Abstract
Coronavirus Disease 2019 (COVID‐19) is a newly emerging infectious disease caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). After its first occurrence in Wuhan of China from December 2019, COVID‐19 rapidly spread around the world. According to the World Health Organization statement on 13 March 2020, there had been over 132 500 confirmed cases globally. Nevertheless, the case reports of children are rare, which results in the lack of evidence for preventing and controlling of children's infection. Here, we report three cases of SARS‐CoV‐2 infected children diagnosed from 3 February to 17 February 2020 in Tianjin, China. All of these three cases experienced mild illness and recovered soon after the treatment, with the nucleic acid of throat swab turning negative within 14, 11, and 7 days after diagnosis, respectively. However, after been discharged, all three cases were tested SARS‐CoV‐2 positive in the stool samples within 10 days, in spite of their remained negative nucleic acid in throat swab specimens. Therefore, it is necessary to be aware of the possibility of fecal‐oral transmission of SARS‐CoV‐2 infection, especially for children cases.
Keywords: children, clinical characteristics, COVID‐19, SARS‐CoV‐2
Highlights
Children with COVID‐19 are mostly mild cases and few severe cases have been reported. Here, we recruited three pediatric patients who experienced mild illness. After treatment, they all recovered soon with negative SARS‐CoV‐2 nucleic acid in throat swab specimens. However, after been discharged, all the three cases were tested SARS‐CoV‐2 positive in the fecal specimens within 10 days.
1. INTRODUCTION
Coronavirus Disease 2019 (COVID‐19) caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has rapidly spread worldwide since its onset in Wuhan throughout December 2019. 1 , 2 , 3 There was a total of 132 536 confirmed cases in 123 countries, areas or territories worldwide, and 4974 deaths till 13 March 2020. 4 The droplet spread or direct contact with fomites is considered to be the major routes of SARS‐CoV‐2 transmission, 3 while the fecal shedding is also considered to be a minor driver of transmission as a viable virus has been identified in a limited number of case reports. 5 , 6 Most patients infected with SARS‐CoV‐2 were mild types and could be cured. About 80% of laboratory‐confirmed cases were mild and moderate cases in adults, including those with or without pneumonia, 13.8% of patients were severe illness, and 6.1% of patients were a critical illness. 7 Children infected with SARS‐CoV‐2 were mostly mild cases and few severe cases have been reported. 8 Given that children are in a special immune state, we need to be alert to the occurrence of severe cases. In this study, we collected three children cases diagnosed with COVID‐19 from 3 February to 17 February 2020 in Tianjin. The clinical characteristics of these children were analyzed and reported to provide a reference for the diagnosis and treatment of children during the epidemic of COVID‐19.
2. MATERIALS AND METHODS
2.1. Study subjects
Children diagnosed with COVID‐19 in Tianjin, China were recruited. The diagnostic criteria for COVID‐19 refer to the “New Coronavirus Infected Pneumonia Diagnosis and Treatment Program (Fifth Edition)” 9 issued by the National Health and Health Commission.
2.2. Research method
Basic information of children who were diagnosed with COVID‐19: clinical characteristics, laboratory tests, chest imaging, etiological examination, treatment and outcome, and so forth were collected. The throat swab and fecal specimens were sent to Centers for Disease Control in Tianjin, China. The real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) nucleic acid tests were used to detect SARS‐CoV‐2 RNA.
2.3. Discharge criteria
This study followed the ethical requirements of biomedical research issued internationally and nationally strictly. All the following criteria 9 had to be met for hospital discharge or discontinuation of quarantine: (a) normal temperature lasting longer than 3 days, (b) resolved respiratory symptoms, (c) substantially improved acute exudative lesions on chest computed tomography (CT) images, and (d) two consecutively negative RT‐PCR test results separated by at least 1 day.
3. RESULTS
3.1. Clinical characteristics of children with COVID‐19
All the three cases exhibited familial aggregation and experienced a history of close contact with their adult relatives who were diagnosed with COVID‐19. All of them were male, aged 9, 6, and 8 years, respectively. Throat swab samples were collected on 1 to 14 days after onset of disease and the results of SARS‐CoV‐2 nucleic acid tests were positive. All the patients were classified as common type. Cases 1 and 3 had fever, nasal obstruction, runny nose, and digestive tract symptoms. Case 2 had mild clinical manifestations included cough, expectoration, and wheezing. As Case 1 combined with supportive tonsillitis, ceftriaxone anti‐infection was used. Case 2 was given ribavirin antivirus treatment. All three cases were given interferon atomization, vitamin C, and oral Chinese medicine treatment. After the treatment, three patients obtained the negative throat swab nucleic acid in 14, 11, and 7 days, and were discharged from hospital with two times of negative results in 16, 13, and 9 days, respectively. Follow‐up of isolation point after discharge: no positive result was found in either of the two times of throat swab nucleic acid tests, but the stool SARS‐CoV‐2 nucleic acid tests were positive after 10 days. The three patients were readmitted to the designated hospital. Neither clinical symptoms nor pathological changes in lung imaging were found in any cases. During the monitoring process, three cases appeared SARS‐CoV‐2 negative of stool specimens in 4, 5, and 10 days, respectively (Table 1).
Table 1.
General information of children with COVID‐19
| Basic features | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age, y | 9 | 6 | 8 |
| Gender | male | male | male |
| Time from onset of symptoms to diagnosis, d | 1 | 14 | 7 |
| Clustered cases within 14 d | Yes | Yes | Yes |
| Contact with fever patients in Wuhan within 14 d | Yes | No | No |
| Fever | Yes | No | Yes |
| Sore throat | Yes | No | No |
| Nasal congestion and runny nose | Yes | No | Yes |
| Fatigue | No | No | No |
| Cough | No | Yes | No |
| Gastrointestinal symptoms | Nausea and gastric appetite | No | Gastric appetite |
| Restless | No | No | No |
| Headache and muscle pain | Yes | No | No |
| Treatment | Interferon, Chinese medicine, and vitamin C | Interferon, Chinese medicine, and vitamin C | Interferon, Chinese medicine, and vitamin C |
| Hormone use | No | No | No |
| Antibiotic application | Yes | No | No |
| Time from diagnosis to throat swab SARS‐CoV‐2 nucleic acid negative, d | 14 | 11 | 7 |
| Length of hospital stay, d | 16 | 13 | 9 |
| Time from discharge to stool SARS‐CoV‐2 nucleic acid positive, d | 13 | 11 | 10 |
| Time form readmission to stool SARS‐CoV‐2 nucleic acid negative, d | 4 | 5 | 10 |
Abbreviations: COVID‐19, Coronavirus Disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.2. Laboratory results of children with COVID‐19
Among the three children, Case 1 was complicated with purulent tonsillitis, with the increase of leukocyte and C‐reactive protein (CRP) with 64.7 mg/L, and these indexes reduced to normal level after anti‐infective treatment. Case 2 and Case 3 showed a normal range of white blood cells (WBC), CRP, and lymphocyte count. The hemoglobin and platelets were normal in three children. There was no obvious abnormality of troponin, blood gas analysis, electrolyte, liver and kidney function, or myocardial enzyme in three children with COVID‐19. Serum procalcitonin, blood lactate dehydrogenase, and interleukin‐6 were normal in three patients. The D‐dimer was normal in all three children, but fibrinogen in Cases 2 and 3 was lower than normal. No bacterial growth was observed in the blood culture of three children, and Mycoplasma pneumoniae, chlamydia, respiratory syncytial virus, and adenovirus were negative. Immunological examination (IgG, IgM, IgA, C3, and C4) were all in the normal range, and lymphocyte subsets (T cells, B cells, and NK cells) were basically normal. Urine and stool are normal. No abnormality was found either in electrocardiogram and echocardiography, or in liver, gallbladder, and kidney ultrasound (Table 2).
Table 2.
Laboratory test results of children with COVID‐19
| Laboratory indicators | Case 1 | Case 2 | Case 3 | Normal range |
|---|---|---|---|---|
| HB, g/L | 114 | 134 | 130 | 120‐160 |
| WBC count, ×9/L | 10.65 | 7.99 | 6.42 | 4‐10 |
| N% | 58.5 | 72.3 | 34.2 | 50‐75 |
| Lymphocyte count, ×9/L | 1.46 | 1.43 | 3.62 | 0.8‐4 |
| L% | 13.7 | 17.9 | 56.4 | 20‐40 |
| CRP, mg/L | 64.7 | 6.28 | 0.2 | 0‐10 |
| PCT, ng/mL | 0.102 | 0.04 | 0.04 | 0‐0.5 |
| ALT, U/L | 26 | 24 | 15 | 21‐72 |
| AST, U/L | 30 | 23 | 25 | 17‐59 |
| CK, U/L | 39 | 45 | 37 | 55‐170 |
| CKMB, µg/L | 7 | 11 | 10 | 0‐25 |
| cTnI, ng/mL | 0.012 | 0.012 | 0.012 | 0‐0.12 |
| IL‐6, pg/mL | 25.7 | 2.7 | 1.5 | 0‐10 |
| LDH, U/L | 467 | 423 | 495 | 313‐618 |
| D‐dimer, mg/L | 0.112 | 0.34 | 0.28 | 0‐0.55 |
| FIB, g/L | 2.9 | 1.78 | 1.85 | 2.0‐4.0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine Kinase; CKMB, creatine kinase Mb isoenzyme; COVID‐19, Coronavirus Disease 2019; CRP, C‐reactive protein; FIB, fibrinogen; HB, hemoglobin; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; L%, lymphocyte ratio; N%, neutrophil ratio; cTnI, marker of myocardial injury; PCT, procalcitonin; WBC, white blood cell.
3.3. Imaging characteristics of children with COVID‐19
In this study, three cases of children with lung CT were found positive, and two of them mainly showed ground‐glass opacity (GGO), and one of them showed new cord shadow, which was located in the lower lung, outer band, near the pleura, and the scope was small and more limited. A review of CT after the treatment showed that the lesions were absorbed to varying degrees (3‐5 days). Specific imaging manifestations and dynamic changes are as follows:
Case 1
Admission lung CT: There was no lesion in anterior medial basal segment of the left lower lobe (Figure 1A). Reexamination of the lung CT in 4 days after admission: new thin strips of anterior medial basal segment of the left lower lobe (Figure 1B). Review of lung CT in 7 days after admission: left lower lobe lesions improved and absorbed (Figure 1C). Review of lung CT in 12 days after admission: left lower lobe anterior interior basal lesions were significantly smaller than before (Figure 1D).
Figure 1.
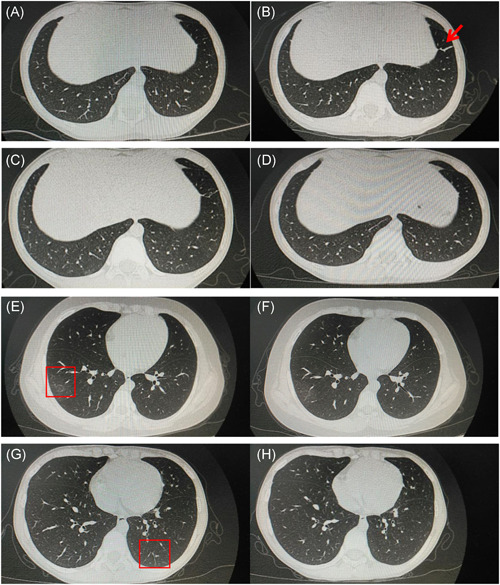
Imaging characteristics of children with COVID‐19. Admission lung CT of Case 1 (A‐D), Case 2 (E and F), and Case 3 (G and H)
Case 2
Admission lung CT: Light GGO in the anterior segment of the outer basal segment of the right lower lobe is located under the pleura (Figure 1E). Ring slightly high‐density shadow can be seen in the lesions of the right lower lobe. Lung CT was rechecked 5 days after admission: the GGO of the two lungs was slightly absorbed and faded (Figure 1F).
Case 3
Admission lung CT: Small piece of GGO under the pleura of the posterior basal segment of the left lower lobe, with uneven density and fuzzy edge (Figure 1G). Reexamination of lung CT 4 days after admission: the range of lesions in the left lower lung was reduced and the density became weak (Figure 1H).
4. DISCUSSION
Since December 2019, there were multiple cases of patients with COVID‐19 pneumonia found in Wuhan city of Hubei province. As the SARS‐CoV‐2 spreading around the whole world, COVID‐19 has become a global pandemic disease. 10 , 11 , 12 Pediatric case reports were gradually emerging, 13 , 14 even newborn COVID‐19 case has been reported. 15 , 16 Childhood infections were mainly caused by family clustering outbreaks and imported cases, 8 , 17 daily prevention at home was the main method to prevent viral spreading among children. Three cases of children in this study contacted with close relatives who were diagnosed with COVID‐19. The three children in this study were classified as a common type of COVID‐19, with no severe symptom. After infection, children's first symptoms were not typical, as most of them only got fever and cough, 6 , 8 and some suffer from gastrointestinal symptoms. 15 Except for Case 1 with supportive tonsillitis, and obvious fever symptoms, no other serious symptoms were found in all of the three cases. Cases 1 and 3 had mild digestive tract symptoms, while Case 2 and Case 3 showed normal range of CRP, WBC, and lymphocyte count, which consistent with other studies. 8 , 17 The lung CT showed ground‐glass density shadow in two cases, which was typical manifestation of COVID‐19 pneumonia. 18 , 19 , 20 As no specific antiviral drug available for SARS‐CoV‐2 infection to date, three patients were all treated with interferon nebulization, vitamin C, and Chinese medicine orally and had good results.
In the follow‐up after discharge, all patients with COVID‐19 had positive stool RT‐PCR test results after 10 days, accompanied by their member of the family. There were no clinical symptoms or imaging discovered in the patients. No positive findings were found by two times of throat swab RT‐PCR tests. Considering that children hardly cooperate in taking samples, further study is still necessary to assess whether two negative throat swab tests can be a feasible indicator to judge children's discharge and isolation. It has been reported that SARS‐CoV‐2 nucleic acid was detectable in the stool of adults and anal swabs of children, 6 , 21 which suggested that live virus exists in the feces discharged by the patients. In our study, we found that the stool nucleic acid was still positive after 10 days of recovery, and compared with anal swab specimens, stool specimens are more representative, suggesting the long existence of viruses in feces and on the surface of objects.
Recovered patients might be possible carriers for the viruses, which force us to reevaluate the current criteria of hospital discharge or discontinuation of quarantine and continued patient management. The study was limited to a small number of patients, longitudinal studies on a larger cohort would help to understand the prognosis of the disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the intellectual content of this manuscript and approved the final manuscript as submitted. TZ, XC, and XZ drafted the initial manuscript. JW, JZ, WG, CC, and GZ cared the patient and collected the clinical samples or data. SH and YX revised the article critically for important intellectual content.
ACKNOWLEDGMENTS
The authors wish to thank the patients and participating investigators and staff associated with the clinical studies discussed here. The authors extend their thanks to Tianjin CDC for helping with the detection of SARS‐CoV‐2 RNA with throat swab and fecal specimens. This study was supported by the Program of Tianjin Science and Technology Plan (grant no. 18ZXDBSY00170).
Zhang T, Cui X, Zhao X, et al. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J Med Virol. 2020;92:909–914. 10.1002/jmv.25795
Tongqiang Zhang and Xiaojian Cui contributed equally to this work.
Contributor Information
Sijia He, Email: she3@gmu.edu.
Yongsheng Xu, Email: xxyyss@126.com.
DATA AVAILABILITY STATEMENT
The data used in this report are available from the corresponding author on reasonable request.
REFERENCES
- 1. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;383:1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Coronavirus disease (COVID‐19) outbreak. 2020.
- 5. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020:1537 ciaa198. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yi Xu, X L, Bing Zhu, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nature Med. 2020. [published online ahead of print March 13, 2020]. 10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inspection Report C‐WNCPC‐J . China‐WHO new coronavirus pneumonia (COVID‐19) joint inspection report.
- 8. Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58(4):E011. [DOI] [PubMed] [Google Scholar]
- 9. General Office of the National Health and Health Commission . Diagnosis and treatment plan forpneumonia caused by new coronavirus (fifth edition), Office of State TCM Administration.
- 10. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China ‐ key questions for impact assessment. N Engl J Med. 2020;382:692‐694. 10.1056/NEJMp2000929 [DOI] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020. [published online ahead of print February 7, 2020]. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:E005. 10.3760/cma.j.issn.0578-1310.2020.0005 [DOI] [PubMed] [Google Scholar]
- 14. Cao Q, Chen YC, Chen CL, Chiu CH. SARS‐CoV‐2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670‐673. 10.1016/j.jfma.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Jin, W D, Chen Guoce, et al. A case of neonatal SARS‐CoV‐2 infection with gastrointestinal symptoms as the first manifestation. Chin J Contemp Pediatr. 2020:211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lingkong Zeng, T, Yuan Wenhao, et al. China's first neonatal coronaviruspneumonia. Chin J Pediatr. 2020;58(4):279‐280. [Google Scholar]
- 17. Wang XF, Yuan J, Zheng YJ, et al. Clinical and epidemiological characteristicsof 34 children with new coronavirus infection in ShenZhen. Chin J Pediatr. 2020;58:E008. 10.3760/cma.j.issn.0578-1310.2020.0008 [DOI] [PubMed] [Google Scholar]
- 18. Chen ZM, Fu JF, Shu Q. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020. [published online ahead of print February 5, 2020]. 10.1007/s12519-020-00345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019‐nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020. [published online ahead of print February 13, 2020]. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID‐19 respiratory infection. Pediatr Radiol. 2020. [published online ahead of print March 11, 2020]. 10.1007/s00247-020-04656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this report are available from the corresponding author on reasonable request.


