Abstract
The COVID‐19 pandemic is creating unique strains on the healthcare system. While only a small percentage of patients require mechanical ventilation and ICU care, the enormous size of the populations affected means that these critical resources may become limited. A number of non‐invasive options exist to avert mechanical ventilation and ICU admission. This is a clinical review of these options and their applicability in adult COVID‐19 patients. Summary recommendations include: (1) Avoid nebulized therapies. Consider metered dose inhaler alternatives. (2) Provide supplemental oxygen following usual treatment principles for hypoxic respiratory failure. Maintain awareness of the aerosol‐generating potential of all devices, including nasal cannulas, simple face masks, and venturi masks. Use non‐rebreather masks when possible. Be attentive to aerosol generation and the use of personal protective equipment. (3) High flow nasal oxygen is preferred for patients with higher oxygen support requirements. Non‐invasive positive pressure ventilation may be associated with higher risk of nosocomial transmission. If used, measures special precautions should be used reduce aerosol formation. (4) Early intubation/mechanical ventilation may be prudent for patients deemed likely to progress to critical illness, multi‐organ failure, or acute respiratory distress syndrome (ARDS).
Keywords: BiPAP, coronavirus, COVID‐19, high flow nasal cannula, high flow oxygen, high velocity nasal insufflation, non‐invasive ventilation, SARS‐CoV‐2, viral pneumonia
1. INTRODUCTION
A novel clinical syndrome caused by a previously unknown coronavirus, SARS‐Cov‐2, was first identified in Wuhan (China) in December 2019. Despite massive efforts to contain viral transmission, a worldwide epidemic has developed from this virus. This disease is presently known as COVID‐19.
The COVID‐19 pandemic has several features that are straining healthcare systems. 1 , 2 The case fatality rate is extremely age dependent with an increase from <0.6% to 2.2% at age 60 and increasing to over 9.3% at age 80. 3 The mode of spread and transmissibility is via respiratory droplets. The high level of contagion combined with the lack of immunity to this virus in the population has resulted in an overwhelming number of severe or critical cases. In Italy, the number of critically ill COVID‐19 cases has exceeded the intensive care unit (ICU) capacity of affected regions, and in NYC, critical illness from COVID‐19 has already far exceeded existing ICU capacity. In the US, critical care capacity was limited even before the COVID‐19 pandemic with over one‐third of patients having to wait 6 hours or more for transfer to an ICU. 4 , 5
A number of non‐invasive options exist to support COVID‐19 patients with mild or moderate respiratory distress and may reduce the numbers of patients requiring intubation, mechanical ventilation, and ICU admission in some severely ill patients. 19 However, all forms of supplemental oxygenation and respiratory support may potentially aerosolize respiratory pathogens. 6 , 7 , 8 Selection of respiratory support for patients affected by COVID‐19 must balance the clinical benefit of the intervention against the risks of nosocomial spread. Complicating this goal is that mitigation of aerosolization by early endotracheal intubation commits to the prolonged use of an ICU bed and mechanical ventilator, which may not be available in the context of a pandemic. Therefore, management of the COVID‐19‐associated respiratory failure must consider the full spectrum of invasive and non‐invasive ventilation options.
In this clinical review, we summarize the options and provide practical recommendations for respiratory support in COVID‐19 patients.
2. ISOLATION AND PERSONAL PROTECTIVE EQUIPMENT
Any patient suspected of having COVID‐19 disease should be managed in a negative pressure room when possible. This is particularly true for patients requiring any form of supplemental oxygen therapy. Staff treating the patient should use maximum PPEs with N‐95 masks and eye protection. When no negative pressure room is available, a closed room may be the only option. Simple measures such as placing a surgical mask over all patients in the ED may be helpful in mitigating the pathogenic spread.
3. SUPPLEMENTAL OXYGEN
Oxygen therapy is recommended by the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) as the first‐line therapy for treating COVID‐19‐induced respiratory distress and hypoxia. 2 , 9 Methods of administration vary and should be determined by severity of illness. The goal of treatment should be maintenance of oxygen saturation >90%. The target of treatment should be SaO2 = 92%–95% for pregnant women. 2 , 10
3.1. Nasal cannula
Supplemental oxygen by nasal cannula provides up to about 5–6 L/min of flow increasing fraction of inspired oxygen (FiO2) to approximately 45%. The actual FiO2 may be variable depending on the patient's inspiratory peak flow. Limitations of flow in the tubing and entrainment of room air prevent higher effective oxygen concentrations regardless of the wall settings. Adequate humidification of the supplemental oxygen is needed to maintain mucociliary action. 11 , 12 While effective for mildly hypoxic patients, supplemental oxygen delivered by nasal cannula can induce significant dispersion of exhaled air, even at low flow rates. In studies using a high fidelity human mannequin model, the reported maximal distance of exhaled air dispersion was 30 cm at 1 L/min, and 40 cm at 5 L/min (Figure 1). 12
FIGURE 1.

Aerosol dispersion distances (cm) for various oxygen supplementation modalities. Distance depicted is the average dispersal for that modality over the range of flow rates typically used for that modality (NC ranges 3‐40 cm, SM at all flows ≈ 30 cm, VM range 33‐40 cm, NRM at all flows < 10 cm, HFNO ranges 4.8‐17 cm, NiPPV ranges 85‐95 cm, and nebulizers < 80 cm). Note that normal tidal breathing was not measured, but the distance measured at a flow rate of 1L/min via nasal cannula was 30 cm.
3.2. Simple mask
Simple masks provide supplemental oxygen with flow rates up to approximately 5–10 L/min. Respiratory rate and exhalation are controlled by the patient and these individually affect the actual FiO2 delivered. Oxygen supplementation via simple mask is only marginally greater than that of nasal cannula. High fidelity human mannequin model studies showed the maximum exhaled dispersion distance using a simple mask at 10 L/min was 40 cm (Figure 1). 12
3.3. Venturi mask
Supplemental oxygen by venturi mask allows more precise oxygen delivery. FiO2 is delivered in discrete levels, typically between 24% and 60% oxygen. The mask uses an air/oxygen entrainment device (venturi) to more precisely mix air and oxygen. Each FiO2 level is achieved with a “snap‐on” venturi, which specifies the oxygen flow rate to achieve the selected FiO2. Oxygen flow rates are specified, and typically vary from 2 to 15 L/min. In studies using a high fidelity human mannequin model, the maximum exhaled dispersion distance varied from 33 cm at FiO2 40%, to 40 cm at FiO2 24% (Figure 1). 12
3.4. Non‐rebreather masks
Non‐rebreather masks (NRBs) offer a safe way to provide supplemental oxygen to COVID‐19 patients as the mask helps to limit the dispersion of droplets. NRB masks can provide supplemental oxygen up to a level of approximately 90% at flow rates approaching 15 L/min. 13 To prevent hypercapnia, the reservoir bag must remain inflated at all times; this requires flow rates of at least 8–10 L/min. 14 High fidelity human mannequin studies demonstrate that the maximum exhaled dispersion distance at 10 L/min is <10 cm, suggesting that this modality generates the least dispersed aerosols (Figure 1). 12
4. INTERMEDIATE THERAPIES
4.1. High flow oxygen systems
High flow nasal oxygen (HFNO) includes high flow nasal cannula and high velocity nasal insufflation. High flow oxygen systems provide oxygen‐rich heated humidified gas to the patient's nose at flow levels sufficient to deliver a constant, precisely set high FiO2. HFNO flow rates reach up to 60 L/min, whereas HVNI delivers flow rates up to 40 L/min due to differing mechanisms of delivery. Exhalation is to the open air. HFNO reduces dead space, provides low levels of PEEP, and decreases breathing frequency and work of breathing. 20 The use of HFNO was associated with lower mortality in hypoxemic respiratory failure. 21 Compared to conventional oxygen therapy, HFNO is associated with decreased risk of subsequent intubation (relative risk [RR] 0.85, 95% confidence interval [CI] 0.74‐0.99) 22 and need for ICU admission. 23 , 24
Initial concern existed on the risk of aerosolization with HFNO leading some to recommend avoiding use of this modality. However, the degree of aerosolization has been shown to minimal with these devices, and it is now recommended as the oxygenation therapy of choice in patients with respiratory distress. Guidelines from the WHO, 2 the Italian Thoracic Society, 15 the Respiratory Care Committee of the Chinese Thoracic Society, 16 The Australian and New Zealand Intensive Care Society, 17 and a joint statement from the German Intensive Care, Anesthesia, and Emergency Medicine Societies, 18 as well as the joint guidelines produced by the European Society of Intensive Care Medicine and The Society of Critical Care Medicine, 19 all recommend HFNO as a therapy for COVID‐19 respiratory failure. Recent publications suggest that newer HFNO and non‐invasive positive pressure ventilation (NIPPV) systems with good interface fitting do not create widespread dispersion of exhaled air and therefore may be associated with low risk of airborne transmissions. 2 , 17
Because of their construction, HF/HV systems demonstrate favorable safety profiles as an AGP. A high‐fidelity mannequin study demonstrated that even at the highest setting of 60 L/min, exhaled air dispersion was 17 cm in a healthy lung scenario and only 4.8 cm in a severely diseased lung scenario. 25 The authors cautioned that if the connection from the tubing to the nasal cannula becomes disconnected there might be a risk of lateral dispersion of oxygen and pathogens. 25 Some guidelines recommend placement of a surgical mask over patients being treated with high flow therapies as a secondary safety measure. 18 High fidelity human mannequin simulation studies show surgical masks do, in fact, reduce exhaled air dispersion (Figure 1). 26
If HFNO oxygen therapy is used, medical staff should use airborne protection, and the patient should be treated in a negative pressure room, if available. 2 , 17
There is no currently published evidence that HFNO is a risk factor for nosocomial transmission of respiratory pathogens. 27 , 28 , 29 , 30 During the 2003 Toronto SARS‐CoV outbreak, HFNO was not found to be a risk factor for transmission to healthcare workers. 29 This is in contrast with endotracheal intubation, which was strongly associated with transmission to healthcare workers during the SARS epidemic. 29
For the treatment of pneumonia, HFNO was associated with reduced mortality compared to NIPPV. 21 Additionally, in a small study of severe 2009 influenza A/H1N1v, 20 of 25 patients could not maintain SpO2 >92% with 9 L/min of oxygen administered by conventional nasal cannula. Of those 20 patients, 9 were successfully treated with HFNO and 11 went on to need mechanical ventilation. 30
Given the current circumstances of an overwhelming pandemic, randomized controlled trials to establish that HFNO reduces the risk of endotracheal intubation (and thus escalation of care to the ICU) in severe COVID‐19 are likely not feasible. However, in a retrospective study of 610 patients from China, where 10% of the affected patients required critical care, 31 a multi‐pronged intervention that included early, and aggressive, use of HFNO was associated with reduced need for mechanical ventilation (<1% vs national average of 2.3%) and lower mortality (3.33% vs 4.34% in a nearby province). 32
There are currently no defined criteria for HFNO failure, but patients who require vasopressor support 30 , 34 and whose respiratory rate and thoracoabdominal asynchrony are not rapidly relieved with HFNO 35 are potentially at high risk of HFNO failure. Recently, the “ROX Index” was developed to aid in the prediction of clinical outcomes of patients treated with HFNO. It is calculated by the ratio of oxygen saturation as measured by pulse oximetry /FiO2 to respiratory rate. A ROX Index >4.88 is predictive of success, meaning the patient is unlikely to progress to needing mechanical ventilation. 36 Patients with established ARDS should move rapidly to mechanical ventilation, and treated per published recommendations. 37 , 38
4.2. Non‐invasive positive pressure ventilation
Continuous positive airway pressure (CPAP) or bi‐level positive airway pressure (BiPAP) are respiratory support devices that deliver positive airway pressure through tight fitting facial or nasal masks. The hallmark of these devices is that they deliver this positive pressure through all phases of the respiratory cycle. The patient continues to breath spontaneously both with and against the positive airway pressure. These devices can provide a FiO2 of up to 100% in a closed circuit.
The risk of aerosol formation and dispersion for CPAP and BiPAP systems are variable depending on setting parameters and model/mask type. Viral filters can be attached to the exhalation line on most newer models. High fidelity human model studies demonstrated that exhaled air dispersed to 40 cm with nasal cannula, and to 64 cm at 10 cmH2O inspiratory air pressure with a BiPAP mask. That distance increased to 85 cm and >95 cm at 18 cmH2O depending on mask style. This work was performed inside a negative pressure room (Figure 1). 11 , 12 , 25 Helmet mask BiPAP is unique and a similar mannequin study showed that it is safer than other models. The maximal measured dispersal distance from the helmet‐neck interface was 2.7 cm when an air cushion was in place around the neck (missing air cushions cause severe dispersion). 11
The use of CPAP or BiPAP is debated in patients with COVID‐19. These modalities (also called NIV or NIPPV) are included in recommendations by the WHO, 2 the Italian Thoracic Society, 15 and the Respiratory Care Committee of the Chinese Thoracic Society, 16 but were not included in a more limited paper by intensive care physicians in France. 34 The Australian and New Zealand Intensive Care Society Guidelines specifically advise against the use of NIPPV. 17 , 34 The joint guidelines by the European Society of Intensive Care Medicine and the Society of Critical Care Medicine advise against the use of NIPPV unless HFNO is not available. 19 NIPPV has been used successfully in COVID‐19 patients in China and Italy, as well as during the SARS epidemic in 2003. In Hong Kong, BiPAP was effective in treating patients with SARS in 2003 with no identified healthcare worker transmissions. 39 However, there are other reports that use of BiPAP was associated with increased rates of nosocomial transmission and higher rates of healthcare worker infection in other situations. 6
Additionally, the use of BiPAP and nebulizer treatments were associated with outbreaks of SARS in hospital wards in China in 2003. In the same studies, high flow oxygen masks (defined as flow rates >6 L/min) and mechanical ventilation were not associated with nosocomial spread. 40 , 41 These data may be of particular relevance to the COVID‐19 outbreak, where 3019 cases have already been reported in healthcare workers including 5 deaths as of February 11, 2020. 42 It is unknown if any of these were associated with the use of any particular respiratory treatment.
These conflicting data suggest that BiPAP should be considered cautiously. Closed circuit systems with appropriate filters in place are important, as are well‐fitting masks and the absence of facial hair on the patient, allowing for tight seals. Helmet BiPAP with an air cushion in place around the neck is safe and should be used if available. All other forms have been associated with higher dispersal distances than high flow oxygen systems and concern for nosocomial and healthcare provider infection. Properly trained personnel are also crucial.
4.3. Nebulizer therapies
Nebulizer treatments should be avoided in the care of patients with COVID‐19. Jet nebulizers were largely responsible for the spread of SARS in a hospital ward in China in 2003. 12 The safety profile regarding APGs for these devices are extremely poor. Modeling with human patient simulation shows that dispersion of particles could be measured beyond 0.8 m when flow rates mimicking severe lung injury were used (oxygen consumption of 500 mL/min, lung compliance 10 mL/cmH2O) (Figure 1). 43
Jet nebulized therapies are possibly some of the highest risk events for nosocomial viral transmission and should only be performed when absolutely necessary in negative pressure environments with highly trained personnel. Some high flow/high velocity systems and closed positive pressure systems have capabilities to add nebulized medications without an increased risk of particle dispersal.
Alternatives to nebulizer therapies include use of metered dose inhalers, or nebulized therapies performed in an adapted oral/nasal mask. 44 While estimates vary due to methodology, 4–6 puffs of a metered dose inhaler is the dose equivalent to a 2.5 mg nebulized dose of albuterol. 45 Placement of a viral filter inline with a nebulizer likely decrease the risk for nosocomial or healthcare worker infection but to our knowledge, no studies have directly measured this effect.
4.4. Mechanical ventilation
Mechanical ventilation through an endotracheal tube may be necessary for patients with frank respiratory failure or multisystem organ dysfunction. The role of mechanical ventilation in COVID treatment is still unclear. While potentially effective, the clinical indications for escalation to mechanical ventilation remain unclear. 10 Furthermore, the process of endotracheal intubation produces high amount of AGPs, contributing to the risks associated with mechanical ventilation. During the SARS epidemic in 2003, intubation was strongly associated with disease transmission to healthcare workers (relative risk, 13.29; 95% CI, 2.99 to 59.04). 34 Thus, the needs of the patient must be balanced against the inherent risks associated with intubation and mechanical ventilation as well as the risks to providers.
A joint statement by German Intensive Care, Anesthesia, and Emergency Medicine Societies suggests direct escalation to intubation and mechanical ventilation if PaO2/FiO2 < 200 mmHg. 18 A group of French experts recommended mechanical ventilation implementation in patients expected to fail other oxygenation/ventilation strategies due to respiratory failure or clinical deterioration (eg, shock, organ failure, etc) with the symptomatic challenges of ARDS. 34 Importantly, observers in China have identified the presence of hypoxemia without signs of respiratory distress (silent hypoxemia), especially in elderly populations. During any respiratory management, patients should regularly be monitored and checked for respiratory deterioration to prevent this occurrence.
There are no clear evidence‐based guidelines for the ideal time to proceed to mechanical ventilation in patients with COVID‐19. Availability of ventilators, intensive care capacity, considerations of palliative care and end‐of‐life resources as well as individual patient characteristics all play a role in decisions to institute mechanical ventilation.
5. INTEGRATING RESPIRATORY THERAPY OPTIONS
Treatment of patients with COVID‐19 who are hypoxic should follow the principles of treatment of hypoxic patients resulting from other etiologies (Figure 2). For patients with O2 sat <90% and mild to moderately increased work of breathing, consider supplemental oxygen (NRB mask preferred) with goal of O2 sat >90%. For those with increasing work of breathing, worsening hypoxia or failure to maintain O2 sat >90%, consider high flow oxygen (HFNO/HVNI). Reassess at least every 30 minutes for the first hour, and then hourly for the next few hours. Monitor closely for clinical deterioration, and look out for the possibility of “silent hypoxemia.” 43
FIGURE 2.
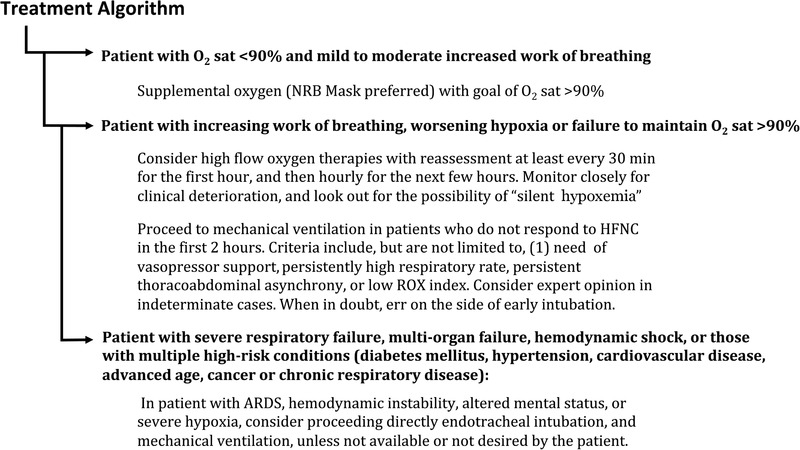
Proposed treatment algorithm for patients with hypoxia due to COVID‐19
Proceed to mechanical ventilation in patients who do not respond to high flow oxygen (HFNO/HVNI) in the first 2 hours. Criteria include, but are not limited to: (1) need of vasopressor support, 30 , 34 (2) persistently high respiratory rate, (3) persistent thoracoabdominal asynchrony, 35 or (4) low ROX index. 36 Consider expert opinion in indeterminate cases. When in doubt, err on the side of early intubation
If clinical findings are consistent with ARDS, hemodynamic instability, altered mental status, or severe hypoxia, proceed directly to endotracheal intubation and mechanical ventilation, unless not available or not desired by the patient.
NIPPV should only be used if helmet masks are available, or if high flow oxygen systems options are not available (summary in Figure 2).
6. CONCLUSION
The COVID‐19 pandemic requires a more comprehensive risk‐benefit analysis with deciding the modality of respiratory support for patients. The risks for healthcare worker infection, nosocomial spread, availability of resources, and clinical spectrum of disease must all be considered.
CONFLICTS OF INTEREST
Jessica S. Whittle has served as a consultant to Vapotherm, Inc., within the last 3 years for the development of educational materials. All compensation totaled less than $1000. Ivan Pavlov has been a speaker for Fisher‐Pakyel within the last 3 years. All compensation was paid to the charitable foundation at the hospital where he works and he did not personally receive any compensation. Alfred D. Sacchetti, Charles Atwood, and Mark S. Rosenberg have no disclosures.
Whittle JS, Pavlov I, Sacchetti AD, Atwood C, Rosenberg MS. Respiratory support for adult patients with COVID‐19. JACEP Open 2020;1:95–101. 10.1002/emp2.12071
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Henry E. Wang, MD, MS.
[Correction added on 24 April 2020, after first online publication: the phrase “silent ischemia” is replaced with “silent hypoxemia” in Figure 2.]
REFERENCES
- 1. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. Journal of Medical Virology. 2020; 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Clinical management of severe acute respiratory infection when Novel coronavirus (2019‐nCoV) infection is suspected: Interim Guidance . WHO/nCoV/Clinical/2020.3 January 28 2020.
- 3. Ferguson N, Laydon D, Nedjati Gilani G, et al. Report 9: Impact of non‐pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. Imperial College London 16 March 2020. 10.25561/77482. [DOI]
- 4. Mullins PM, Goyal M, Pines JM. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Acad Emerg Med. 2013;20(5):479‐486. [DOI] [PubMed] [Google Scholar]
- 5. Herring AA, Ginde AA, Fahimi J, et al. Increasing critical care admissions from U.S. emergency departments, 2001–2009. Crit Care Med. 2013;41(5):1197‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UK . Guidance on infection prevention and control for COVID‐19. In: England PH, ed. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control2020.
- 8. HPSC . Aerosol Generating Procedures (AGPs) . https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/infectioncontroladvice/File,3625,en.pdf.: Scotland NHS National Services; 2020.
- 9. CDC . Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID‐19) in Healthcare Settings. 2020. https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/dialysis.html
- 10. WHO . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 11. Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147(5):1336‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hui DS, Chan MT, Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20(suppl 4):9‐13. [PubMed] [Google Scholar]
- 13. Farias E, Rudski L, Zidulka A. Delivery of high inspired oxygen by face mask. J Crit Care. 1991;6(3):119‐124. [Google Scholar]
- 14. Herren T, Achermann E, Hegi T, Reber A, Staubli M. Carbon dioxide narcosis due to inappropriate oxygen delivery: a case report. J Med Case Rep. 2017;11(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harari SA, Vitacca M, Blasi F, Centanni S, Santus PA, Tarsia P. Managing the Respiratory care of patients with COVID‐19 . http://www.aiponet.it: Italian Thoracic Society ‐ Associazione Italiana Pneumologi Ospedalieri ‐ Societa Italiana Di Pneumologia;2020.
- 16. Respiratory care committee of Chinese Thoracic S . [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020. [DOI] [PubMed] [Google Scholar]
- 17. ANZICS . COVID‐19 Guidelines. Australian and New Zealand Intensive Care Society. Melbourne: ANZICS; 2020. http://cec.health.nsw.gov.au/__data/assets/pdf_file/0004/572512/ANZICS-COVID-19-Guidelines-Version-1.pdf [Google Scholar]
- 18. Kluge S, Janssens U, Welte T, et al. [Recommendations for critically ill patients with COVID‐19]. Medizinische Klinik, Intensivmedizin und Notfallmedizin. 2020. 10.1007/s00063-020-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‐19). Intensive Care Medicine. 2020; 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishimura M. High‐flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529‐541. [DOI] [PubMed] [Google Scholar]
- 21. Frat JP, Thille AW, Mercat A, et al. High‐flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185‐2196. [DOI] [PubMed] [Google Scholar]
- 22. Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta‐analysis. Intensive Care Med. 2019;45(5):563‐572. [DOI] [PubMed] [Google Scholar]
- 23. Nagata K, Morimoto T, Fujimoto D, et al. Efficacy of high‐flow nasal cannula therapy in acute hypoxemic respiratory failure: decreased use of mechanical ventilation. Respir Care. 2015;60(10):1390‐1396. [DOI] [PubMed] [Google Scholar]
- 24. Plate JDJ, Leenen LPH, Platenkamp M, Meijer J, Hietbrink F. Introducing high‐flow nasal cannula oxygen therapy at the intermediate care unit: expanding the range of supportive pulmonary care. Trauma Surg Acute Care Open. 2018;3(1):e000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high‐flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4). 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 26. Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7(12):e50845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leung CCH, Joynt GM, Gomersall CD, et al. Comparison of high‐flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101(1):84‐87. [DOI] [PubMed] [Google Scholar]
- 28. Kotoda M, Hishiyama S, Mitsui K, et al. Assessment of the potential for pathogen dispersal during high‐flow nasal therapy. J Hosp Infect. 2019. 10.1016/j.jhin.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5(5):e10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rello J, Perez M, Roca O, et al. High‐flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012;27(5):434‐439. [DOI] [PubMed] [Google Scholar]
- 31. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID‐19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouadma L, Lescure F‐X, Lucet J‐C, et al. Severe SARS‐CoV‐2 infections: practical considerations and management strategy for intensivists. Intensive Care Medicine. 2020;46 (4):579–582. 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37(11):1780‐1786. [DOI] [PubMed] [Google Scholar]
- 36. Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high‐flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368‐1376. [DOI] [PubMed] [Google Scholar]
- 37. Fielding‐Singh V, Matthay MA, Calfee CS. Beyond low tidal volume ventilation: treatment adjuncts for severe respiratory failure in acute respiratory distress syndrome. Crit Care Med. 2018;46(11):1820‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID‐19. The Lancet Respiratory Medicine. 2020; 10.1016/s2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung TM, Yam LY, So LK, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126(3):845‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fowler RA, Guest CB, Lapinsky SE, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169(11):1198‐1202. [DOI] [PubMed] [Google Scholar]
- 41. Yu IT, Xie ZH, Tsoi KK, et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44(8):1017‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine. 2020; 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie J, Tong Z, Guan X, et al. Critical care crisis and some recommendations during the COVID‐19 epidemic in China. Intensive Care Med. 2020. 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weingart S. Some Additional COVID Airway Management Thoughts. Vol March 16 2020. https://emcrit.org/emcrit/some-additional-covid-airway-management-thoughts/ EMCrit Blog; 2020.
- 45. Moriates C, Feldman L. Nebulized Brochodilator Instead of MDI. J. Hosp. Med 2015;10;691‐693. [DOI] [PubMed] [Google Scholar]


