SUMMARY:
Pathogenic variants in the polymerase γ gene (POLG) cause a diverse group of pathologies known as POLG-related disorders. In this report, we describe brain MR imaging findings and electroencephalogram correlates of 13 children with POLG-related disorders at diagnosis and follow-up. At diagnosis, all patients had seizures and 12 had abnormal MR imaging findings. The most common imaging findings were unilateral or bilateral perirolandic (54%) and unilateral or bilateral thalamic signal changes (77%). Association of epilepsia partialis continua with perirolandic and thalamic signal changes was present in 86% and 70% of the patients, respectively. The occipital lobe was affected in 2 patients. On follow-up, 92% of the patients had disease progression or fatal outcome. Rapid volume loss was seen in 77% of the patients. The occipital lobe (61%) and thalamus (61%) were the most affected brain regions. Perirolandic signal changes and seizures may represent a brain imaging biomarker of early-onset pediatric POLG-related disorders.
Polymerase γ (Polγ) is the only DNA polymerase active during human mitochondrial DNA (mtDNA) genome replication.1 Pathogenic variants in the Polγ gene (DNA polymerase γ [POLG]) cause a group of clinical syndromes known as POLG-related disorders (POLG-RDs).1 Patients with POLG-RDs fall into a heterogeneous clinical spectrum. At the least severe end of the spectrum, patients present in adulthood with ptosis and ophthalmoplegia, whereas those most severely affected present with progressive and severe neurologic impairment and liver involvement in early childhood.2
Pathogenic variants of POLG are the most frequently detected genetic forms of mitochondrial epilepsy.3 Seizures are described as the first clinical manifestation in up to 50% of patients. Seizure types include myoclonus, focal motor seizures, generalized seizures, status epilepticus, refractory febrile seizures, and epilepsia partialis continua (EPC).4
Neuroimaging findings in POLG-RDs have been described primarily in the later stages of the disease,5 with the occipital lobe being the most commonly involved region.4 Brain MR imaging findings in the early onset of pediatric POLG-RDs are not well-known. The primary goal of this study was to describe the early brain MR imaging findings in children, including the “perirolandic sign,” defined as signal changes in the brain parenchyma surrounding the central sulcus of POLG-RDs, and to correlate them with electroencephalogram (EEG) findings. Our secondary goal was to describe the evolution of brain MR imaging findings on follow-up imaging.
Case Series
This retrospective institutional review board–approved study was conducted in a single academic pediatric hospital, Children’s Hospital of Philadelphia. Medical records were searched for primary mitochondrial disorders from January 2001 to July 2018. Patients with brain MR imaging and a confirmed molecular diagnosis of POLG-RDs (confirmed biparental inheritance for autosomal recessive disease when possible) were included. Exclusion criteria consisted of the unavailability of brain MR imaging or confirmed molecular diagnosis.
Demographic data, clinical history, brain MR imaging at diagnosis, and the EEG most contemporaneous with the MR imaging at diagnosis were reviewed. On the basis of prior observation of an index case (which was included in the analysis), we also explicitly assessed the presence of MR imaging signal changes around the central sulcus involving the pre- or postcentral gyri perirolandic sign. Brain MR imaging was reviewed (at diagnosis and follow-up) by 3 pediatric neuroradiologists in consensus (F.G.G., A.V., and G.Z.). T1 and T2 imaging and DWI were available in at least 1 plane in all examinations. T2* and SWI, MR spectroscopy, and arterial spin-labeling (ASL) were partially available across the studies. When obtained, MR spectroscopy was acquired with the point resolved spectroscopy protocol, using at least a voxel in the right basal ganglia with the following parameters: TR/TE =1700/20 ms.
From a cohort of 117 patients with primary mitochondrial disorders, 13 met the criteria for a POLG-RD. Demographic information, age at onset, brief relevant history at presentation, EEG findings, elapsed time between the first brain MR imaging and the most contemporaneous EEG, and pathogenic variants of each patient’s pathogenic variants are shown in On-line Table 1. The presence of the perirolandic sign involving either the pre- or postcentral gyri, MR imaging signal changes in the thalami, additional brain MR imaging findings, and the specific sequences in which the signal changes were depicted in each patient are shown in On-line Table 2. All patients had follow-up imaging, which varied significantly in number among patients. Thus, instead of being mentioned individually, their follow-up findings were pooled and summarized in 1 single column in On-line Table 2, called “Pooled Follow-Up Imaging.”
Most patients were female (n = 9, 69%). The median age was 3 years (interquartile range, 0.7–7.5 years). All patients presented clinically with seizures (n = 13, 100%). Other common associated symptomatology included regression, developmental delay, hypotonia, vomiting, and signs of liver damage. All patients had an EEG available around the time of their first brain MR imaging (n = 13, 100%); all the scans had abnormal findings. Clinical or EEG evidence of EPC was detected in most patients (n = 8, 61%). The mean elapsed time between the most contemporaneous EEG and brain MR imaging at diagnosis was 6 days. The most common pathogenic variant found from each parent with another variant was c.1399G>A:p.A467T (n = 7, 54%), which is consistent with findings in previous literature.6
At diagnosis, brain MR imaging findings were abnormal in most patients (n = 12, 92%). The most common brain MR imaging findings were unilateral or bilateral perirolandic signal changes (n = 7, 54%) (Fig 1) and unilateral or bilateral thalamic signal changes (n = 10, 77%) (Fig 2). Perirolandic signal abnormalities were unilateral in most cases (n = 5, 71%) and were more frequently seen only affecting the precentral or both the pre- and postcentral gyri. Half of the thalamic changes were unilateral (n = 5, 50%). Simultaneous perirolandic and thalamic signal changes occurred in 6 patients (n = 6, 46%). An association of EPC (clinically/EEG) and perirolandic signal changes was present in 6 patients (n = 6, 75%), and an association of EPC (clinically/EEG) and thalamic signal changes, in 7 patients (n = 7, 87.5%). Two patients with EPC (clinically/EEG) did not present with perirolandic signal abnormalities. The findings positive for lesions overall were on the DWI of 10 patients (n = 10, 83%), on the T2WI of 9 patients (n = 9, 75%), and on FLAIR of 7 patients (n = 7, 58%). In 4 patients, DWI was the only sequence with abnormal findings. The occipital lobe was affected in the early brain MR imaging in 2 patients (n = 2, 15%). Signal changes in other brain regions were found in 5 patients (n = 5, 42%), involving multiple regions, namely the cerebral white matter, insula, putamen, caudate nucleus, fornix, cerebellar vermis, and also the frontal and occipital lobes. One patient did not present with either perirolandic or thalamic changes but instead presented with a diffuse pattern similar to that of leukoencephalopathy, with restricted diffusion in the white matter and white matter tracts (Fig 3). Three patients had an abnormally high lactate peak on MR spectroscopy. None of our patients had ASL or other perfusion-weighted imaging at the time of diagnosis. T1 and T2* imaging and SWI had negative findings at the time of diagnosis.
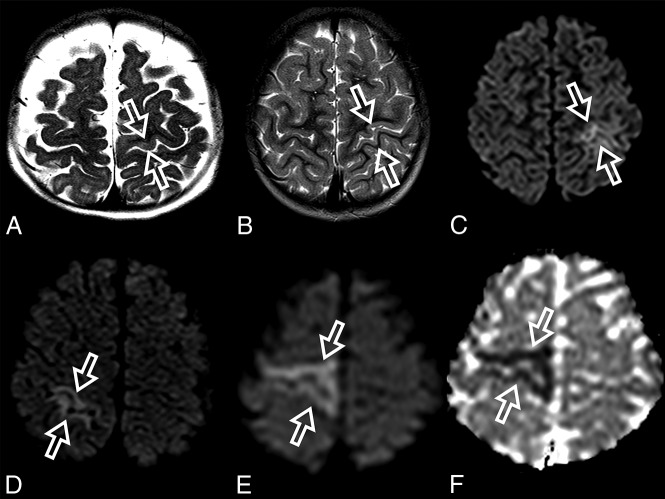
Fig 1.
Perirolandic sign in 4 different patients with POLG-related disorders (A, An 8-month-old female), (B and C, A 3 year-old-male), (D, An 1-year-old male) and (E and F, A 9-month-old female). Signal changes around the central sulcus were variable with varying degrees of conspicuity. A, T2WI. Signal changes are subtle and focal, evident only in the left precentral gyrus (open arrows). B, T2WI. Signal changes are subtle and focal, evident in the left pre- and postcentral gyrus (open arrows), but more conspicuous in the DWI (open arrows, C). D, DWI. Linear signal changes involving mainly the cortex surrounding the right central sulcus (open arrows). E and F, DWI and ADC map, respectively. Marked signal changes in both right pre- and postcentral gyri.
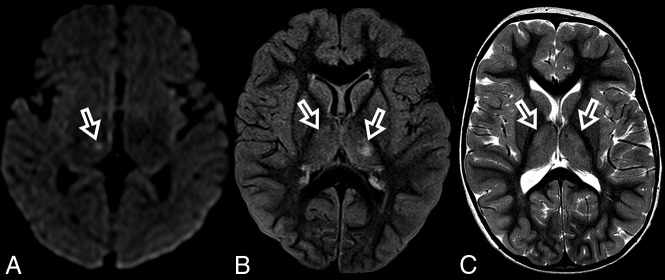
Fig 2.
MR imaging thalamic signal changes in 3 different patients with POLG-related disorders (A, A 9-month-old female), (B, A 3-year-old female), and (C, A 3-year-old male). Thalamic signal changes were also variable with varying degrees of conspicuity. A, DWI. Signal changes were subtle and focal with restricted diffusion in the right thalamus (open arrow). B, FLAIR. Signal changes involved both thalami, more conspicuous on the left side (open arrows). C, T2WI. Signal changes were bilateral and symmetric involving both thalami (open arrows).
Fig 3.
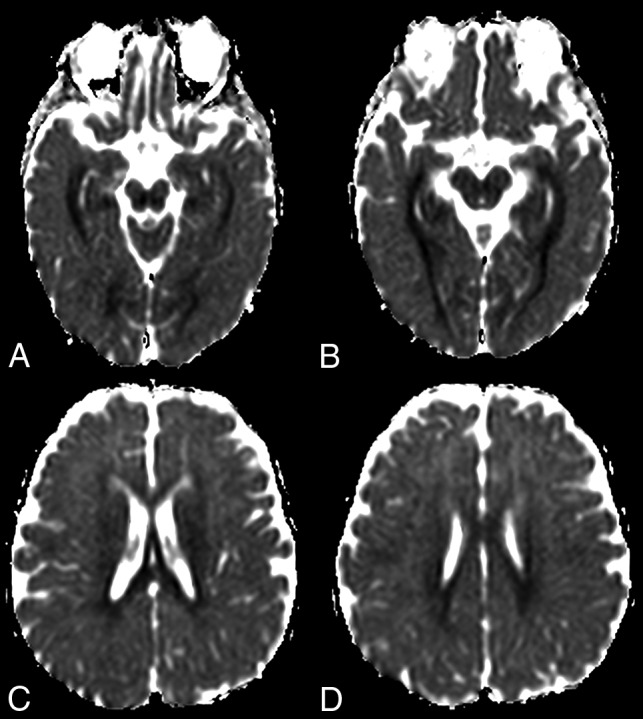
ADC map images of a 7-month-year old male with POLG-related disorder, demonstrating a diffuse pattern of leukoencephalopathy with restricted diffusion of the periventricular white matter of the bilateral temporal lobes in A, the occipital lobes in B, and also of the bilateral fornix in C and corpus callosum in D.
All patients had at least 1 follow-up brain MR imaging. Most patients had imaging findings suggesting disease progression (n = 12, 92%) or a fatal outcome. On follow-up imaging, most demonstrated volume loss (n = 10, 77%), which was typically very rapid, in a matter of a few weeks in most patients. On the follow-up brain MR imaging, the perirolandic and thalamic signal changes had various evolutions, with resolution, stability, contralateral involvement (unilateral → bilateral), or progression with time. The most affected brain regions at follow-up were the occipital lobe (n = 8, 61%) and thalamus (n = 8, 61%). The perirolandic sign was detected in 3 patients who did not have any evidence of perirolandic involvement at diagnosis. Other affected brain regions included the hippocampus, brain stem, dentate nucleus, cerebellar vermis, cerebellar hemispheres, other regions of the frontal and parietal lobes, and the occipital (Fig 4) and temporal lobes. ASL perfusion was obtained in only some of the patients at follow-up imaging. Regions more commonly found with increased ASL perfusion included the thalamus, the perirolandic region, and the occipital and parietal lobes. The areas with increased ASL perfusion also had signal changes visible on T2WI, FLAIR, or DWI. An increased lactate peak was seen in all patients in whom MR spectroscopy was performed on follow-up. Diffuse brain edema was noted in 1 patient, who died shortly thereafter. One patient had unremarkable brain MR imaging on follow-up with resolution of findings.
Fig 4.
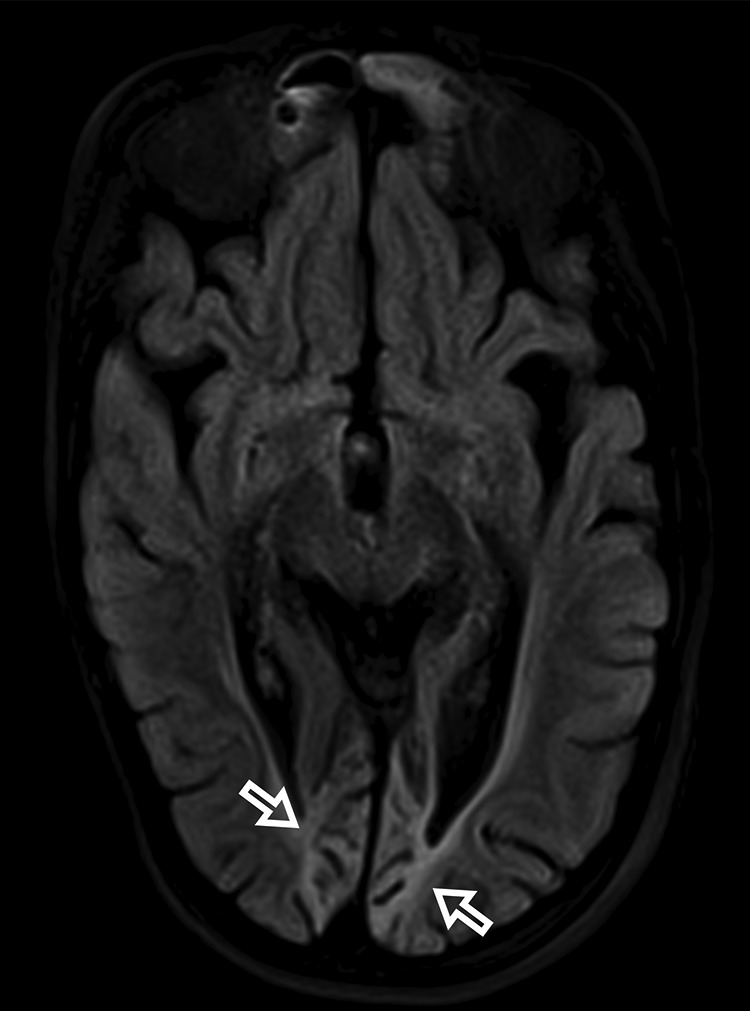
Bilateral occipital volume loss, gliosis, and encephalomalacia in a 16-year-old female patient with POLG-related disorder (open arrows).
DISCUSSION
Polγ is the only human DNA polymerase active during mtDNA replication.1 POLG is a nuclear gene that encodes for Polγ, and pathogenic variants lead to errors in mtDNA replication, resulting in multiple small mtDNA deletions, an overall reduction in the number of mtDNA genome copies (mtDNA depletion), and decreased adenosine triphosphate production leading to chronic loss of cellular energy.2
Patients with POLG-RD have been grouped into multiple distinct clinically-defined syndromes, which were later attributed to different subtypes of pathogenic variants. The prototypical clinical phenotypes associated with POLG pathogenic variants are the following: 1) Alpers–Huttenlocher syndrome; 2) myocerebrohepatopathy spectrum; 3) myoclonic epilepsy myopathy sensory ataxia; 4) ataxia neuropathy spectrum, which includes mitochondrial recessive ataxia syndrome, spinocerebellar ataxia with epilepsy, and sensory ataxia neuropathy dysarthria and ophthalmoplegia; 5) autosomal recessive progressive external ophthalmoplegia; and 6) autosomal dominant progressive external ophthalmoplegia.12 Nevertheless, it is increasingly recognized that there is a broad clinical spectrum across these clinical syndromes, in which a patient with POLG-RD may present with clinical features that may not fit within a single classically described syndrome.7
POLG pathogenic variants are the most common cause of mitochondrial epilepsy.8 Seizures are reported as the first clinical presentation in 50% of all patients with POLG-RDs.4 The most critical factor that triggers epileptic activity is the loss of mtDNA (mtDNA depletion). mtDNA depletion causes loss of the respiratory chain components, which restricts normal energy metabolism, causing a continued neurodegenerative process that interferes with neuronal function. This causes a vicious cycle that ultimately leads to neuronal death and parenchymal necrosis.8
EPC is a distinct type of focal motor seizure, first described in 1894.9 EPC characteristically involves repetitive, sometimes rhythmic, unilateral focal motor twitching of the limbs and/or face, with preservation of consciousness, suggesting an underlying brain lesion.10 EPC presentation is variable, occurring as single or multiple episodes, and it may be chronic, progressive, or nonprogressive.11 Several neurologic entities are associated with EPC, such as tuberous sclerosis, Sturge-Weber syndrome, and cortical dysplasia. Metabolic abnormalities such as hyponatremia; hypoglycemia; hyperglycemia; hyperuricemia; uremia; brain tumors such as oligodendroglioma, meningioma, and high-grade glioma; autoimmune processes; and infections. Rasmussen encephalitis also has a well-known association with EPC.11,12
A comprehensive literature review of 136 patients with epilepsy with POLG-RDs has shown that stroke-like changes were the most common imaging findings. The lesions were more commonly located in the occipital lobes. Other structures involved were the parietal, temporal, and frontal lobes; thalamus; basal ganglia; and cerebellum.4 The age of the patients in this study varied from younger than 30 days to 64 years, with a median of 2 years (first quartile = 0.75 and third quartile = 13.50).4 Before our study, reports of perirolandic MR imaging signal changes in POLG-RDs have only been mentioned in a few isolated case reports, including a 10-month-old child with Alpers-Huttenlocher syndrome who showed multifocal diffusion restriction areas in the left insula, deep gray matter, and bilateral perirolandic area, which were initially related to a nonspecific metabolic process.13 Right pre- and post-central gyri T2 and DWI signal abnormalities were also detected in a 7-month-old girl with POLG-RD who developed focal clonic status epilepticus, mainly of her left arm and occasionally also involving the left leg.14 Additional case reports described subtle signal changes on FLAIR in a 15-year-old girl with EPC15 and a 13-month-old child with bilateral perirolandic restricted diffusion with a history of hypotonia, mild motor delay, and EPC.16
In this current study of children with POLG-RDs, all patients presented initially with seizures. Most initial brain MR imaging findings were abnormal. A temporal association between seizures and brain MR imaging abnormalities was found, suggesting lesion-related seizures. The primary brain MR imaging abnormalities included unilateral or bilateral signal changes in the perirolandic region, unilateral or bilateral signal changes in the thalamus, or a combination of the 2 in most of our patients. At diagnosis, the perirolandic region or the thalamus was spared in only 2 patients in our cohort, one with unremarkable brain MR imaging findings and the other in whom diffuse white matter with restricted diffusion was noted (Fig 3).
The perirolandic sign was common and present in most patients at diagnosis. Moreover, the sign was seen in most patients during the course of the disease because half of the patients who did not have the sign initially presented with the sign later. The presence of perirolandic signal changes would reflect neuronal death following acute energy failure in a metabolically demanding region (primary motor and sensory cortex) within the dominant hemisphere. Further studies are needed to confirm this hypothesis.
The MR imaging appearance of the perirolandic sign was varied. Signal changes involved both the pre- and postcentral gyri, more commonly in the precentral gyri. The conspicuity of the perirolandic sign was also variable (Fig 1). In the more notable cases, signal changes were ribbon-like following the course of the gyri, which were readily detectable as T2 hyperintensities and restricted diffusion. DWI was the most sensitive MR imaging sequence to detect signal changes and, therefore, should always be included in the protocol and carefully evaluated when a case of POLG-RD is suspected.
Thalamic signal changes were also frequent at the time of diagnosis and on follow-up imaging. Unilateral or bilateral thalamic involvement was identified in most patients during the onset of their disease. In our cohort, there was no new thalamic involvement at follow-up. On follow-up imaging, thalamic changes had different outcomes: complete resolution, progression from unilateral to bilateral involvement, progression accompanied by volume loss, and fluctuation with periods of an almost-complete resolution and frank progression. Thalamic signal changes were variably detected on DWI, T2WI, FLAIR, or ASL.
Volume loss was detected almost always on follow-up imaging. Volume loss varied from mild to severe, showing rapid evolution in most cases. Most important, volume loss showed no lobar predominance in most patients. However, in severe cases, the occipital lobes were affected asymmetrically and with encephalomalacic changes. Signal changes were detected virtually in any part of the brain, except the medulla. The spinal cord was not part of the scope of this study.
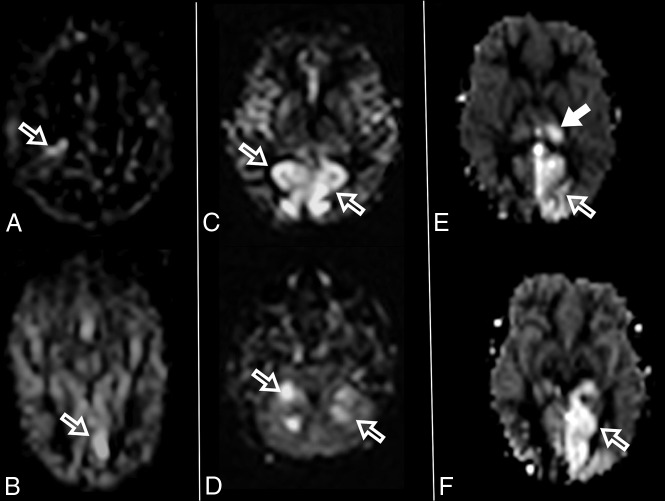
The occipital lobe was rarely affected at diagnosis, though frequently involved on follow-up imaging. Advanced MR imaging sequences such as MR spectroscopy and ASL, albeit not being acquired in all the patients, were useful to demonstrate increased lactate peaks and areas of localized hyperperfusion, respectively. An increased lactate peak is an expected finding in primary mitochondrial disorders and has already been described in patients with POLG-RDs, reflecting mitochondrial dysfunction.8 ASL perfusion has mostly been described in patients with mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS), but rarely in POLG-RDs. Increased ASL perfusion in MELAS preceded signal changes on conventional MR imaging in 3 patients, indicating that ASL imaging has the potential for predicting the emergence of stroke-like lesions.17 In our cohort, increased ASL perfusion was coincident with either T2WI, FLAIR, or DWI signal changes and may have been related to seizure activity. ASL, however, was useful to indicate areas that would undergo further volume loss in some of our patients (Fig 5). The complete role of ASL perfusion in patients with POLG-RDs needs further investigation.
Fig 5.
Increased ASL perfusion in 3 different patients with POLG-related disorders (A and B), (C and D), and (E and F). Increased perfusion regions are seen in the right perirolandic region in A (open arrow) and in the left occipital lobe in B (open arrow). Bilateral parietal and occipital increased perfusion is seen in C (open arrows) and in the bilateral cerebellar hemispheres (open arrows) in D. Increased ASL perfusion is seen in the left thalamus in E (solid arrow) and in the left parietal and occipital lobes in E and F (open arrows).
Valproic acid is an effective antiepileptic drug to manage epilepsy. However, it is strictly contraindicated to treat seizures in patients with POLG-RD due to its potential to cause fulminant hepatotoxicity.5 The best diagnostic test to prevent valproic acid–induced liver toxicity is POLG gene testing before the use of this medication.18 The presence of a unilateral or bilateral perirolandic sign, unilateral or bilateral thalamic MR imaging signal changes, or a combination of both in a child with seizures/EPC may be a POLG-RD biomarker, which can also be useful for early suspicion of this diagnosis and to prevent the use of valproic acid in these children.
MR imaging findings in our cohort are not pathognomonic for POLG-RD, and the differential diagnosis includes postictal changes and stroke-like episodes similar to MELAS. At this time, we can only speculate about their nature, and further laboratory, genetic, and imaging correlation are necessary to establish their pathogenesis. Bilateral perirolandic cortical involvement can also be seen in patients with hypoxic-ischemic injury,19 urea-cycle disorders,20 and Wernicke encephalopathy,21 though these patients often have a different clinical context. Perirolandic white matter involvement can be seen in cases of hypoxic-ischemic injury19 and maple syrup urine disease.22 Unilateral cortical perirolandic involvement can be seen in vascular infarcts, focal cortical dysplasia, and primary and metastatic tumors.23 Unilateral or bilateral thalamic signal changes can be seen in multiple entities such as arterial infarction, hypoxic-ischemic injuries, several metabolic diseases, infection, inflammatory processes, neoplasms, and veno-oclusive syndromes.24,25
Limitations of our study include the small number of patients with this rare disorder and the fact that this was a retrospective analysis. However, this study is about the first and largest cohort, describing the very early brain MR imaging findings in pediatric patients with confirmed POLG-RD. EPC, in association with perirolandic signal abnormalities, may serve as an early sign and a potential biomarker of POLG-RD. This clinical presentation should increase awareness and prompt testing for POLG pathogenic variants because the discovery of POLG-RD has relevant implications for acute clinical management and long-term prognosis.
ABBREVIATIONS:
- ASL
arterial spin-labeling
- EEG
electroencephalogram
- EPC
epilepsia partialis continua
- MELAS
mitochondrial encephalomyopath with lactic acidosis and stroke-like episodes
- mtDNA
mitochondrial DNA
- Polγ
polymerase γ
- POLG-RD
DNA polymerase γ–related disorder
Footnotes
G.Z. is the Principal Investigator (P.I.).
This work was funded, in part, by the Mitochondrial Medicine Frontier Program at Children’s Hospital of Philadelphia.
Disclosures: Zarazuela Zolkipli-Cunningham—UNRELATED: Employment: Children’s Hospital of Philadelphia. Marni J. Falk—UNRELATED: Board Membership: United Mitochondrial Disease Foundation, Comments: travel reimbursement for serving on scientific and medical advisory boards and board of trustees of the United Mitochondrial Disease Foundation; Consultancy: several pharma companies, Comments: paid consultant for Mitobridge/Astellas, NeuroVive, Cyclerionc Therapeutics, Reneo Pharmaceuticals, Mission Therapeutics, Stealth BioTherapeutics, and Imel Therapeutics; Grants/Grants Pending: several pharma companies and foundations, Comments: grants from Mitobridge/Astellas, NeurovVive, Cyclerion Therapeutics, Mission Therapeutics (pending), Stealth BioTherapeutics, Minovia Therapeutics (pending)*; Patents (Planned, Pending or Issued): several, Comments: several patents (no current value) at home institution in mitochondrial disease diagnostic and therapeutic development*; Royalties: Elsevier, Comments: editor of book now in press at Elsevier, Mitochondrial Disease Genes Compendium; Stock/Stock Options: Mitocureia, Comments: co-founder and scientific advisory board co-Chair of Mitocureia; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: education travel, Comments: reimbursed for travel to speak at meetings on mitochondrial diagnostics and therapeutics including at CardioInfantil, Bogota, Columbia; Pediatric Neurology Society, Mexico; American College of Medical Genetics; Emory/Atlanta Children’s Hospital; New York University; Cornell University; Washington University in St Louis; Seattle Children’s Hospital; scientific and family meetings hosted by United Mitochondrial Disease Foundation; European Society of Human Genetics; Society for the Study of Inborn Errors of Metabolism; Society of Inherited Metabolic Disease; Hebrew University; Asian Society Of Inborn Errors of Metabolism; Wellcome Genome Campus; Cold Spring Harbor Laboratory. Amy Goldstein—UNRELATED: Board Membership: United Mitochondrial Disease Foundation, Comments: Scientific and Medical Advisory Board. *Money paid to the institution.
References
- 1.Copeland WC. The mitochondrial DNA polymerase in health and disease. Subcell Biochem 2010;50:211–22 10.1007/978-90-481-3471-7_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stumpf JD, Saneto RP, Copeland WC. Clinical and molecular features of POLG-related mitochondrial disease. Cold Spring Harb Perspect Biol 2013;5:a011395 10.1101/cshperspect.a011395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zsurka G, Kunz WS. Mitochondrial dysfunction and seizures: the neuronal energy crisis. Lancet Neurol 2015;14:956–96 10.1016/S1474-4422(15)00148-9 [DOI] [PubMed] [Google Scholar]
- 4.Anagnostou ME, Ng YS, Taylor RW, et al. Epilepsy due to mutations in the mitochondrial polymerase gamma (POLG) gene: a clinical and molecular genetic review. Epilepsia 2016;57:1531–45 10.1111/epi.13508 [DOI] [PubMed] [Google Scholar]
- 5.Rahman S, Copeland WC. POLG-related disorders and their neurological manifestations. Nat Rev Neurol 2019;15:40–52 10.1038/s41582-018-0101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nurminen A, Farnum GA, Kaguni LS. Pathogenicity in POLG syndromes: DNA polymerase gamma pathogenicity prediction server and database. BBA Clin 2017;7:147–56 10.1016/j.bbacli.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen BH, Chinnery PF, Copeland WC. POLG-related disorders In: Adam MR, Ardinger HH, Pagon RA. eds. GeneReviews. University of Washington; 2018 [PubMed] [Google Scholar]
- 8.Hikmat O, Eichele T, Tzoulis C, et al. Understanding the epilepsy in POLG related disease. Int J Mol Sci 2017;18 10.3390/ijms18091845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vein AA, van Emde Boas W. Kozhevnikov epilepsy: the disease and its eponym. Epilepsia 2011;52:212–18 10.1111/j.1528-1167.2010.02900.x [DOI] [PubMed] [Google Scholar]
- 10.Thomas JE, Reagan TJ, Klass DW. Epilepsia partialis continu: a review of 32 cases. Arch Neurol 1977;34:266–75 10.1001/archneur.1977.00500170020003 [DOI] [PubMed] [Google Scholar]
- 11.Mameniškienė R, Wolf P. Epilepsia partialis continua: a review. Seizure 2017;44:74–80 10.1016/j.seizure.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 12.Bien CG, Widman G, Urbach H, et al. The natural history of Rasmussen’s encephalitis. Brain 2002;125:1751–59 10.1093/brain/awf176 [DOI] [PubMed] [Google Scholar]
- 13.Park S, Kang HC, Lee JS, et al. Alpers-Huttenlocher syndrome first presented with hepatic failure: can liver transplantation be considered as treatment option? Pediatr Gastroenterol Hepatol Nutr 2017;20:259–62 10.5223/pghn.2017.20.4.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf NI, Rahman S, Schmitt B, et al. Status epilepticus in children with Alpers’ disease caused by POLG1 mutations: EEG and MRI features. Epilepsia 2009;50:1596–1607 10.1111/j.1528-1167.2008.01877.x [DOI] [PubMed] [Google Scholar]
- 15.Lagan NC, Gorman KM, Shahwan A, et al. Teaching Video Neuro: epilepsia partialis continua in an adolescent with preexisting focal epilepsy. Neurology 2017;89:e274–75 10.1212/WNL.0000000000004713 [DOI] [PubMed] [Google Scholar]
- 16.Papandreou A, Rahman S, Fratter C, et al. Spectrum of movement disorders and neurotransmitter abnormalities in paediatric POLG disease. J Inherit Metab Dis 2018;41:1275–83 10.1007/s10545-018-0227-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikawa M, Yoneda M, Muramatsu T, et al. Detection of preclinically latent hyperperfusion due to stroke-like episodes by arterial spin-labeling perfusion MRI in MELAS patients. Mitochondrion 2013;13:676–80 10.1016/j.mito.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Saneto RP, Lee I-C, Koenig MK, et al. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure 2010;19:140–46 10.1016/j.seizure.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White ML, Zhang Y, Helvey JT, et al. Anatomical patterns and correlated MRI findings of non-perinatal hypoxic-ischaemic encephalopathy. Br J Radiol 2013;86:20120464 10.1259/bjr.20120464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takanashi JI, Barkovich AJ, Cheng SF, et al. Brain MR imaging in neonatal hyperammonemic encephalopathy resulting from proximal urea cycle disorders. AJNR Am J Neuroradiol 2003;24:1184–87 [PMC free article] [PubMed] [Google Scholar]
- 21.Zuccoli GE, Siddiqui N, Bailey A, et al. Neuroimaging findings in pediatric Wernicke encephalopathy: a review. Neuroradiology 2010;52:523–29 10.1007/s00234-009-0604-x [DOI] [PubMed] [Google Scholar]
- 22.Shah T, Purohit S, Raval M. Imaging in maple syrup urine disease. Indian J Pediatr 2018;85:927–28 10.1007/s12098-018-2696-y [DOI] [PubMed] [Google Scholar]
- 23.Magill ST, Han SJ, Li J, et al. Resection of primary motor cortex tumors: feasibility and surgical outcomes. J Neurosurg 2018;129:961–72 10.3171/2017.5.JNS163045 [DOI] [PubMed] [Google Scholar]
- 24.Smith AB, Smirniotopoulos JG, Rushing EJ, et al. Bilateral thalamic lesions. AJR Am J Roentgenol 2009;192:W53–62 10.2214/AJR.08.1585 [DOI] [PubMed] [Google Scholar]
- 25.Tuttle C, Boto J, Martin S, et al. Neuroimaging of acute and chronic unilateral and bilateral thalamic lesions. Insights Imaging 2019;10:24 10.1186/s13244-019-0700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]