Abstract
The rapid spread of the epidemic has aroused widespread concern in the international community. Severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2) was first reported in China, with bats as the likely original hosts and pangolins as potential intermediate hosts. The current source of the disease is mainly patients infected with SARS‐COV‐2. Patients in the incubation period may also become sources of infection. The virus is mainly transmitted via respiratory droplets and contact, and the population is generally susceptible. The epidemic has progressed through the local outbreak stage and community transmission stage due to exposure at Wuhan's Huanan wholesale seafood market and is now in the stage of large‐scale transmission due to the spread of the epidemic. The basic productive number (R0) at the beginning of the epidemic was 2.2, with an average incubation period of 5.2 days. The proportion of critically ill patients was 23.4%, the mortality rate was lower than those of SARS and Middle East respiratory syndrome, and 96.5% of deaths occurred in Hubei Province, where the outbreak occurred first. Among them, elderly men with underlying diseases had a higher mortality rate. Chinese medical staff have summarized a set of effective strategies and methods in the diagnosis and treatment of this disease that are worthy of reference for their international counterparts. With powerful government intervention and the efforts of Chinese medical staff, China's outbreak has gradually improved.
Keywords: COVID‐19, epidemic, mortality, treatment, virus pneumonia
Research Highlights
The current source of the disease is mainly patients infected with SARS‐COV‐2. Patients in the incubation period may also become sources of infection.
The virus is mainly transmitted via respiratory droplets and contact, and the population is generally susceptible.
The proportion of critically ill patients was 23.4%, the mortality rate was lower than those of SARS and Middle East respiratory syndrome. Among them, elderly men with underlying diseases had a higher mortality rate.
1. INTRODUCTION
Since December 2019, patients with unexplained pneumonia have been found in Wuhan, Hubei Province, China. 1 On 7 January 2020, Chinese authorities confirmed that the cause was a novel coronavirus that had not been previously identified, different from other coronaviruses, such as Middle East respiratory syndrome coronavirus (MERS‐CoV) in 2012 to 2015 and severe acute respiratory syndrome coronavirus (SARS‐CoV) in 2003. On 12 January 2020, the World Health Organization (WHO) temporarily named this new virus 2019 novel coronavirus (2019‐nCoV). With the spread of the disease, novel coronavirus cases have been found in other parts of China and other countries in the world. As an ongoing coronavirus outbreak, the Chinese government has paid great attention to this situation. On 21 January 2020, the National Health Commission of the People's Republic of China announced 2019‐nCoV pneumonia as a category B infectious disease, and preventive and control measures were taken according to category A infectious disease.
On 30 January 2020, the WHO declared that the 2019‐nCoV epidemic constitutes a Public Health Emergency of International Concern. Soon after that, the novel coronavirus‐infected pneumonia was officially named "COVID‐19" (coronavirus disease 2019) by the WHO on 11 February 2020. At the same time, the International Virus Classification Commission announced that the novel coronavirus was named "SARS‐CoV‐2." With the development of pathogenic testing and the continuous accumulation of clinical cases, people have acquired a certain degree of knowledge and experience in the diagnosis and treatment of the disease. This article presents the currently known epidemiological characteristics, clinical manifestations, laboratory findings, and experiences in the prevention and treatment of COVID‐19, providing references for the prevention and treatment of this disease.
2. SOURCE OF INFECTION
SARS‐CoV‐2 is the seventh type of coronavirus isolated in humans. This coronavirus belongs to the genus β and has an envelope; its particles are round or oval, often polymorphic, and 60 to 140 nm in diameter. The genetic characteristics of SARS‐CoV‐2 are significantly different from those of SARS‐CoV and MERS‐CoV. Current research shows that SARS‐CoV‐2 has more than 85% homology with bat SARS‐like coronavirus (Bat‐SL‐CoV ZC45). 2 Therefore, it is now believed that the original host of SARS‐CoV‐2 may have been Rhinolophus sinicus. 3 A study team from South China Agricultural University pointed out that pangolins may be one of the intermediate hosts of SARS‐CoV‐2, through which the virus may spread to humans. Relevant research teams are being organized by the Chinese government to further support this view. The outbreak is considered to be closely related to Wuhan's Huanan wholesale seafood market. 4 , 5 , 6 , 7 Epidemiological surveys have shown that 27 of 41 people with the earliest onset have visited the market. 8 Post hoc investigations also revealed that there was a wildlife trade in this market, and SARS‐CoV‐2 has also been isolated from the market's environment. Human‐to‐human transmission emerged shortly thereafter. 9 Patients with COVID‐19 have become the main source of infection. COVID‐19 may be capable of transmission during the incubation period. 10 , 11 These patients are difficult to diagnose and isolate in time, which causes great difficulties in the prevention and control of the disease. However, up to date, there is no enough evidence to support the role of asymptomatic patients in the transmission of COVID‐19. In addition, the presence of the virus can be detected in patients during the recovery stage, especially in fecal specimens requiring longer detoxification times, indicating the possible risk of fecal‐oral transmission. 11 SARS‐CoV‐2 is a positive‐stranded RNA virus that is susceptible to gene mutation and recombination, but so far, no significant mutations have been found in humans or the environment. 2 , 12
3. METHOD OF SPREAD
At present, droplets inhaled into the respiratory tract and contact transmission are considered to be the most important routes of transmission. There is no direct evidence showing that COVID‐19 can be transmitted through aerosols. The WHO believes that further evidence is needed to assess the possibility of aerosol transmission. However, some of the observations suggest that aerosol propagation is possible under the condition of long exposure to high concentrations of aerosols in a relatively closed environment. In the early stages of the outbreak of SARS‐CoV‐2 pneumonia, it is uncertain whether stool samples from patients are infectious. However, Lu et al, 2 using a computer structural simulation, found that the S‐protein of SARS‐CoV‐2 can bind to human angiotensin‐converting enzyme 2 (ACE2) in the spatial conformation. After that, Letko et al 13 synthesized the S‐protein of SARS and the receptor‐binding domain (RBD) of SARS‐CoV‐2 in vitro and reacted with known human coronavirus receptors, respectively. As a result, the RBD of SARS‐CoV‐2 is similar to the S protein in SARS, and it can directly bind to human ACE2. ACE2 is a metalloproteinase belonging to a type I transmembrane glycoprotein, and it can be expressed in the small intestine. 14 , 15 In our laboratory, it was also found that nucleic acid amplification testing of stool samples can still be positive for a long time after oral swab samples become negative. In addition, a large number of clinical studies have shown that a certain proportion of SARS‐CoV‐2 pneumonia patients develop symptoms of diarrhea. 10 , 16 It can be concluded that during the development of the disease, there is a greater possibility of the virus in the stool. Recently, SARS‐CoV‐2 has been isolated from the stool of confirmed patients in Wuhan and Shenzhen, China, and in the United States, 17 indicating that the virus can replicate and survive in the digestive tract. However, it is not clear whether the infection and transmission are caused by eating food contaminated with the virus. Through the analysis of the current epidemic situation, some scholars believe that viruses in feces may be retransmitted by the aerosol formation of virus‐containing droplets. SARS‐CoV‐2 also has adverse effects on fetuses and infants, including restricted intrauterine growth, premature birth, spontaneous abortion, and perinatal death. 18 However, the possibility of intrauterine mother‐to‐child transmission of COVID‐19 is very low, and neither of the two existing retrospective clinical studies have found direct evidence of intrauterine vertical transmission. 19 , 20
4. SUSCEPTIBLE INDIVIDUALS
COVID‐19 is an emerging infectious disease, so the population is not immune and is generally susceptible. A report 16 delineated 1099 patients with SARS‐CoV‐2 pneumonia from 552 hospitals in 31 provinces/province‐level municipalities of China and found that the median age was 47.0 years old (interquartile range [IQR]: 35.0‐58.0) and that 41.9% of patients were females. SARS‐CoV‐2 pneumonia was diagnosed throughout the whole spectrum of age. A total of 0.9% of patients were aged below 15 years old. Another Chinese analysis of 4021 confirmed cases showed that people of all ages were generally susceptible. The study found that 71.45% of patients were 30 to 65 years old, and children under 10 years old accounted for 0.35%. 21 Those in close contact with patients with SARS‐CoV‐2 pneumonia, including the family members of patients, are at high risk for developing the SARS‐CoV‐2 infection. Medical staff who treat patients also have a high risk of infection. Nine percent of the patients admitted to Zhongnan Hospital of Wuhan University from 1 to 28 January 2020, were the medical staff. 22 By the end of 29 January 2020, survey data from 552 hospitals in 31 provinces in China showed that the infection rate of medical staff was as high as 2.09%. 16
5. MORTALITY
According to a WHO report, as of 23 March 2020, the world has reported 332 930 confirmed cases of COVID‐19, including 81 601 cases in China and 251 329 cases in other countries outside China. The average mortality rate in China was 4.0% (3276/81 601), while the average mortality rate outside China was 4.5% (11 233/251 329). Hubei Province, the worst‐affected province in China, had an average mortality rate of 4.7% (3160/67 801). The area in Hubei Province with higher‐than‐average mortality rates in Hubei Province was Wuhan (5.0%, 2524/50 006).
The mortality rates in other severely affected provinces in China were relatively lower than those in Hubei Province, with mortality rates of 0.6% (8/1428) in Guangdong, 1.7% (22/1274) in Henan, 0.1% (1/1240) in Zhejiang, and 0.4% (4/1018) in Hunan. The above results are shown in Figures 1, 2, 3. According to the available data, the mortality rate of COVID‐19 in China is lower than those of SARS (10%) 23 and MERS (40%). 24 By 23 March 2020, 96.5% (3160/3276) of the deaths caused by SARS‐CoV‐2 pneumonia in China was in Hubei Province, and the earlier the onset of disease was, the higher the mortality rate, suggesting that the pathogenicity may gradually decrease as the virus spreads, which may also occur because of the improving ability of medical staff to diagnose and treat the disease. The National Health Commission of China notified on 4 February 2020, that the population is generally susceptible to the virus, and more than 80% of the deaths are elderly people over 60 years old; at the same time, more than 75% of the deaths have underlying diseases. Overall, men are linked to a higher risk of death. The specific data in this section are shown in Table 1.
Figure 1.

Distribution of coronavirus disease 2019 cases as of 23 March 2020 worldwide. The boundaries and names are shown and the designations used on this map do not imply the expression of any opinion whatever on the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. *“Confirmed” cases reported between 13 and 19 February 2020 include both laboratory‐confirmed and clinically diagnosed (only applicable to Hubei province); for all other dates, only laboratory‐confirmed cases are shown. &712 cases are identified on a cruise ship currently in Japanese territorial waters. Data source: World Health Organization
Figure 2.
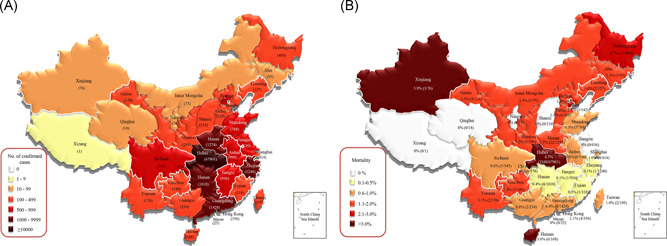
Distribution of coronavirus disease 2019 cases as of 23 March 2020 in China. *“Confirmed” cases reported between 13 and 19 February 2020 include both laboratory‐confirmed and clinically diagnosed (only applicable to Hubei province); for all other dates, only laboratory‐confirmed cases are shown. Data source: World Health Organization
Figure 3.

Distribution of coronavirus disease 2019 cases as of 23 March 2020 in Hubei, China. Date source: as reported by China
Table 1.
Clinical features of adult novel coronavirus pneumonia
| Wuhan | Wuhan | Wuhan | Wuhan | Hubei | Beijing | Wuhan | Nationwide | |
|---|---|---|---|---|---|---|---|---|
| (n = 41) 8 | (n = 99) 6 | (n = 138) 22 | (n = 29) 25 | (n = 137) 26 | (n = 61) 27 | (n = 141) 28 | (n = 1099) 16 | |
| Definite diagnosis time | 16 December 2019 to 2 January 2020 | 1 January 2020 to 20 January 2020 | 1 January 2020 to 28 January 2020 | 14 January 2020 to 29 January 2020 | 30 December 2019 to 24 January 2020 | 13 January 2020 to 31 January 2020 | 20 January 2020 to 28 January 2020 | 1 December 2019 to 29 January 2020 |
| Huanan seafood market exposure | 27 (65.9%) | 49 (49.5%) | 12 (8.7%) | 2 (6.9%) | 0 (0.0%) | NA | NA | NA |
| Characteristics | ||||||||
| Age | 49 (41‐58) | 56 (21‐82) | 56 (42‐68) | 56 (26‐79) | 57 (20‐83) | 40 (1‐86) | 49 (9‐87) | 47 (35‐58) |
| Sex | ||||||||
| Male | 30 (73.2%) | 67 (67.7%) | 75 (54.3%) | 21 (72.4%) | 61 (44.5%) | 31 (50.8%) | 77 (54.6%) | 640 (58.2%) |
| Female | 11 (26.8%) | 32 (32.3%) | 63 (45.7%) | 8 (27.6%) | 76 (55.5%) | 30 (49.2%) | 64 (45.4%) | 459 (41.8%) |
| Underlying disease | 13 (31.7%) | 50 (50.5%) | 64 (46.4%) | 16 (55.2%) | 27 (19.7%) | NA | 44 (31.2%) | 255 (23.2%) |
| Hypertension | 6 (14.6%) | NA | 43 (31.2%) | 8 (27.6%) | 13 (9.5%) | 12 (19.7%) | 27 (19.2%) | 164 (14.9%) |
| Cardiovascular disease | 6 (14.6%) | NA | 20 (14.5%) | NA | 10 (7.3%) | 1 (1.6%) | 21 (14.9%) | 27 (2.5%) |
| Cerebrovascular disease | NA | NA | 7 (5.1%) | NA | NA | NA | NA | 15 (1.4%) |
| Chronic obstructive pulmonary disease | 1 (2.4%) | NA | 4 (2.9%) | NA | 2 (1.5%) | 5 (8.2%) | NA | 12 (1.1%) |
| Diabetes | 8 (19.5%) | NA | 14 (10.1%) | 5 (17.2%) | 14 (10.2%) | 5 (8.2%) | 25 (17.7%) | 81 (7.4%) |
| Malignancy | 1 (2.4%) | 1 (1.0%) | 10 (7.2%) | 1 (3.4%) | 2 (1.5%) | NA | NA | 10 (0.9%) |
| Chronic kidney disease | NA | NA | 4 (2.9%) | NA | NA | NA | NA | 8 (0.7%) |
| Chronic liver disease | 1 (2.0%) | NA | 4 (2.9%) | 2 (6.9%) | NA | NA | NA | NA |
| Clinical features | ||||||||
| Fever | 40 (97.6%) | 82 (82.8%) | 136 (98.6%) | 28 (96.6%) | 112 (81.8%) | 60 (98.4%) | 139 (98.6%) | 966 (87.9%) |
| Cough | 31 (75.6%) | 81 (81.8%) | 82 (59.4%) | 21 (72.4%) | 66 (48.2%) | 39 (63.9%) | 106 (75.2%) | 744 (67.7%) |
| Myalgia or fatigue | 18 (43.9%) | NA | 96 (69.6%) | 12 (41.4%) | 44 (32.1%) | 35 (57.4%) | NA | 582 (53.0%) |
| Expectoration | 11/39 (28.2%) | NA | 37 (26.8%) | NA | 6 (4.4%) | 27 (44.3%) | 41 (29.1%) | 367 (33.4%) |
| Headache | 3/38 (7.9%) | 8 (8.1%) | 9 (6.5%) | 2 (6.9%) | 13 (9.5%) | 21 (34.4%) | 11 (7.8%) | 150 (13.6%) |
| Diarrhea | 1/38 (2.6%) | 2 (2.0%) | 14 (10.1%) | 4 (13.8%) | 11 (8.0%) | 6 (9.8%) | 4 (2.8%) | 41 (3.7%) |
| Dyspnea | 22/40 (55.0%) | 31 (31.3%) | 43 (31.2%) | 17 (58.6%) | 26 (19.0%) | 3 (4.9%) | NA | 204 (18.6%) |
| Muscle ache | NA | 11 (11.1%) | NA | 12 (41.4%) | NA | NA | NA | NA |
| Pharyngalgia | NA | 5 (5.1%) | 24 (17.4%) | NA | NA | NA | NA | 153 (13.9%) |
| Nausea and vomiting | NA | 1 (1.0%) | 19 (13.8%) | NA | NA | 5 (8.2%) | NA | 55 (5.0%) |
| Laboratory test | ||||||||
| White blood cell count | ||||||||
| Decreased | 10/40 (25.0%) | 9 (9.1%) | NA | 6 (20.7%) | 51 (37.2%) | NA | 38 (27.0%) | 330/978 (33.7%) |
| Increased | 12/40 (30.0%) | 24 (24.2%) | NA | 6 (20.7%) | 26 (19.0%) | NA | NA | 58/978 (5.9%) |
| Lymphocyte count | ||||||||
| Decreased | 26 (63.4%) | 35 (35.4%) | NA | 20 (69.0%) | 99 (72.3%) | NA | 71 (50.4%) | 731/890 (82.1%) |
| Platelets count | ||||||||
| Decreased | 2/40 (5.0%) | 12 (12.1%) | NA | 5 (17.2%) | NA | NA | NA | 315/869 (36.2%) |
| D‐dimer | ||||||||
| Increased | NA | 36 (36.4%) | NA | NA | NA | NA | NA | 260/560 (46.4%) |
| C‐reactive protein level | ||||||||
| Increased | NA | 63/73 (86.3%) | NA | 27 (93.1%) | 115 (83.9%) | NA | NA | 481/793 (60.7%) |
| Procalcitonin level | ||||||||
| Increased | NA | 6 (6.1%) | 49 (35.5%) | NA | NA | NA | NA | 35/633 (5.5%) |
| Albumin | ||||||||
| Decreased | NA | 97 (98.0%) | NA | 15 (51.7%) | NA | NA | NA | NA |
| Aspartate aminotransferase | ||||||||
| Increased | 15 (36.6%) | 35 (35.4%) | NA | 7 (24.1%) | NA | NA | NA | 168/757 (22.2%) |
| Alanine aminotransferase | ||||||||
| Increased | NA | 28 (28.3%) | NA | 5 (17.2%) | NA | NA | NA | 158/741 (21.3%) |
| Lactate dehydrogenase | ||||||||
| Increased | 29/40 (72.5%) | 75 (75.8%) | NA | 20 (69.0%) | NA | NA | NA | 277/675 (41.0%) |
| Creatine kinase | ||||||||
| Increased | 13/40 (32.5%) | 13 (13.1%) | NA | NA | NA | NA | NA | 90/657 (13.7%) |
| Creatinine | ||||||||
| Increased | 4/41 (9.8%) | 6 (6.1%) | NA | 2 (6.9%) | NA | NA | NA | 12/752 (1.6%) |
| Abnormalities on chest CT or X‐ray | NA | NA | 138 (100.0%) | NA | NA | NA | 141 (100.0%) | 840 (76.4%) |
| Location | ||||||||
| Unilateral pneumonia | NA | 25 (25.3%) | NA | NA | NA | NA | 35 (24.8%) | NA |
| Bilateral pneumonia | 40 (97.6%) | 74 (74.7%) | 138 (100.0%) | NA | 116 (84.7%) | 48 (78.7%) | 106 (75.2%) | 505 (46.0%) |
| Severity | ||||||||
| Multiple patch‐ like shadows | NA | 14 (14.1%) | NA | NA | 36 (31.0%) | NA | 71 (50.4%) | 409 (37.2%) |
| Ground‐glass opacity in both lungs | NA | NA | NA | 55 (47.4%) | NA | 47 (33.3%) | 550 (50.0%) | |
| Consolidation shadow | NA | NA | NA | NA | 25 (21.6%) | NA | 23 (16.3%) | NA |
| Complications | NA | 33 (33.3%) | NA | NA | NA | NA | NA | NA |
| Septic shock | 3 (7.3%) | 4 (4.0%) | 12 (8.7%) | NA | NA | NA | NA | 11 (1.0%) |
| Acute respiratory distress syndrome | 12 (29.3%) | 17 (17.2%) | 27 (19.6%) | NA | NA | NA | NA | 37 (3.4%) |
| Acute cardiac injury | 5 (12.2%) | NA | 10 (7.2%) | NA | NA | NA | NA | NA |
| Acute kidney injury | 3 (7.3%) | 3 (3.0%) | 5 (3.6%) | NA | NA | NA | NA | 6 (0.5%) |
| Prognosis | ||||||||
| Discharged | 28 (68.3%) | 31 (31.3%) | NA | NA | 44 (32.1%) | 3 (4.9%) | NA | 55 (5.0%) |
| Death | 6 (14.6%) | 11 (11.1%) | NA | 2 (6.9%) | 16 (11.7%) | NA | NA | 15 (1.4%) |
Abbreviations: CT, computed tomography; NA, not available.
A single‐centered, retrospective, observational study from one hospital of Wuhan 29 found among 52 critically ill patients with SARS‐CoV‐2 infection, 32 (61·5%) patients had died at 28 days, and the median duration from intensive care unit admission to death was 7 (IQR: 3‐11) days in the nonsurvivors. Compared with survivors, nonsurvivors were more likely to develop acute respiratory distress syndrome (ARDS) (26 [81%] vs 9 [45%]) and were more likely to receive mechanical ventilation (30 [94%] vs 7 [35%]). A total of 30 (81%) of 37 patients requiring mechanical ventilation had died by 28 days.
According to the data, 30 by 11 February 2020, among the 422 medical institutions involved in the diagnosis and treatment of SARS‐CoV‐2 pneumonia in China, a total of 1716 medical workers were diagnosed with confirmed SARS‐CoV‐2 pneumonia. Among the 1688 confirmed cases with severity grading data, 87.3% (1474/1688) of the infected medical staff were in Hubei Province, and 10.4% of the infected medical staff were seriously ill. A total of 64.0% (1080/1688) of new coronavirus infections among medical workers occurred in Wuhan, and the proportion of critically ill patients was 17.7%. Medical workers in other parts of China accounted for 12.7% (214/1688), with a proportion of critically ill patients of 7.0%. The proportion of critically ill patients among medical workers in Wuhan has gradually decreased from a peak of 38.9% to 12.7% by early February 2020. The average mortality rate for the 1688 diagnosed medical staff was 0.3%.
6. EPIDEMIOLOGICAL CHARACTERISTICS
The spread of SARS‐CoV‐2 pneumonia in China can be roughly divided into three stages: 55% of the patients who developed symptoms before the end of December 2019 are related to the exposure at Wuhan's Huanan wholesale seafood market; therefore, the first stage of epidemic transmission mainly occurred in a local outbreak among people with a direct contact history at the seafood market. Only 8.6% of the patients who became ill after 1 January 2020, had a history of exposure to Wuhan's Huanan wholesale seafood market, suggesting that the epidemic of SARS‐CoV‐2 pneumonia had now shifted to the second stage: the community dissemination stage. 31 Interpersonal and cluster transmission occurred in multiple communities and families in Wuhan during this period. 4 , 5 , 6 , 7 , 10
25 January 2020 was Chinese New Year, also known as the Spring Festival, which is the most important festival in China. With the festival approaching, a large number of migrant workers and people new to Wuhan began to leave Wuhan to travel to their hometowns in other provinces in China for family reunions or to start a vacation in other countries. With the movement of people, the epidemic quickly spread to other parts of China and other countries around the world and reached the stage of large‐scale transmission, causing widespread concern in the international community. 32 , 33
A retrospective study of 425 patients with SARS‐CoV‐2 pneumonia at the beginning of the epidemic before 22 January 2020, found that the average incubation period of the disease was 5.2 days (95% confidence interval: 4.1‐7.0) and the P95 was 12.5 days; in the early stages, the epidemic doubling time was 7.4 days, which means the number of infections doubled every 7.4 days; the R0 was estimated to be 2.2 (which means infecting two healthy people for every sick person). 31 Additionally, the WHO assessed the R0 of this epidemic as between 1.4 and 2.5. 7
7. CLINICAL FEATURES
At the beginning of the outbreak, the average time from onset to the first visit and the average time from onset to hospitalization was 5.8 and 12.5 days, respectively (for patients who developed the disease before 1 January 2020), and 4.6 and 9.1 days, respectively (for patients who developed disease between 1 and 11 January 2020). 31 A retrospective analysis of approximately 4021 confirmed SARS‐CoV‐2 pneumonia patients from various regions in China found that the average time from onset to confirmed diagnosis was 5 days, and this time window was getting shorter as the epidemic progresses. 21 The confirmed times for patients who developed the disease before 14 January, during 14 to 22 January, and after 22 January were 14, 6, and 1 day, respectively.
This shows that with the development of the epidemic situation, the diagnostic ability of Chinese medical staff is rapidly improving. The average time from symptom onset to the hospitalization of critically ill patients was 7 days, and the average time from symptom onset to confirmed diagnosis was 8 days, which was significantly longer than that of mildly ill patients. The average time from symptom onset to confirmed diagnosis in death cases was 9 days, and the average time interval from symptom onset to death was 9.5 days. Therefore, early detection, early diagnosis, and early treatment are effective methods for reducing the severity and mortality of the disease. 21
Based on the analysis of existing retrospective studies, 6 , 8 , 16 , 22 , 25 , 26 , 27 , 28 the main manifestations of SARS‐CoV‐2 pneumonia patients were fever (89.6%, 1563/1745) and dry cough (67.0%, 1170/1745). Additionally, a small number of patients had symptoms such as the stuffy nose, runny nose, sore throat, myalgia, and diarrhea. A total of 90.2% (1157/1283) of patients had normal or decreased leukocyte counts, 73.4% (982/1337) of patients had decreased lymphocyte counts, and 81.2% (1119/1378) of patients had abnormal pulmonary imaging. It can be seen that patients with SARS‐CoV‐2 pneumonia had a fever and/or respiratory symptoms; in the early stages of onset, the white blood cells were normal or reduced, or with lymphocytopenia; the proportion of abnormalities on chest computed tomography or X‐ray was high. 34 Therefore, meeting two of the above three clinical manifestations at the same time with an epidemiological history, or both conforming to the above three clinical manifestations was presented as the diagnostic criteria for suspected cases in China's guidelines for the diagnosis and treatment of COVID‐19. If the suspected case has a positive result for a nucleic acid amplification assay or serological evidence (immunoglobulin M [IgM] antibody specific to SARS‐CoV‐2 was detected 3‐5 days after onset, or the titer of IgG antibody specific to SARS‐CoV‐2 was increased by four times or more in the recovery period compared with the acute phase), the case can be classified as confirmed. According to statistics, the proportion of critically ill patients among the confirmed cases is approximately 23.4% (1198/5120). 16 , 21 The above results are shown in Table 1. Most of the 14 cases in children reported in the existing literature (Table 2) had an epidemiological history related to Wuhan, and almost all had family members that were confirmed patients. Except for one child who failed to see a doctor in time, the others were all in mild condition. Among the seven cases reported in the existing literature outside China, except for one case where the patient died, the remaining cases were in stable condition (Table 3).
Table 2.
Clinical features of children novel coronavirus pneumonia
| Patient 1 35 | Patient 2 36 | Patient 3 36 | Patient 4 37 | Patient 5 38 | Patient 6 38 | Patient 7 39 | Patient 8 39 | Patient 9 39 | Patient 10 46 | Patient 11 39 | Patient 12 39 | Patient 13 39 | Patient 14 39 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| District | Wuhan | Wuhan | Xian | Haikou | Shanghai | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Definite diagnosis time | 3 March 2020 | 29 January 2020 | 30 January 2020 | 26 January 2020 | 19 January 2020 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Characteristics | ||||||||||||||
| Age | 1 y 1 mo | 3 y 10 mo | 13 y | 4 mo | 7 y | 9 mo | 11 mo | 8 mo | 10 mo | 7 mo | 1 mo 26 d | 3 mo | 3 mo 22 d | 6 mo |
| Sex | Male | Female | Male | Female | Male | Female | Female | Female | Male | Female | Female | Female | Female | Male |
| Linkage to Wuhan | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | No |
| No. of family members infected | 0 | 2 | 1 | 2 | 1 | 2 | 1 | 5 | 1 | 2 | 2 | 2 | 1 | 1 |
| Clinical features | ||||||||||||||
| Fever | Yes | Yes | No | Yes | Yes | Yes | Yes | No | NA | Yes | No | No | Yes | NA |
| Cough | No | No | No | No | Yes | No | No | No | NA | No | Yes | Yes | No | NA |
| Rhinorrhea | No | No | No | No | No | No | No | No | NA | No | Yes | No | No | NA |
| Fatigue | Yes | No | No | No | No | No | No | No | NA | No | No | No | No | NA |
| Expectoration | No | No | No | No | No | No | No | No | NA | No | No | Yes | No | NA |
| Headache | No | No | No | No | No | No | No | No | NA | No | No | No | No | NA |
| Diarrhea | Yes | No | No | No | No | No | No | No | NA | No | No | No | No | NA |
| Dyspnea | No | No | No | No | No | No | No | No | NA | No | No | No | No | NA |
| Pharyngalgia | No | No | Yes | No | No | No | No | No | NA | No | No | No | No | NA |
| Nausea and vomiting | Yes | No | No | No | Yes | No | No | No | NA | No | No | No | No | NA |
| Laboratory test | ||||||||||||||
| White blood cell count, ×109/L | 7.5 | 4.8 | 5.1 | 9.7 | 16.0 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Neutrophil (%) | 52.2 | 20.8 | 35.8 | 44.6 | 70.2 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lymphocyte count (%) | 23.5 | 69.8 | 56.8 | 44.3 | 22.9 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Red blood cell count, ×1012/L | 4.0 | 4.5 | 4.5 | NA | 4.7 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hemoglobin, g/L | 108.0 | NA | NA | 113.0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Platelets count, ×109/L | 183.0 | 225.0 | 287.0 | 494.0 | 138.0 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Prothrombin time, s | 14.3 | Normal | NA | NA | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| D‐dimer, mg/L | Normal | 0.4 | NA | NA | 0.6 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C‐reactive protein level, mg/L | 24.8 | 12.0 | <10.0 | 5.7 | 15.0 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Procalcitonin level, μg/L | 430.0 | Normal | <0.05 | 0.1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Aspartateaminotransferase, U/L | Normal | 23.3 | 20.9 | Normal | 33 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Alanineaminotransferase, U/L | Normal | 10.6 | 12.3 | Normal | 17 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lactate dehydrogenase | Normal | NA | NA | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Creatine kinase, U/L | 62.0 | 75.3 | 83.8 | Normal | 127.0 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Urea nitrogen, mmol/L | 15.9 | NA | NA | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Uric acid, μmol/L | 701.0 | NA | NA | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Creatinine, μmol/L | 224.0 | 27.3 | 35.3 | Normal | 29.0 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Potassium, mmol/L | 5.5 | Normal | Normal | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Sodium, mmol/L | 129.2 | Normal | Normal | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chloride, mmol/L | 94.7 | Normal | Normal | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Calcium, mmol/L | 1.9 | Normal | Normal | Normal | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IgA, g/L | 0.66 | NA | NA | 0.01 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IgM, g/L | 1.18 | NA | NA | 0.20 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IgG, g/L | 4.43 | NA | NA | 3.10 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C3 | 0.66 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C4 | 0.10 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CD3+ | 0.38 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CD8+ | 0.17 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CD4+ | 0.20 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CD4+/CD8+ | 1.21 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CD16+ | 0.06 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| CD19+ | 0.51 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IL‐2, ng/L | 1.03 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IL‐4, ng/L | 4.02 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IL‐6, ng/L | 120.31 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IL‐10, ng/L | 33.38 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| TNF‐α, ng/L | 4.47 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IFN‐γ, ng/L | 1.92 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Abnormalities on chest CT or X‐ray | ||||||||||||||
| Pneumonia of location | Bilateral pneumonia | Normal | Normal | Bilateral pneumonia | Bilateral pneumonia | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pneumonia of severity | Ground‐glass opacity, consolidation shadow | Normal | Normal | Multiple patch‐like shadows | Increased lung texture | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Days from illness onset to admission time | 6 d | 1 d | 3 d | 4 h | 3 h | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Time between admission and diagnosis | 8 d | 2 d | 1 d | 1 d | 1 d | 1 d | 1 d | 3 d | 3 d | 1 d | 1 d | 1 d | 1 d | 2 d |
Abbreviations: CT, computed tomography; IFN‐γ, interferon γ; IgA, immunoglobulin A; IL‐6, interleukin 6; NA, not available; TNF‐α, tumor necrosis factor α.
Table 3.
Clinical features of patients out of China novel coronavirus pneumonia
| Patient 1 17 | Patient 2 40 | Patient 3 41 | Patient 4 41 | Patient 5 42 | Patient 6 43 | Patient 7 44 | |
|---|---|---|---|---|---|---|---|
| Definite diagnosis time | 20 January 2020 | NA | 22 January 2020 | 23 January 2020 | 28 January 2020 | 13 January 2020 | 1 January 2020 |
| The district of illness | United States | Canada | Vietnam | Vietnam | Thailand | NA | Wuhan |
| Nationality | United States | Canada | Vietnam | Vietnam | Thailand | Arabia | Nepal |
| Characteristics | |||||||
| Age | 35 | 56 | 65 | NA | 51 | 60 | 32 |
| Sex | Male | Male | Male | Male | Male | Male | Male |
| Huanan seafood market exposure | No | No | No | No | No | NA | No |
| Linkage to Wuhan | Yes | Yes | Yes | Met patient 3 | Contact with Chinese tourist | NA | Yes |
| Underlying disease | Hypertriglyceridemia | NA | Hypertension, diabetes, coronary heart disease, lung cancer | NA | Hypertension, diabetes | None | None |
| Clinical features | |||||||
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Cough | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Rhinorrhea | Yes | No | No | No | No | No | No |
| Fatigue | Yes | No | Yes | No | No | No | No |
| Expectoration | No | No | No | No | No | Yes | No |
| Headache | No | No | No | No | No | No | No |
| Diarrhea | Yes | No | No | No | No | No | No |
| Dyspnea | No | No | Yes | No | Yes | Yes | Yes |
| Pharyngalgia | No | No | No | No | No | No | No |
| Nausea and vomiting | Yes | No | No | No | No | No | No |
| Myalgia | No | No | No | No | Yes | No | No |
| Laboratory test | |||||||
| White blood cell count, ×109/L | Decreased | Normal | NA | Normal | NA | Normal | Normal |
| Neutrophil (%) | Decreased | NA | NA | Normal | NA | Increased | Normal |
| Lymphocyte count (%) | Normal | NA | NA | Normal | NA | Decreased | Normal |
| Red blood cell count, ×1012/L | Normal | NA | NA | Normal | NA | NA | Normal |
| Hemoglobin, g/L | Decreased | Normal | NA | Normal | NA | NA | Normal |
| Platelets count, ×109/L | Decreased | Decreased | NA | Normal | NA | NA | Normal |
| Prothrombin time, s | Decreased | NA | NA | Normal | NA | NA | Normal |
| C‐reactive protein level, mg/L | NA | NA | NA | Increased | NA | NA | Normal |
| Procalcitonin level, μg/L | Normal | NA | NA | Normal | NA | NA | Normal |
| Aspartate aminotransferase, U/L | Increased | NA | NA | Normal | NA | NA | Normal |
| Alanine aminotransferase, U/L | Increased | Normal | NA | Normal | NA | NA | Normal |
| Lactate dehydrogenase, U/L | Increased | NA | NA | Normal | NA | NA | Normal |
| Lactate, mmol/L | Normal | Normal | NA | Normal | NA | NA | Normal |
| Creatine kinase, U/L | Increased | NA | NA | Normal | NA | NA | Normal |
| Urea nitrogen, mmol/L | Normal | NA | NA | Normal | NA | Increased | Normal |
| Uric acid, μmol/L | NA | NA | NA | Normal | NA | NA | Normal |
| Creatinine, μmol/L | Normal | Normal | NA | Normal | NA | Increased | Normal |
| Potassium, mmol/L | Decreased | NA | NA | Normal | NA | NA | Normal |
| Sodium, mmol/L | Decreased | NA | NA | Normal | NA | NA | Normal |
| Chloride, mmol/L | Normal | NA | NA | Normal | NA | NA | Normal |
| Calcium, mmol/L | Decreased | NA | NA | Normal | NA | NA | Normal |
| Abnormalities on chest CT or X‐ray | No abnormalities | Ill‐defined opacities in all lung zones | An infiltrate in the upper lobe of the left lung, progressive infiltrate and consolidation | No abnormalities | Reticular, patchy infiltration of the left lower lung | Low lung volumes, bilateral enhanced pulmonary hilar vascular shadows more prominent on the left | An infiltrate in the upper lobe of the left lung |
| Days from illness onset to admission time | 4 d | NA | 4 d | 2 d | 8 d | 7 d | 10 d |
Abbreviations: CT, computed tomography; NA, not available.
8. TREATMENT PROGRAMS
According to the latest data as of 23 March 2020, more than 73 159 patients with SARS‐CoV‐2 pneumonia have recovered and been discharged. Hubei and Wuhan reported no new confirmed or suspected cases for 5 consecutive days, and the number of confirmed cases continued to drop. These results indicate that China's preventions and treatments against COVID‐19 have achieved good results. In the past month or so, China has issued a series of national recommendations for the diagnosis and treatment of respiratory infections caused by SARS‐CoV‐2. 45 These recommendations have now been updated to version 7. Recommendations for the diagnosis and treatment of respiratory infection caused by SARS‐CoV‐2 in children were also drafted by the National Clinical Research Center for Child Health, Children's Hospital, Zhejiang University School of Medicine, to further standardize the protocol for diagnosis and treatment of respiratory infection in children caused by SARS‐CoV‐2. 46 Chinese medical workers have gradually formed some effective diagnosis and treatment strategies and methods in clinical practice of antiviral therapy and symptomatic treatment, including a comprehensive series of diagnosis and treatment methods such as respiratory support, circulatory support, and immunity improvement, to accelerate recovery. 47 Antiviral drugs (ganciclovir, acyclovir, and ribavirin) and neuraminidase inhibitors (oseltamivir, paramivir, zanamivir, etc) commonly used in clinical practice, are ineffective on COVID‐19 and are not recommended. Drugs that are possibly effective on COVID‐19 include remdesivir, lopinavir/ritonavir, lopinavir/ritonavir combined with interferon‐β, convalescent plasma, and monoclonal antibodies. 48 However, the efficacy and safety of these drugs for SARS‐CoV‐2 pneumonia patients need to be assessed by further clinical trials. Chinese researchers recently discovered that the anti‐malarial drug chloroquine phosphate has an effect on the treatment of SARS‐CoV‐2 pneumonia through drug screening and clinical trials. Preliminary evidence suggests that chloroquine phosphate can significantly reduce the exacerbation rate of patients with SARS‐CoV‐2 pneumonia, has an antipyretic function, can accelerate pulmonary inflammation recovery, shorten the negative time and increase the negative conversion rate of viral nucleic acids, and shorten the course of the disease. In addition, chloroquine phosphate is relatively safe, and no serious adverse reactions related to the drug have been found so far. The drug has been included in the latest edition of the Chinese diagnosis and treatment guidelines for SARS‐CoV‐2 pneumonia treatment updated on 3 March 2020, to expand the scope of clinical trials. However, the efficacy of this drug should be further evaluated in clinical application. Use should be discontinued when there is an intolerable side effect. Patients with COVID‐19 treated with chloroquine phosphate must have a normal electrocardiogram before medication. The combination uses chloroquine phosphate with macrolides including azithromycin or other drugs that may cause a prolonged QT interval are prohibited in China. However, a study from France showed that azithromycin added to hydroxychloroquine was significantly more efficient for virus elimination in COVID‐19 patients. In this study, among hydroxychloroquine‐treated patients six patients received azithromycin to prevent bacterial super‐infection under daily electrocardiogram control, 100% of patients were virologicaly cured. 49 Although the study sample size was small, the results require further confirmation. However, the study offers an insight: whether this compound could be useful as chemoprophylaxis to prevent the transmission of the virus, especially for health care workers.
The principle of treatment for severe and critically ill cases is to actively prevent and treat complications, treat underlying diseases, prevent infection, and provide timely organ function support based on symptomatic treatment; clinical warning indicators and early intervention for patients were explored to reduce mortality; additionally, new treatments such as convalescent plasma therapy were developed. The convalescent plasma therapy was developed using a virus‐free plasma containing high‐titer SARS‐CoV‐2 specific antibodies donated from rehabilitated patients. This therapy will be applied clinically after virus inactivation, antibody titer determination, and multiple pathogenic microorganism determination. On 22 January the Chinese research group incorporated the preparation of exempt plasma from rehabilitated SARS‐CoV‐2 pneumonia patients into the scientific research emergency project. The first plasma from patients with a recovery period was collected on 1 February 2020, and coronavirus‐free plasma was then prepared. On 9 February 2020, the first severely ill patient received SARS‐CoV‐2‐free plasma treatment at a hospital in Wuhan, and more patients subsequently received the therapy. Clinical studies have shown that SARS‐CoV‐2‐free plasma is safe and effective for critically ill patients. The therapeutic method has been added to the latest guidelines (version 6) for critically ill patients. In addition, China has organized several research groups to explore the role of Chinese medicine in the prevention and treatment of COVID‐19 and has achieved a certain effect.
Zhejiang Province is one of the provinces with the largest number of SARS‐CoV‐2 pneumonia patients in China. As of 23 March 2020, a total of 1240 patients with SARS‐CoV‐2 pneumonia were diagnosed, of which 1193 recovered and were discharged. The most important thing is that to date, only one patient has died from SARS‐CoV‐2 infection in this province. The medical staff in this province have formed a unique "Zhejiang experience”. (a) Within 72 hours of disease onset, a combination of lopinavir/ritonavir/abidol and interferon nebulization is used for antiviral treatment to reduce the damage to the cells caused by viral expansion and replication. (b) Glucocorticoids are mainly used in critically ill patients suffering inflammatory cytokine storm. Inhibition of excessive inflammation through timely administration of glucocorticoids in the early stage of inflammatory cytokine storm effectively prevents the occurrence of ARDS and protects the functions of the patients’ organs. For patients with progressive deterioration of oxygenation indicators, rapid imaging progress, and excessive inflammatory response, the use of glucocorticoid in the short term (3‐5 days) is appropriate, and the recommended dose is no more than equivalent to methylprednisolone 1 to 2 mg/kg/d. Some critically ill patients should use methods, such as, artificial liver and plasma exchange to block the cytokine storm. (c) Effective oxygen therapy is performed for patients. (d) Infection is actively prevented. (e) Nutritional support is strengthened.
9. RISK FACTORS FOR DISEASE PROGRESSION FROM ORDINARY COVID‐19 TO SEVERAL CASES
In clinical practice, some mild patients can quickly progress to critical patients. Some characteristics of these patients were summarized in the diagnosis and treatment of COVID‐19 (trial version 7) from China. In adults, the following conditions indicate a worsening of the disease. (a) Progressive decline of peripheral blood lymphocytes; (b) peripheral inflammatory factors, such as interleukin 6 and C‐reactive protein, increased progressively. (c) Progressive increase of lactic acid; (d) intrapulmonary lesions progress rapidly in a short period of time.
Children with the following conditions are prone to develop into critical cases. (a) Increased respiratory rate; (b) poor mental response and lethargy; (c) progressive increase of lactic acid; (d) imaging findings showed bilateral or multilobular infiltration, pleural effusion, or rapid progression in the short term; (e) infants under 3 months of age or have underlying diseases (congenital heart disease, pulmonary dysplasia of the airway, malformation of the respiratory tract, abnormal hemoglobin, severe malnutrition, etc), or have immunodeficiency (long‐term use of immunosuppressive agents).
10. REAPPEARANCE OF NUCLEIC ACID OF COVID‐19 PATIENTS AFTER DISCHARGE FROM HOSPITALS
After treatment, COVID‐19 patients can be discharged if they meet the following conditions: temperature returns to normal for more than 3 days; respiratory symptoms improve significantly; pulmonary imaging shows significant improvement of acute exudative lesions; negative nucleic acid test of respiratory tract specimens, such as sputum and nasopharyngeal swab, for two consecutive times (sampling time should be at least 24 hours apart). However, it is now found that about 0.1% of patients will be tested positive for nucleic acid again after discharge. 50 There are many reasons for the positive nucleic acid test of discharged patients. 51 The reagent itself, detection method, sampling method, and so on will affect the positive rate. In addition, the possibility of disease recurrence cannot be ruled out too. Genomic analysis of the virus from different regions, different periods and different sources of patients revealed that SARS‐CoV‐2 was stable and showed no major variation. So once the patient is cured with immunity against the virus, there is little chance of reinfection. The detection of SARS‐CoV‐2 specific IgM and IgG antibodies is an effective means to determine whether a patient has immunity against SARS‐CoV‐2. But these patients still have the potential to infect others. Therefore, after the patient is discharged from the hospital, it is recommended to continue the isolation management and health condition monitoring for 14 days. Here are some tips and considerations for self‐isolation: wear a mask, live in a single room with good ventilation, reduce close contact with family members, separate meals, good hand hygiene, and avoid outdoor activities. It is also recommended to visit the hospital for reexamination on the 14th and 28th days after discharge.
11. FUNCTION OF GOVERNMENT
In the early stages of the epidemic, the Chinese government adopted the principles of "four early" and "four centralization" to address the epidemic so that the epidemic situation was effectively controlled. The so‐called "four early" principle is early detection, early reporting, early isolation, and early treatment. Early detection and isolation of SARS‐CoV‐2‐infected people, moving the prevention and control barrier forward, accelerating the grid‐based management of the community, improving detection capabilities, optimizing the diagnosis and treatment process, and shortening the diagnosis time can effectively block the spread of the epidemic and reduce the infection rate. The principle of "four centralization" is to centralize patients, centralize experts, centralize resources, and centralize treatment. Patients with severe illness are centralized at the best hospital with the strongest comprehensive capacity for treatment. At the same time, to centralize resources and experts to treat critically ill patients, multidisciplinary and comprehensive individualized diagnosis and treatment are applied in accordance with "one person, one strategy," and finally, the mortality rate is effectively reduced.
In addition, more than 37 000 medical staff from other parts of China participated in the treatment of patients with SARS‐CoV‐2 pneumonia in Wuhan, Hubei, which fully reflects the noble qualities and good professionalism of Chinese medical staff.
12. LIMITATIONS
It has not been long since the occurrence of SARS‐CoV‐2 pneumonia, there are relatively few reports in the literature, and some opinions need to be further verified by multicenter big data. In addition, infectious diseases have different characteristics at different stages. Whether some of the findings and opinions have always been applicable needs further evaluation.
13. CONCLUSIONS
The SARS‐CoV‐2 was first reported in China, with bats as the likely original hosts and pangolins as potential intermediate hosts. The current source of the disease is mainly patients infected with SARS‐CoV‐2. Patients in the incubation period may also become sources of infection. COVID‐19 is mainly transmitted through respiratory droplets and contact, and the population is generally susceptible. The epidemic has experienced the local outbreak stage and community transmission stage caused by the Wuhan's Huanan wholesale seafood market exposure and is now in the stage of large‐scale transmission caused by the spread of the epidemic. The R0 at the beginning of the epidemic was 2.2, with an average incubation period of 5.2 days. The proportion of critically ill patients was 23.4%, the mortality was lower than those of SARS and MERS, and 96.5% of the deaths occurred in Hubei Province, where the outbreak first occurred. Among them, elderly men with underlying diseases had a higher mortality rate. In the process of fighting against the COVID‐19, Chinese medical staff have summarized a set of effective strategies and methods that are worthy of reference for their international counterparts. Because of powerful government intervention and the efforts of Chinese medical staff, the situation in China has now been controlled and has gradually improved.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Dr. SS and QS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: SS and QS; acquisition, analysis, or interpretation of data: BW, JM, JF, TZ, SS and QS; drafting of the manuscript: QY; critical revision of the manuscript for important intellectual content: SS and QS; administrative, technical, or material support: QY.
ACKNOWLEDGMENT
This study was supported by the Zhejiang University special scientific research fund for COVID‐19 prevention and control.
Ye Q, Wang B, Mao J, et al. Epidemiological analysis of COVID‐19 and practical experience from China. J Med Virol. 2020;92:755–769. 10.1002/jmv.25813
Contributor Information
Shiqiang Shang, Email: shangsq@zju.edu.cn.
Qiang Shu, Email: shuqiang@zju.edu.cn.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. York A. Novel coronavirus takes flight from bats? Nat Rev Microbiol. 2020;18(4):191. 10.1038/s41579-020-0336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707. 10.1001/jama.2020.0757 [DOI] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahase E. China coronavirus: what do we know so far? BMJ. 2020;368:m308. [DOI] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishiura H, Linton NM, Akhmetzhanov AR. Initial cluster of novel coronavirus (2019‐nCoV) infections in Wuhan, China is consistent with substantial human‐to‐human transmission. J Clin Med. 2020;9(2):488. 10.3390/jcm9020488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Letko MC, Munster V. Functional assessment of cell entry and receptor usage for lineage B‐coronaviruses, including 2019‐nCoV. BioRxiv. 2020. 10.1101/2020.01.22.915660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perlot T, Penninger JM. ACE2—from the renin‐angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15(13):866‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020. 10.1101/2020.02.06.20020974 [DOI] [Google Scholar]
- 17. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019‐nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9(1):51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020. 10.1101/2020.02.10.20021675 [DOI] [Google Scholar]
- 22. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:e201585. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parry J. WHO warns that death rate from SARS could reach 10%. BMJ. 2003;326(7397):999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L, Liu H‐G, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin J Tuberc Respir Dis. 2020;43:E005. [DOI] [PubMed] [Google Scholar]
- 26. Liu K, Fang Y‐Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020. 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 2020. 10.1101/2020.02.10.20021584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu X‐F, Gong W, Wang L, et al. Clinical features and high resolution CT imaging findings of preliminary diagnosis novel coronavirus pneumonia. Chin J Radiol. 2020;54:E006. [Google Scholar]
- 29. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. 10.1016/S2213-2600(1020)30079-30075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in china. Chin J Epidemiol. 2020;41(2):145‐151. [PMC free article] [PubMed] [Google Scholar]
- 31. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan J, Li M‐Y, Sun A‐H, Peng Y‐H. 2019 novel coronavirus and infectious pneumonia. Chin J Microbiol Immunol. 2020;40(1):1‐6. [Google Scholar]
- 35. Chen F, Liu Z‐S, Zhang F‐R, et al. China's first child severe new coronavirus pneumonia. Chin J Pediatr. 2020;58:E005. [DOI] [PubMed] [Google Scholar]
- 36. Deng H‐L, Zhang Y‐F, Wang Y, Li F‐Y. The reportes of two cases of new coronavirus infection in children. Chin Pediatr Emerg Med. 2020;27(22):81‐83. [Google Scholar]
- 37. Zhang Y‐H, Lin D‐J, Xiao M‐F, et al. A case of novel coronavirus infection in a 3‐month‐old infant. Chin J Pediatr. 2020;58:E006. [DOI] [PubMed] [Google Scholar]
- 38. Cai J‐H, Wang X‐S, Ge Y‐L, et al. First case of 2019 novel coronavirus infection in children in Shanghai. Chin J Pediatr. 2020;58:E002. [DOI] [PubMed] [Google Scholar]
- 39. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020. 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silverstein WK, Stroud L, Cleghorn GE, Leis JA. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;395(10225):734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phan LT, Nguyen TV, Luong QC, et al. Importation and human‐to‐human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pongpirul WA, Pongpirul K, Ratnarathon AC, Prasithsirikul W. Journey of a Thai taxi driver and novel coronavirus. N Engl J Med. 2020;382(11):1067‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 44. Bastola A, Sah R, Rodriguez‐Morales AJ, et al. The first 2019 novel coronavirus case in Nepal. Lancet Infect Dis. 2020;20(3):279‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin L, Li TS. Interpretation of "Guidelines for the diagnosis and treatment of novel coronavirus (2019‐nCoV) infection by the National Health Commission (trial version 5)". Zhonghua Yi Xue Za Zhi. 2020;100:E001. [DOI] [PubMed] [Google Scholar]
- 46. Chen Z‐M, Fu J‐F, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020. 10.1007/s12519-12020-00345-12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li H, Wang YM, Xu JY, Cao B. [Potential antiviral therapeutics for 2019 novel coronavirus]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E002. [DOI] [PubMed] [Google Scholar]
- 49. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020:105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Chen D, Xu W, Lei Z, et al. Recurrence of positive SARS‐CoV‐2 RNA in COVID‐19: a case report. Int J Infect Dis. 2020;93:297‐299. 10.1016/j.ijid.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou L, Liu K, Liu HG. Cause analysis and treatment strategies of "recurrence" with novel coronavirus pneumonia (COVID‐19) patients after discharge from hospital. Chin J Tuberc Respir Dis. 2020;43:E028. [DOI] [PubMed] [Google Scholar]


