed the field of oncology, offering sustained cancer control and even clearance, where previously few successful therapeutic options existed. Immune checkpoint inhibitor (ICI) therapy causes T cell activation allowing for targeted eradication of immunogenic cancer cells. This immune response is modulated and controlled through non-redundant signalling events in the immune cellular cycle termed immune checkpoints. Two such checkpoints have emerged as effective therapeutic targets in metastatic melanoma: cytotoxic T-lymphocyte-associated antigen protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1).1 CTLA-4, expressed by activated T cells, competes with the costimulatory molecule CD28 for binding with CD80/CD86 on antigen presenting cells (APC). T cell and APC costimulation via CD28 and CD80/86 attenuates T cell activation and proliferation. CTLA-4 is also found in regulatory T cells (Tregs) and inhibits their function. Conversely, PD-1 on T cells binds to PD-1 ligand expressed in the tumour micro-environment to further blunt T cell function, promote peripheral T cell exhaustion and conversion of T effector cells to Tregs. Combined blockade of both CTLA-4 and PD-1 demonstrated improved survival in metastatic melanoma compared with either agent alone.2
Checkpoint inhibition from T cell activation is associated with spectrum of side effects termed immune-related adverse events (irAEs). These include endocrinopathies, hepatitis, colitis and rarely myocarditis and neurological manifestations such as myasthenia gravis (MG).3 We report a case of ICI-mediated subclinical focal myocarditis as part of a broader presentation of MG, hepatitis and thyroiditis.
Case presentation
A 55-year-old man with a background of localised melanoma excised from his back 3 years prior was diagnosed with cerebral and pulmonary melanoma metastases. His cardiovascular risk factors included hypertension and previous smoking in the absence of family history of coronary artery disease (CAD). He was commenced on dual ICI therapy with nivolumab and ipilimumab. One week following the second three weekly cycle of this therapy, he was admitted with blurred vision, mild bilateral ptosis and fatigable left arm abduction. There was no family history of relevant diseases.
MRI of the brain demonstrated reduction in metastatic size since commencing immunotherapy. Two daily doses of 50 mg prednisolone were administered to facilitate discharge with a neurology follow-up.
Outpatient single fibre electromyography demonstrated one fibre pair with jitter and a markedly elevated mean consecutive difference, without block. Antimuscle-specific kinase and antiacetylcholine receptor antibodies were not detected. A CT scan showed no thymic mass. Clinical findings were deemed to be consistent with ICI-mediated MG.
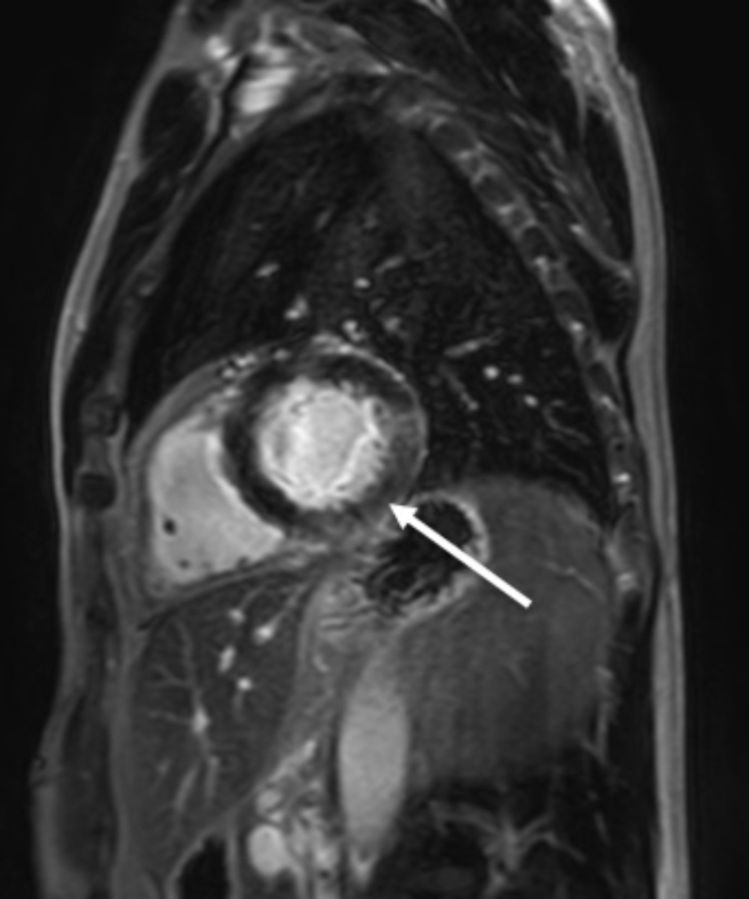
Subsequently he was re-admitted with progressive ocular weakness and diplopia. As part of investigations for MG, creatine kinase and high sensitivity troponin I levels were found to be elevated at 613 U/L (45–200) and 240 ng/L (<26), respectively. There was no clinical evidence of ischaemic heart disease or cardiac failure. An ECG demonstrated non-dynamic deep anterolateral T wave inversion. A transthoracic echocardiogram (TTE) showed normal left ventricular function with preserved ejection fraction and no wall motion abnormalities. However, a cardiac MRI (figure 1) demonstrated a small focal area of subendocardial late gadolinium enhancement in the basal inferolateral wall with preserved left ventricular systolic function. A CT coronary angiogram (CTCA) showed mild (<25% luminal stenosis) diffuse soft plaque identified throughout the right coronary artery and left anterior descending (LAD) artery with a tiny eccentric focus of calcification in the proximal LAD. Coronary artery calcium score was 3 Agatston units (34 centile for age/sex). This did not correspond to the region of the MRI abnormality.
Figure 1.
Cardiac MRI demonstrates a focal area of subendocardial late gadolinium enhancement in the inferolateral wall which extends into the basal inferior segments (indicated by white arrow).
Autoimmune serology (including anti-nuclear antibody (ANA), extractable-nuclear antigen antibody (ENA), anti-double stranded DNA, rheumatoid factor and coeliac serology) performed due to the association of systemic and organ-specific autoimmune diseases was found to be negative. Epstein–Barr virus, cytomegalovirus and parvovirus B19 DNA, hepatitis C viral RNA and hepatitis B surface antigen were not detected in peripheral blood. HIV screen was negative. The patient was diagnosed with ICI-mediated MG with subclinical focal myocarditis.
ICI therapy was discontinued and the patient was commenced on prednisone 0.2 mg/kg daily and intravenous immunoglobulin (IVIG) 0.5 g/kg/day for 4 days. Immunosuppression was escalated after 4 days due to non-resolving diplopia and persistently elevated troponin I levels between 100 and 200 ng/L. Prednisone was increased to 1 mg/kg/day and mycophenolate mofetil (MMF) was commenced, with a final daily dose of 2 g. Further IVIG therapy (0.5 g/kg/day for 4 days) was scheduled in 4 weeks. Aspirin was continued as secondary prevention for mild CAD identified on CTCA. The patient’s Myasthenia Gravis Activity Score decreased from 15 on admission to 2 on discharge. Troponin I levels returned to normal levels at 4 ng/L but T wave changes were persistent on the ECG. The patient was discharged after 2 weeks with mild improvement in myasthenic symptoms and no signs of heart failure.
Outpatient review 1 month post discharge showed persistent ocular MG despite IVIG and corticosteroid therapy. Troponin I had normalised. Treatment was escalated to plasma exchange with resolution of diplopia and ptosis after five daily induction followed by two monthly exchanges. Prednisone and MMF were weaned after 8 weeks.
Four months after presentation, the patient developed worsening dyspnoea on exertion. The troponin I level remained normal. TTE findings were consistent with heart failure with preserved ejection fraction, with reduced global longitudinal strain (−12), a dilated left atrium (volume index 35 mL/m2) and lateral mitral annular e′ velocity of 8 cm/s. He was commenced on perindopril and bisoprolol.
Differential diagnosis
The patient’s presentation with MG and subclinical myocarditis following nivolumab and ipilimumab was highly suggestive of multisystem irAEs from ICI therapy.
The differential diagnoses included systemic autoimmune rheumatic diseases, organ-specific autoimmune diseases, primary MG and viral infections. However, autoimmune serological studies were unremarkable for our patient. Selected viral studies also returned negative.
During his initial presentation with neurological symptoms, there were concerns for a space-occupying lesion affecting the brainstem which was excluded from the MRI scan. Amyotrophic lateral sclerosis was considered but also excluded, since ptosis and ocular dysmotility causing diplopia (as observed in MG) are not present in this condition. Unlike our patient, Lambert-Eaton myasthenic syndrome involves proximal leg weakness as an early and prominent symptom. Furthermore, he was not taking medications such as statin that can induce MG, and denied any history of congenital myasthenic syndrome.
Regarding to the patient’s high sensitivity troponin I rise, myocardial infarction was considered as the primary differential, but was ruled out based on the following factors. First, the high sensitivity troponin I levels remained steadily elevated for a period of 10 days, not following the standard rise fall pattern observed with myocardial infarction. They then returned to normal with immunosuppression and discontinuation of ICI therapy. The CTCA findings showed no significant coronary artery stenosis and assessment of systolic function demonstrated preserved biventricular systolic function on both echocardiography and cardiac MRI. The cardiac MRI demonstrated a small focal area of subendocardial late gadolinium enhancement in the basal inferolateral wall. There was no increase in T2 on STIR images, however, in the clinical setting these changes were deemed to likely represent acute myocarditis.
In relation to the subsequent diagnosis of heart failure with a preserved ejection fraction, persistent inflammatory myocarditis was considered as a differential. The symptoms, however, developed in the context of ongoing immunosuppressive therapy with prednisone, and were associated with a normal troponin I level of 7 ng/L. This diagnosis was supported by echocardiographic findings.
Outcome and follow-up
Follow-up has extended to 12 months post-treatment. A recent positive emission tomography (PET) scan showed a sustained response to the ICI therapy and the patient remains in disease remission. While there are no objective signs of MG, he continues to complain of left eye weakness. He has been maintained on pharmacotherapy for heart failure with preserved ejection fraction and his symptoms are well managed.
Discussion
We report a case of ICI-induced MG associated with focal subclinical myocarditis that progressed to symptomatic heart failure. Recent literature suggests a relationship between MG and myocarditis as irAEs. A systematic review of ICI-induced neuromuscular complications reported that 10% of patients with ICI-induced MG had concomitant myocarditis.4 A study from the WHO database showed that the most common irAEs associated with myocarditis were myositis (25%) and MG (11%).5 The association of ICI-induced MG and myocarditis is also found rarely in thymoma-associated multi-organ autoimmunity suggesting that this manifestation following ICI is due to dysregulation of autoreactive T cells.
Myocarditis is recognised as a common and potentially fulminant and fatal complication of ICIs.6 Murine studies demonstrate that cardiac PD-1 protects the heart against T cell-mediated inflammation and that mice, on PD-1 inhibition, develop myocarditis.7 Furthermore, sequencing of the T cell receptor CD3 shows shared sequences in tumour, skeletal and cardiac muscle suggesting fatal myocarditis can result from common antigen response.6 This is a case of smouldering’ or subclinical myocarditis as reported in the literature, however, offers consideration of progression to symptomatic disease.8 It is possible that earlier studies have under-reported the incidence of subclinical and focal myocarditis due to the limited utilisation of cardiac MRI.9 An autopsy study revealed that not all immunologic effects of ICIs on the heart become clinically apparent, despite some degree of myocarditis with a CD8+ T cell predominant lymphocytic infiltrate on histology.10
Mahmood et al showed that the prevalence of myocarditis was 1.14% when confirmed by imaging or histology.11 A major adverse cardiac event (MACE) occurred in 46% of those affected. Interestingly, and as observed in our case, left ventricular ejection fraction was preserved in ICI-induced myocarditis (38%), whereas troponin elevation above 15 ng/dL was 95% specific for prediction of MACE. In this study the regional distribution was not reported, with the main focus being depth of myocardial inflammation.11
A study by Sarocchi et al stratified 59 patients receiving nivolumab therapy into estimated risk of troponin release. The risk was stratified on the presence of existing cardiac disease and extra cardiac target organ disease. Cardiac disease included cardiomyopathy, valvular pathology, chronic arrhythmias, pulmonary hypertension or known CAD. Extra cardiac disease included stroke/transient ischemic attack, peripheral vascular disease and diabetes mellitus.9 Serial screening of troponins was performed during the first 3 weeks of nivolumab therapy. Six patients had significant troponin elevations, five of which were attributable to pre-existing heart disease. The remaining case did not have pre-existing cardiac disease and developed subclinical myocarditis based on troponin elevation and a time course temporally related to ICI therapy. This patient was followed out to 22 weeks and did not develop any symptoms of heart failure. In conclusion, they proposed baseline risk stratification alongside serial screening troponins with the use of ICIs.9
Further studies are required to elucidate the true incidence of subclinical myocarditis and the proportion of cases that are either subclinical or clinical, with a long-term follow-up to fully understand the significance of smouldering disease. We recommend investigating for subclinical myocarditis with troponin screening stratified according to risk, as demonstrated by Sarocchi et al, and/or associated manifestations such as MG.9 Subsequent detection of subclinical focal myocardial involvement requires close monitoring for development of symptomatic disease. Our case of subclinical myocarditis progressed to symptomatic disease with cardiac failure that responded to beta-blocker and angiotensin-converting enzyme inhibitor therapy. Treatment might require consideration of antiarrhythmic drugs and immunosuppressive agents such as corticosteroids, adjunctive mycophenolate, infliximab or consideration of a pacing wire, plasma exchange and rabbit antithymocyte globulin for life-threatening manifestations such as ventricular arrhythmias.12
Patient’s perspective.
About a week after my first Ipi/Nivo (ipilimumab plus nivolumab) infusion I developed a mild rash, sometimes elevated temperature, mild headaches (curable with paracetamol) and mild diarrhoea. None of this really affected my sleep quality and I was able to get on with almost a normal routine. Prior to my second infusion the headaches and fever had gone, but I still had mild diarrhoea and mild itchy skin. Nothing that I was concerned about. Two days after my second Ipi/Nivo infusion my eyelids, especially left, had started drooping and my diarrhoea had become quite severe. As it was a Sunday I went in to the emergency, in fact I went in because I was worried about the diarrhoea, not my droopy eyes (at this stage I did not know that ‘droopy eyes’ could be an irAE, and I’d never heard of MG). I was discharged that evening with a low dose prescription of prednisone. Anyhow the diarrhoea had gone by that afternoon and I had told the doctors I was feeling ok, and I would contact my oncologist the next day.
The next day I sent a picture of my face with the heavily drooping eyes to my oncologist who subsequently ordered me to have an MRI the following morning followed by an appointment with them, and later that day a neurologist. Even though I was finding it difficult to see and required assistance to walk around outside, I was overjoyed by the results of the MRI which indicated a substantial size decrease in all my brain tumours (only after a little over 3 weeks of starting treatment). Now, I’m not sure if this is entirely correct, but the neurologist realising that I may have immune related MG and that it could have severe effects on other organs, immediately sent me to the emergency. I presume he knew how severe it could be even though I was not feeling any discomfort, except for my eyes.
So I spent the next 10 days in hospital starting with a blood test after which I was hooked up to a heart monitor (I did not know why at that point). Every day I was being told about my heart and what they were doing to control the symptoms. They were also concerned about my breathing. Actually I never felt anything wrong, and no doubt it’s because the doctors were doing everything to prevent anything going wrong, which I am incredibly grateful for. After 10 days, when I was in a stable condition, I was discharged, only to again start having double vision, and 3 days later I was back in hospital. This time I only spent 3 or 4 days and was discharged after some high dose methylprednisolone. I was later started on a course of plasma exchange along with my prednisone and mycophenalate. I think the plasma exchange was most effective in getting my eyes back to almost normal (both droopy eyelids and double vision), still feel my left eye is slightly weak although other people including doctors do not notice it. Once out of hospital, I felt while walking around especially uphill, even only a slight grade, I was easily out of breath, so I mentioned this to my oncologist during a regular check-up who then referred me to a cardiologist. I was put on low doses of bisoprolol and perindopril and am still taking them both. I have also had two heart echo’s. For quite some time now I have no issues with breathlessness. I swim and ride a few times per week with no breathing difficulties.
It’s been a year now and my latest PET/CT and brain MRI are extremely encouraging indicating words like ‘no FDG uptake’, ‘Total Metabolic Response’, and ‘Not Seen’ so no matter what I have been through and still continue feel, to get this kind of result I would do it all again. I have other less severe immune related issues which have cropped up in the last 6 months but I am extremely fortunate to have responded so well to immunotherapy and grateful to the team of doctors who managed my irAE’s so well to ensure they did not become severe.
I hope that ways will be found so that more patients will respond as well as I have, and at the same time doses can be individualised so that irAE’s become less common or less severe. For the time being I could not return to my previous occupation, as I am cognitively or physically still not able to hold a full time responsible position. Looking to the future I will look for part time or consultancy employment.
Learning points.
Immune checkpoint inhibitor (ICI)-induced myocarditis is an under-recognised immune-related adverse event and can progress from a subclinical to clinical and potentially life-threatening outcome.
Patients with ICI-induced myasthenia gravis should be screened and monitored for associated myocarditis.
Patients having received ICI, especially nivolumab, may also benefit from serial screening of troponins and monitoring for clinical and sub-clinical myocarditis. Alternate diagnosis including coronary heart disease, pulmonary emboli, arrhythmias, valvular heart disease, cerebrovascular disease and/or pulmonary hypertension should also be considered.
Cardiac MRI is a particularly useful tool for its diagnosis and to exclude other causes and determine the extent of cardiac involvement.
Footnotes
Contributors: PJL, SLF, HS-IJ and STV had a joint role in conducting a literature review, drafting and editing of the manuscript. PJL was directly involved in the patients acute care and STV continues to have a role in the patients follow up.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med Overseas Ed 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–80. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 4.Puwanant A, Isfort M, Lacomis D, et al. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul Disord 2019;29:127–33. 10.1016/j.nmd.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Moslehi JJ, Salem J-E, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. 10.1016/S0140-6736(18)30533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarrio ML, Grabie N, Bu D-xiu, et al. Pd-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 2012;188:4876–84. 10.4049/jimmunol.1200389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thibault C, Vano Y, Soulat G, et al. Immune checkpoint inhibitors myocarditis: not all cases are clinically patent. Eur Heart J 2018;39:3553. 10.1093/eurheartj/ehy485 [DOI] [PubMed] [Google Scholar]
- 9.Sarocchi M, Grossi F, Arboscello E, et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist 2018;23:936–42. 10.1634/theoncologist.2017-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 2007;116:2062–71. 10.1161/CIRCULATIONAHA.107.709360 [DOI] [PubMed] [Google Scholar]
- 11.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mir H, Alhussein M, Alrashidi S, et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol 2018;34:1059–68. 10.1016/j.cjca.2018.03.012 [DOI] [PubMed] [Google Scholar]