Abstract
Background
Currently, the epidemic of coronavirus disease 2019 (COVID‐19) has begun to spread worldwide. We aim to explore reliable evidence for the diagnosis and treatment of the COVID‐19 by analyzing all the published studies by Chinese scholars on the clinical and imaging features in novel coronavirus pneumonia caused by SARS‐CoV‐2.
Methods
We searched five medical databases including two Chinese and three English databases for all published articles on COVID‐19 since the outbreak. A random‐effects model was designed, and the imaging and clinical data from all studies were collected for meta‐analysis.
Results
Overall, 31 articles and 46 959 patients were included, including 10 English articles and 21 Chinese articles. The results of meta‐analysis showed that the most common clinical manifestations were fever (87.3%; 0.838‐0.909), cough (58.1%; 0.502‐0.660), dyspnea (38.3%; 0.246‐0.520), muscle soreness or fatigue (35.5%; 0.253‐0.456), and chest distress (31.2%; −0.024 to 0.648). The main imaging findings were bilateral pneumonia (75.7%; 0.639‐0.871) and ground‐glass opacification (69.9%; 0.602‐0.796). Among the patients, the incidence that required intensive care unit (ICU) was (29.3%; 0.190‐0.395), the incidence with acute respiratory distress syndrome was (28.8%; 0.147‐0.429), the incidence with multiple organ dysfunction syndrome was (8.5%; −0.008 to 0.179), and the case fatality rate of patients with COVID‐19 was (6.8%; 0.044‐0.093).
Conclusion
COVID‐19 is a new clinical infectious disease that mainly causes bilateral pneumonia and lung function deteriorates rapidly. Nearly a third of patients need to be admitted to the ICU, and patients are likely to present respiratory failure or even death.
Keywords: 2019 novel coronavirus pneumonia, clinical features, imaging finding, SARS‐CoV‐2
Research Highlights
In 2019, coronavirus disease (covid‐19) has started to spread globally. How to quickly identify influenza and covid‐19 is the key to ensure timely and effective treatment.
The fever and cough were the main clinical manifestations of the patients with novel coronavirus pneumonia in China, and the main complications are respiratory failure, acute respiratory distress syndrome.
Nearly a third of patients need to be admitted to the ICU, and some patients are likely to cause respiratory failure or even death.
1. INTRODUCTION
The 2019 novel coronavirus pneumonia (NCP) initially broke out in China, especially in Hubei province. The NCP is caused by a new coronavirus (SARS‐COV‐2) of the Sarbe virus subgenus, a member of orthocoronavirus subfamily. 1 SARS‐COV‐2 is a member of the coronavirus family along with SARS‐CoV and MERS‐CoV. With the deepening of research, more and more evidence show that its transmission channels are diversified, and its transmission speed and infectivity are stronger than SARS‐CoV and MERS‐CoV. 2 , 3 , 4 Since the outbreak of the epidemic, China has taken active prevention and control measures and achieved good results, but, recently, the epidemic situation abroad has begun to develop into an uncontrollable situation. As of 28 February 2020, the epidemic of NCP has affected six continents, and the epidemic situation in South Korea, Italy, Japan, and other countries is extremely serious. On 29 February, the “China‐WHO NCP (COVID‐19) Joint Inspection Report” stated that the NCP is almost susceptible to everyone on the same day. On 11 March, the WHO declared the SARS‐CoV‐2 outbreak as pandemic. Currently, published studies and case reports indicate that patients with NCP have very different clinical manifestations, laboratory tests, and imaging tests, making clinical diagnosis and treatment limited. Therefore, it is urgent to improve the understanding of the clinical characteristics of patients with NCP to further guide clinical and scientific research through evidence‐based medicine.
2. MATERIALS AND METHODS
2.1. Search strategy and study selection
This study was approved by the Ethics Committee of the Tongji Medical College, Huazhong University of Science and Technology. The literature search was performed according to the PRISMA (preferred reporting items for systematic reviews and meta‐analyses) process. The search was conducted in five popular medical databases including three English databases (PubMed, Cochrane Library, and Embase) and two Chinese databases (National Knowledge Infrastructure [CNKI] and China Biology Medicine disc [CBMdisc]). The searches were concluded by 1 March 2020. The language limit is English and Chinese. The retrieval is a combination of subject words and free words, and the keywords are as follows: “2019 novel coronavirus pneumonia,” “COVID‐19,” “Coronavirus,” “SARS‐CoV‐2,” “Wuhan Coronavirus,” “clinical features,” “2019 novel coronavirus pneumonia,” and “imaging features.”
2.2. Inclusion/exclusion criteria
Inclusive criteria are as follows: (a) research types: cross‐sectional studies and case series; (b) research subjects: patients with confirmed NCP, including patients with clinical diagnosis; and (c) data items: including clinical characteristics, biochemical indicators, and imaging signs. Exclusive criteria are as follows: (a) the type of study is case report, review, and so forth; (b) repeated research; and (c) lack of the above case data.
2.3. Data extraction and paper quality evaluation
The titles and abstracts of all retrieved references were independently reviewed by two investigators, and if there was any ambiguity in the search process, the decision was made by a third investigator. (a) The basic characteristics of the included literature are as follows: author, publication date, journal, research type, number of patients, quality score, and so forth. (b) The basic characteristics of the research subjects are as follows: age, sex, comorbidities, clinical manifestations, laboratory test results, imaging manifestations, and so on. The quality of all included literature was assessed using the Institute of Health Economics (IHE) scale. 5
2.4. Statistical analysis
The statistical software Stata version 14.0 and Open Meta‐Analyst were used for meta‐analysis of single‐arm studies. We first unified all units of variables and, then, expressed classified variables as percentages and expressed continuous variables as mean ± standard deviation. The combined prevalence and 95% CI were calculated using a random‐effects model. We performed the Egger test to assess publication bias in all literature works, and P < .05 was considered as publication bias.
3. RESULTS
3.1. Literature inclusion and characteristics
A total of 956 articles were retrieved. After deleting duplicates, 96 studies remained, of which 860 were excluded based on the title or abstract. Finally, 65 were eliminated after reading the full text, and a total of 31 articles 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 and 46 959 patients were included in this meta‐analysis (Figure 1). The main characteristics of the included studies are shown in Table 1. Publication bias was assessed with a funnel plot for the standard error by logit event, with no evidence of bias. Additionally, the Egger test (P = .091) suggested that there was no notable evidence of publication bias.
Figure 1.

Diagram of documents retrieval
Table 1.
The characteristics of the included literature
| References | Journal | Year | Date (M/D) | Country | No. patient | Sex (male) | Average age | Research type | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Huang et al 6 | Lancet | 2020 | 01/24 | China | 41 | 30 | 49 | Retrospective study | 8 |
| Chen et al 7 | Lancet | 2020 | 01/30 | China | 99 | 67 | 55.5 | Retrospective study | 8 |
| Yu et al 35 | J Pract Med | 2020 | 01/31 | China | 40 | 22 | 45.9 | Retrospective study | 5 |
| Michael et al 8 | Radiology | 2020 | 02/04 | China | 21 | 13 | 51 | Retrospective study | 5 |
| Wang et al 9 | JAMA | 2020 | 02/07 | China | 138 | 75 | 56 | Retrospective study | 8 |
| Liu et al 10 | Chin J Pediatr | 2020 | 02/07 | China | 137 | 61 | 57 | Retrospective study | 5 |
| Chang et al 11 | JAMA | 2020 | 02/07 | China | 13 | 10 | 34 | Retrospective study | 6 |
| Zheng et al 12 | Shanghai Med J | 2020 | 02/10 | China | 70 | … | Retrospective study | 4 | |
| Liu et al 13 | Sci China Life Sci | 2020 | 02/12 | China | 12 | 8 | … | Retrospective study | 6 |
| Gao et al 14 | J Xi'an Jiaotong Univ (Med Sci) | 2020 | 02/13 | China | 10 | 6 | 41.8 | Retrospective study | 5 |
| Gong et al 15 | Radiol Prac | 2020 | 02/13 | China | 33 | 13 | 51 | Retrospective study | 5 |
| Pan et al 16 | Eur Radiol | 2020 | 02/13 | China | 63 | 33 | … | Retrospective study | 6 |
| Liu et al 17 | Preprint Lancet | 2020 | 02/13 | China | 24 | 8 | 43 | Retrospective study | 6 |
| Pan et al 18 | Radiology | 2020 | 02/13 | China | 21 | 6 | 40.9 | Retrospective study | 5 |
| Zhang et al 19 | Chin J Tuberc Respir Dis | 2020 | 02/15 | China | 9 | 5 | 36 | Case series | 5 |
| Feng et al 20 | Chin J Pediatr | 2020 | 02/17 | China | 15 | 5 | … | Case series | 5 |
| Wang et al 21 | Chin J Pediatr | 2020 | 02/17 | China | 34 | 14 | 8 | Retrospective study | 5 |
| Zhang et al 22 | J. Chin Epi | 2020 | 02/17 | China | 44 672 | 22 981 | … | Retrospective study | 6 |
| Liu et al 23 | Radiol Prac | 2020 | 02/18 | China | 41 | 32 | 48.45 | Retrospective study | 5 |
| Zhuang et al 24 | Chin J Nosocomiology | 2020 | 02/19 | China | 26 | 18 | … | Retrospective study | 6 |
| Wang et al 25 | J Clin Med | 2020 | 02/19 | China | 30 | 16 | … | Retrospective study | 5 |
| Chen et al 26 | Herald Med | 2020 | 02/19 | China | 54 | 27 | 58.5 | Retrospective study | 5 |
| Zhong et al 27 | Med J Wuhan Univ | 2020 | 02/19 | China | 30 | 18 | 50.17 | Retrospective study | 5 |
| Fu et al 28 | Med J Wenzhou Univ | 2020 | 02/20 | China | 35 | 21 | 47 | Retrospective study | 5 |
| Yang et al 29 | Lancet Respir Med | 2020 | 02/21 | China | 52 | 35 | 59.7 | Retrospective study | 7 |
| Ji et al 30 | Chin J Med Imaging Technol | 2020 | 02/24 | China | 45 | 27 | 45.4 | Retrospective study | 6 |
| Chen et al 36 | Chin J Tuberc Respir Dis | 2020 | 02/25 | China | 29 | 21 | 56 | Retrospective study | 5 |
| Chen et al 31 | J Clin Med | 2020 | 02/26 | China | 12 | 8 | 63 | Retrospective study | 4 |
| Zeng et al 32 | J Emerg Tradit Chin Med | 2020 | 02/27 | China | 18 | 10 | 45.94 | Retrospective study | 5 |
| Cao et al 33 | Med J Wuhan Univ | 2020 | 02/28 | China | 36 | 20 | 72.45 | Retrospective study | 5 |
| Guan et al 34 | NEJM | 2020 | 02/29 | China | 1099 | 640 | 47 | Retrospective study | 8 |
3.2. Meta‐analysis results
3.2.1. Demographical characteristics and comorbidities
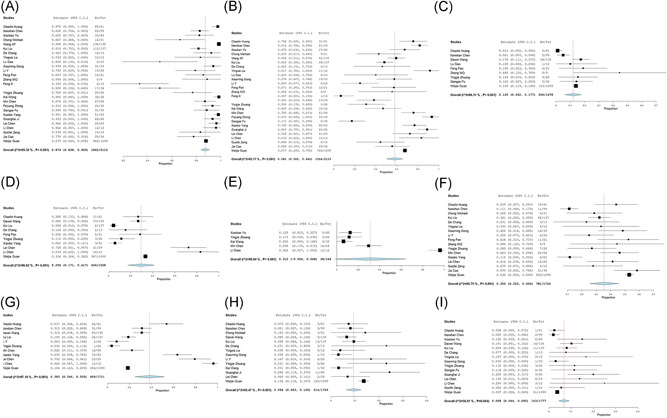
The mean age of the patients with SARS‐COV‐2 infection was 46.62 (95% CI, 31.710‐61.531) and 55.6% (95% CI, 0.530‐0.602) were male. About 35.6% (0.267‐0.444) of patients had comorbidities, including 18.3% (0.130‐0.236) with hypertension, 11.2% (0.078‐0.145) with cardiovascular disease, 10.3% (0.069‐0.136) with diabetes, 3.9% (0.011‐0.067) with chronic obstructive pulmonary disease, 3.0% (0.021‐0.039) with chronic hepatonephropathy, and 1.1% (0.003‐0.020) with tumor (Table 2 and Figures 2 and 3).
Table 2.
Meta‐analysis results of the incidence of demographical and comorbidities
| Variable | N a | Estimate | 95% CI | N b | Standard error | P | T 2 | Q | P c | I 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex, male | 30 | 0.556 | 0.530 to 0.602 | 24 250 | 0.018 | <.001 | 0.004 | 104.391 | <.001 | 72.22 |
| Age, mean | 14 | 46.62 | 31.71 to 61.531 | 334 | 7.608 | <.001 | 801.948 | 2756.956 | <.001 | 99.528 |
| ICU | 9 | 0.293 | 0.190 to 0.395 | 2371 | 0.052 | <.001 | 0.022 | 487.408 | <.001 | 98.359 |
| Comorbidities | 10 | 0.356 | 0.267 to 0.444 | 464 | 0.045 | <.001 | 0.015 | 75.378 | <.001 | 88.06 |
| Tumor | 8 | 0.011 | 0.003 to 0.020 | 135 | 0.004 | .009 | 0.000 | 22.143 | .002 | 68.387 |
| Diabetes | 13 | 0.103 | 0.069 to 0.136 | 1261 | 0.017 | <.001 | 0.002 | 97.488 | <.001 | 87.691 |
| Hypertension | 12 | 0.183 | 0.130 to 0.236 | 2964 | 0.027 | <.001 | 0.006 | 160.717 | <.001 | 93.156 |
| Cardiovascular disease | 11 | 0.112 | 0.078 to 0.145 | 1023 | 0.017 | <.001 | 0.002 | 136.694 | <.001 | 92.684 |
| Phthisis | 3 | 0.021 | −0.005 to 0.047 | 515 | 0.013 | .120 | 0.000 | 2.655 | .265 | 24.672 |
| COPD | 8 | 0.039 | 0.011 to 0.067 | 46 | 0.014 | .006 | 0.001 | 53.971 | <.001 | 87.03 |
| Chronic hepatonephropathy | 7 | 0.030 | 0.021 to 0.039 | 46 | 0.005 | <.001 | 0.000 | 5.144 | .525 | 0 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
Number of studies.
Number of patients.
Heterogeneity P value.
Figure 2.
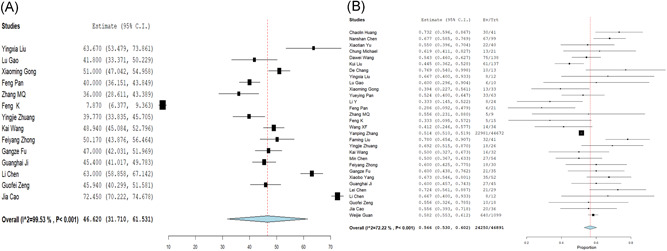
The forest plots of age and sex. A, age and (B) sex
Figure 3.

The forest plots of the incidence of comorbidities and intensive care unit (ICU). A, Comorbidities; (B) tumor; (C) diabetes; (D) hypertension; (E) cardiovascular disease; (F) phthisis; (G) chronic obstructive pulmonary disease; (H) chronic hepatonephropathy; (I) ICU
3.2.2. Clinical features
The incidence of fever was 87.3% (0.838‐0.909), that of cough was 58.1% (0.502‐0.660), that of sore throat was 12% (0.062‐0.177), that of expectoration was 29.4% (0.171‐0.417), that of chest distress was 31.2% (−0.024 to 0.648), that of muscle soreness or fatigue was 35.5% (0.253‐0.456), that of headache was 9.4% (0.063‐0.126), that of diarrhea was 6.8% (0.044‐0.092), and that of dyspnea was 38.3% (0.246‐0.520) (Table 3 and Figure 4).
Table 3.
Meta‐analysis results of the incidence of clinical manifestations
| Variable | N a | Estimate | 95% CI | N b | Standard error | P | T 2 | Q | P c | I 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | 27 | 0.873 | 0.838 to 0.909 | 1842 | 0.018 | <.001 | 0.006 | 177.086 | <.001 | 85.318 |
| Cough | 27 | 0.581 | 0.502 to 0.660 | 1354 | 0.040 | <.001 | 0.037 | 332.025 | <.001 | 92.169 |
| Sore throat | 9 | 0.120 | 0.062 to 0.177 | 200 | 0.029 | <.001 | 0.005 | 58.432 | <.001 | 86.309 |
| Expectoration | 10 | 0.294 | 0.171 to 0.417 | 466 | 0.063 | <.001 | 0.035 | 266.04 | <.001 | 96.617 |
| Chest distress | 5 | 0.312 | −0.024 to 0.648 | 38 | 0.172 | .069 | 0.144 | 204.480 | <.001 | 98.044 |
| Muscle soreness or fatigue | 18 | 0.355 | 0.253 to 0.456 | 781 | 0.052 | <.001 | 0.038 | 220.594 | <.001 | 92.747 |
| Headache | 14 | 0.094 | 0.063 to 0.126 | 214 | 0.016 | <.001 | 0.002 | 37.648 | <.001 | 65.47 |
| Diarrhea | 15 | 0.068 | 0.044 to 0.092 | 103 | 0.012 | <.001 | 0.001 | 32.263 | .004 | 56.607 |
| Dyspnea | 11 | 0.383 | 0.246 to 0.520 | 409 | 0.070 | <.001 | 0.051 | 351.966 | <.001 | 97.159 |
Abbreviation: CI, confidence interval.
Number of studies.
Number of patients.
Heterogeneity P value.
Figure 4.
The forest plots of the incidence of clinical features. A, Fever; (B) cough; (C) sore throat; (D) expectoration; (E) chest distress; (F) muscle soreness or fatigue; (G) headache; (H) diarrhea; (I) dyspnea
3.2.3. Laboratory tests
The laboratory findings showed leukocytosis in 11.0% (0.070‐0.150), leukopenia in 36.9% (0.146‐0.593), lymphocytopenia in 57.4% (0.410‐0.737), high C‐reactive protein (CRP) in 61.3% (0.451‐0.774), high lactate dehydrogenase (LDH) in 57.0% (0.360‐0.780), and high erythrocyte sedimentation rate (ESR) in 42.2% (0.076‐0.767) (Table 4 and Figure 5).
Table 4.
Meta‐analysis results of the incidence of laboratory tests
| Variable | N a | Estimate | 95% CI | N b | Standard error | P | T 2 | Q | P c | I 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocytosis | 13 | 0.110 | 0.070‐0.150 | 141 | 0.020 | <.001 | 0.003 | 115.035 | <.001 | 89.568 | |
| Leukopenia | 16 | 0.369 | 0.146‐0.593 | 541 | 0.114 | .001 | 0.204 | 5837.766 | <.001 | 99.743 | |
| Lymphocytopenia | 16 | 0.574 | 0.410‐0.737 | 1157 | 0.083 | <.001 | 0.105 | 1113.409 | <.001 | 98.653 | |
| High CRP | 15 | 0.613 | 0.451‐0.774 | 910 | 0.082 | <.001 | 0.089 | 564.423 | <.001 | 97.697 | |
| High LDH | 7 | 0.570 | 0.360‐0.780 | 477 | 0.107 | <.001 | 0.076 | 236.597 | <.001 | 97.464 | |
| High ESR | 5 | 0.422 | 0.076‐0.767 | 132 | 0.176 | .017 | 0.151 | 188.792 | <.001 | 97.881 |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase.
Number of studies.
Number of patients.
Heterogeneity P value.
Figure 5.

The forest plots of the incidence of laboratory test features. A, Leukocytosis; (B) leukopenia; (C) lymphocytopenia; (D) high C‐reactive protein; (E) high lactate dehydrogenase; (F) high erythrocyte sedimentation rate
3.2.4. Imaging features
At the chest computed tomography (CT), the pneumonia compromise was predominantly bilateral in 75.5% (0.639‐0.871) and unilateral 20.4% (0.106‐0.302). The most common patterns on chest CT were ground‐glass (69.9%, 0.602‐0.796), followed by irregular or halo sign (54.4%, 0.255‐0.833), air bronchogram (51.3%, 0.326‐0.701), bronchovascular bundle thickening (39.5%, 0.082‐0.708), grid‐form shadow (24.4%, 0.116‐0.371), and hydrothorax (18.5%, 0.001‐0.370) (Table 5 and Figure 6).
Table 5.
Meta‐analysis results of the incidence of chest imaging
| Variable | N a | Estimate | 95% CI | N b | Standard error | P | T 2 | Q | P c | I 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Unilateral | 19 | 0.204 | 0.106‐0.302 | 522 | 0.050 | <.001 | 0.043 | 751.641 | <.001 | 97.605 |
| Bilateral | 21 | 0.755 | 0.639‐0.871 | 1196 | 0.059 | <.001 | 0.068 | 1582.357 | <.001 | 98.736 |
| Lung consolidation | 9 | 0.369 | 0.215‐0.523 | 122 | 0.079 | <.001 | 0.050 | 96.579 | <.001 | 91.717 |
| Ground‐glass | 21 | 0.699 | 0.602‐0.796 | 1413 | 0.049 | <.001 | 0.047 | 1482.862 | <.001 | 98.651 |
| Air bronchogram | 6 | 0.513 | 0.326‐0.701 | 119 | 0.096 | <.001 | 0.048 | 49.183 | <.001 | 89.834 |
| Grid‐form shadow | 6 | 0.244 | 0.116‐0.371 | 64 | 0.065 | <.001 | 0.022 | 39.574 | <.001 | 87.365 |
| Bronchovascular bundles thickening | 4 | 0.395 | 0.082‐0.708 | 41 | 0.160 | .013 | 0.097 | 68.065 | <.001 | 95.592 |
| Hydrothorax | 7 | 0.185 | 0.001‐0.370 | 23 | 0.094 | .049 | 0.059 | 281.788 | <.001 | 97.871 |
| Irregular or halo sign | 5 | 0.544 | 0.255‐0.833 | 107 | 0.148 | <.001 | 0.104 | 105.731 | <.001 | 96.217 |
Abbreviation: CI, confidence interval.
Number of studies.
Number of patients.
Heterogeneity P value.
Figure 6.

The forest plots of the incidence of imaging features. A, Unilateral; (B) bilateral; (C) lung consolidation; (D) ground‐glass; (E) air bronchogram; (F) grid‐form shadow; (G) bronchovascular bundles thickening; (H) hydrothorax; (I) irregular or halo sign
3.2.5. Complications and outcomes
Among the infected patients, severe cases who required intensive care unit (ICU) were 29.3% (0.190‐0.395), and the incidence of acute respiratory distress syndrome (ARDS) was 28.8% (0.147‐0.429), that of acute cardiac injury was 14.1% (0.079‐0.204), that of acute renal injury was 7.1% (0.031‐0.110), that of shock was 4.7% (0.009‐0.086), that of multiple organ dysfunction syndrome (MODS) was 8.5% (−0.008 to 0.179), and the case fatality rate was 6.8% (0.044‐0.093) (Table 6 and Figure 7).
Table 6.
Meta‐analysis results of the incidence of complications
| Variable | N a | Estimate | 95% CI | N b | Standard error | P | T 2 | Q | P c | I 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| ARDS | 8 | 0.288 | 0.147 to 0.429 | 160 | 0.072 | <.001 | 0.037 | 195.606 | <.001 | 96.421 |
| ACI | 7 | 0.141 | 0.079 to 0.204 | 151 | 0.032 | <.001 | 0.005 | 49.732 | <.001 | 87.935 |
| ARI | 8 | 0.071 | 0.031 to 0.110 | 58 | 0.02 | <.001 | 0.002 | 36.801 | <.001 | 80.979 |
| Shock | 5 | 0.047 | 0.009 to 0.086 | 31 | 0.020 | .016 | 0.001 | 15.319 | .004 | 73.889 |
| MODS | 4 | 0.085 | −0.008 to 0.179 | 9 | 0.048 | .074 | 0.004 | 5.050 | .080 | 60.392 |
| Mortality | 8 | 0.068 | 0.044 to 0.093 | 1111 | 0.012 | <.001 | 0.001 | 110.944 | <.001 | 93.69 |
Abbreviations: ACI, acute cardiac injury; ARDS, acute respiratory distress syndrome; ARI, acute renal injury; CI, confidence interval; MODS, multiple organ dysfunction syndrome.
Number of studies.
Number of patients.
Heterogeneity P value.
Figure 7.
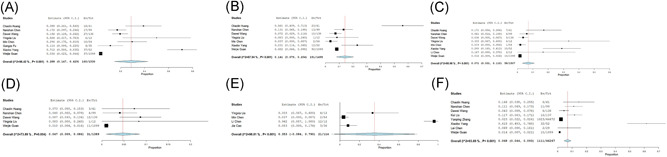
The forest plots of the incidence of complication. A, acute respiratory distress syndrome; (B) acute cardiac injury; (C) acute renal injury; (D) shock; (E) multiple organ dysfunction syndrome; (F) mortality
4. DISCUSSION
The results of this study showed that fever (87.3%) and cough (58.1%) were the main clinical manifestations in the patients with NCP in China. This was followed by dyspnea (38.3%), myalgia or weakness (35.5%), and chest tightness (31.2%), and some patients also presented other clinical symptoms such as chills, cough, conjunctival discomfort, headache, shortness of breath, and joint pain. A few patients had nausea, vomiting, diarrhea, and other abdominal discomfort symptoms, whereas very few patients showed hemoptysis symptoms. Most patients with NCP required hospitalization, of which 29.3% required intensive care. The main complications are respiratory failure, ARDS (28.8%) and multiple organ failure (8.5%), and heart failure, shock, renal injury, sepsis, striated muscle lysis, and diffuse intravascular coagulation are rare. According to the severity, the patients with NCP can be divided into mild, normal type (80%), medium type, and severe type (13.8%). The clinical manifestations of patients with different severity vary greatly. According to statistics, the fatality rate in China is about 3.8%, 37 lower than that of SARS (9.6%) and MERS (35%). The main causes of death are massive alveolar damage and progressive respiratory failure. Generally, viral pneumonia mainly involves pulmonary interstitium, producing pulmonary interstitial fibrosis. The autopsy report of the first NCP patient in China found that coronavirus disease 2019 (COVID‐19) mainly caused the inflammatory response characterized by deep airway and alveolar damage, accompanied by a large amount of viscous secretions in the airway. The pulmonary transparent membrane became less obvious, and the degree of fibrosis was not as severe as SARS. 38 However, the degree of effect of COVID‐19 on pulmonary fibrosis still needs to be paid close attention, which is also an important factor influencing pulmonary function in the prognosis of patients with NCP.
In this meta‐analysis, white blood cells were normal or decreased in most patients, lymphocytes were mostly decreased, and CRP, LDH, ESR level was elevated in some patients. A few patients had elevated creatine kinase procalcitonin bilirubin, whereas some had decreased albumin and elevated ALT, AST. The pathological results of patients with SARS‐COV‐2 suggested that the excessive activation of T lymphocytes, which is characterized by increased Th17 cells and high toxicity of CD8+ T cells, has caused severe immune damage to a certain extent. 4 This may be the main reason for the loss of lymphocytes in patients. Sequence comparison analysis showed that the S spike protein of SARS‐COV‐2 contains a SARS‐CoV‐like receptor binding domain, which indicates that ACE2 may be the main receptor of SARS‐COV‐2. 3 ACE2 was highly expressed in gastric and testicular epithelial cells, and also enriched in colon, heart, kidney, and so on. Over‐expressed ACE2 may be related to the elevated liver enzyme. 39 The similarity of SARS‐COV‐2 and SARS‐CoV gene sequences suggests that the mechanism of action may also be similar. SARS‐COV‐2 enters host cells through dense S protein, 40 , 41 acts on bronchial epithelial cells through ACE2 receptor, and then infects other cells, causing a series of immune responses or inflammatory cytokine storm in severe cases. In addition, the sequence alignment showed that the SARS‐COV‐2 and SARS‐CoV S2 subunits are highly conserved, and the overall identity in the HR1 and HR2 domains is 92.6% and 100%, respectively. This suggested that novel coronary pneumonia drugs research may base on this site. 42
In imaging results, this meta‐analysis showed that 75.7% of the patients had lesions involving both lungs, and 69.9% showed ground‐glass shadows on imaging, mostly interstitial pulmonary lesions. Chest CT showed consolidation shadow nodular or patchy shadow in some patients, whereas there also existed other characteristics in few patients, such as chest‐shaped shadows, thick cord‐like shadows, pleural reactions, thickened blood vessels, pleural effusion, and bronchial inflation, subpleural line, halo sign, antihalo sign, mosaic sign, and so on. The course of the critically ill patient progressed rapidly, and chest CT could cause “white lung” changes within a few days. Because the sensitivity of nucleic acid test is closely related to the detection sample and testing the sample of lower respiratory tract is more sensitive, 43 nucleic acid test shows partial false negative result. Chest CT examination, as an important examination method for NCP, is highly sensitive to SARS‐COV‐2 (even up to 97% in epidemic areas) and is an important supplement to nucleic acid detection. 44 In patients with negative nucleic acid test reports, chest CT results are still of high auxiliary diagnostic value. In addition, imaging manifestations of patients also show dynamic evolution in the course of disease progression.
Current research showed that COVID‐19, which source may be Chinese chrysanthemum head bats and pangolin may be a potential intermediate host, can cause a zoonotic disease. 3 Since late February 2020, the number of confirmed cases of NCP abroad has increased rapidly, which may indicate a pandemic. The “three early” principle (early detection, early diagnosis, and early treatment) followed by disease prevention and treatment is particularly important in the prevention and treatment of SARS‐COV‐2. In addition, the clinical manifestations of patients with neocoronary pneumonia are diverse and the atypical symptoms also account for part of the proportion. Therefore, we systematically analyzed the clinical manifestations and auxiliary examination results of patients with COVID‐19, so as to reflect the disease characteristics more comprehensively, increase the discrimination of the disease, and strive for early diagnosis, early isolation, and early treatment.
The number of newly diagnosed cases of NCP has been rising worldwide recently, especially in South Korea, Italy, Iran, and Japan. To control the further spread of the epidemic, it is still necessary to strictly follow the management measures for the prevention and treatment of infectious diseases and follow the WHO declaration on public health emergencies of international concern. Certainly, prevention of imported cases is also extremely important. 45 Particularly, in some densely populated markets, stations, large ports, and other places, protective deployment measures should be strengthened to ensure that protective equipment, drugs, medical supplies, and so on are sufficient. 46 National public health capabilities and infrastructure remain at the core of global health security, as they are the first line of defense for infectious disease emergencies. 47 The International Health Organization, all countries, and all humanity need to pay great attention to SARS‐COV‐2.
This meta‐analysis, with large enough sample size, relatively high literature quality, and more comprehensive analysis, included a total of 31 literature studies, including 46 959 patients with NCP. The conclusions are very credible to some extent. This article still has the following limitations, for example, (a) the samples are domestic cases, without foreign cases; (b) different data sources may lead to some bias in the results; and (c) there exists some publication bias. Therefore, the conclusions of this article need to be further verified.
5. CONCLUSION
COVID‐19 is a new clinical infectious disease, which mainly causes bilateral pneumonia and lung function deteriorates rapidly. Nearly a third of patients need to be admitted to the ICU, and patients are likely to present respiratory failure or even death.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
We are grateful to all the medical staff who worked on the front line to fight the epidemic and even gave their precious lives. This study was supported by grants from the Science and Technology Department of Hubei Province (No. 2018CFC884); free innovation pre‐research fund and platform scientific research fund in 2019 (02.03.2019‐111).
Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: A systematic review and meta‐analysis. J Med Virol. 2020;92:1449–1459. 10.1002/jmv.25822
Yinghao Cao and Xiaoling Liu contributed equally to this study.
Contributor Information
Lijuan Xiong, Email: lijuanxiong2016@126.com.
Kailin Cai, Email: caikailin@hust.edu.cn.
REFERENCES
- 1. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Institute of Health Economics . Quality Appraisal of Case Series Studies Checklist; 2014.
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology. 2020;295(1):202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;233(3):104‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu K, Fang Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;3(4):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng YY, Ma X, Wang HY, et al. Computed tomography features of patients with novel coronavirus pneumonia. Shanghai Med J. 2020;43(2):1‐10. [Google Scholar]
- 13. Liu YX, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury (Chinese). Sci China Life Sci. 2020;50(3):258‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao L, Zhang JP, Du YH, et al. CT features of patients with imported 2019‐nCoV‐pneumonia. J Xi'an Jiaotong Univ (Med Sci) 2020;41(2):187. [Google Scholar]
- 15. Gong XM, Li H, Song L, et al. Preliminary explore on CT characteristics of coronavirus disease 2019 (COVID‐19). Radiol Prac. 2020;35(3):261‐265. [Google Scholar]
- 16. Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019‐nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;133(21):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Zhang D, Tang S, et al. The epidemiological and clinical characteristics of 2019 novel coronavirus infection in Changsha, China. Preprint Lancet. 2020;50(2):342‐346. [Google Scholar]
- 18. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology. 2020;35(1):200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang MQ, Wang XH, Chen YL, et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua Jiehe He Huxi Zazhi. 2020;43(0):E013. [DOI] [PubMed] [Google Scholar]
- 20. Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Chin J Pediatr. 2020;58(3):E007. [DOI] [PubMed] [Google Scholar]
- 21. Wang XF, Yuan J, Zheng YJ, et al. Retracted: Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Chin J Pediatr. 2020;58(1):E008. [DOI] [PubMed] [Google Scholar]
- 22. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. J. Chin Epi. 2020;41(2):145‐151. [PMC free article] [PubMed] [Google Scholar]
- 23. Liu FM, Ding HL, Gong XM, et al. Chest CT performance and clinical characteristics of coronavirus disease 2019 (COVID‐19). Radiol Prac. 2020;35(3):266‐268. [Google Scholar]
- 24. Zhuang YJ, Chen Z, Li J. Clinical and epidemiological characteristics of 26 patients dia gnosed with novel coronavirus pneumonia. Chin J Nosocomiology. 2020;23(6):826‐829. [Google Scholar]
- 25.Wang K, Kang SR, Tian RH, et al. CT characteristic appearances of patients with novel coronavirus pneumonia. J Clin Med. 2020;1(27):27‐31.
- 26. Chen M, An W, Xia F, et al. Retrospective analysis of COVID‐19 patients with different clinical subtypes. Herald Med. 2020;41(5):1‐12. [Google Scholar]
- 27. Zhong FY, Zhang HF, Wang BC, et al. CT findings in 2019 novel coronavirus disease (COVID‐19) patients. Med J Wuhan Univ. 2020;31(1):1‐5. [Google Scholar]
- 28. Fu GZ, Xu CY, Sun HC, et al. Application of chest CT examination in screening of patients with novel coronavirus pneumonia. Med J Wenzhou Univ. 2020;20(2):1‐9. [Google Scholar]
- 29. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(4):E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ji GH, Huang MH, Zhang Q, et al. CT manifestations and dynamic changes of coronavirus disease 2019. Chin J Med Imaging Technol. 2020;36(2):242‐247. [Google Scholar]
- 31. Chen L, Feng SY, Wang FX, et al. Clinical diagnosis and treatment of critical patients with novel coronavirus pneumonia (report of 12 cases). J Clin Med. 2020;27(1):32‐35. [Google Scholar]
- 32. Zeng GF, Yang HP, Zhang XY, et al. Analysis of clinical and imaging features of novel coronavirus pneumonia in Chongqing traditional Chinese medicine system. J Emerg Tradit Chin Med. 2020;29(3):377‐380. [Google Scholar]
- 33. Cao J, Zhou J, Liao XN, et al. Clinical characteristics and CT signs of coronavirus disease 2019 (COVID‐19) in the elderly. Med J Wuhan Univ. 2020;4(2):1‐4. [Google Scholar]
- 34. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;213(42):543‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu XT, Ye H, Yang SF, et al. Chest CT features of COVID‐19. J Pract Med. 2020;2(3):1‐3. [Google Scholar]
- 36. Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin J Tuberc Respir Dis. 2020;43(0):E005. [DOI] [PubMed] [Google Scholar]
- 37. Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine . An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID‐19). J. Chin Epi. 2020;41(2):139. [Google Scholar]
- 38. Liu Q, Wang RS, Qu GQ, et al. Systematic solution of new coronavirus pneumonia in dead cadavers. J Forensic Sci. 2020;36(1):19‐21. [Google Scholar]
- 39. Guan GW, Gao L, Wang JW, et al. Study on the mechanism of liver enzyme abnormality in pneumonia infected by novel coronavirus. J Hepatol. 2020;28(2):E002. [DOI] [PubMed] [Google Scholar]
- 40. Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3(1):237‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bj B, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801‐8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chu DKW, Pan Y, Cheng SMS, et al. Molecular diagnosis of a novel coronavirus (2019‐nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020;24(3):200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boldog P, Tekeli T, Vizi Z, Dénes A, Bartha FA, Röst G. Risk assessment of novel coronavirus COVID‐19 outbreaks outside China. J Clin Med. 2020;9(2):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. The Lancet. 2020;395(10225):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sands P, Mundaca‐Shah C, Dzau VJ. The neglected dimension of global security—a framework for countering infectious‐disease crises. N Engl J Med. 2016;374(13):1281‐1287. [DOI] [PubMed] [Google Scholar]


