Abstract
Background
Children are commonly affected by respiratory tract infections. Based on clinical symptoms, laboratory evaluation, and imaging, the causative pathogen often cannot be delineated. Point‐of‐care‐testing systems that provide an opportunity for fast detection of common viruses and some bacteria can therefore influence treatment's options. We aimed to examine whether the Biofire® FilmArray® has an effect on antibiotic treatment, duration of antibiotic therapy, and length of hospital stay within a pediatric cohort.
Methods
We included children who were admitted to inpatient treatment with an acute respiratory tract infection from 02/2017 to 04/2018 using the FA respiratory panel for pathogen detection. The study group data were compared to the retrospective data of children admitted from 02/2016 to 02/2017, using a proprietary multiplex RT‐PCR.
Results
A total of 322 children of the study group and 464 children of the control group were analyzed for clinical symptoms, laboratory findings, antibiotic treatment, and length of hospital stay. There was no significant reduction (P < .05) of antibiotic treatment and length of hospital stay. CRP, prehospital antibiotic treatment, antibiotic treatment, past medical history, age, and further pathogen detection showed a significant impact on antibiotic therapy, duration of antibiotic treatment, and length of hospital stay.
Conclusion
The use of the FA did not result in a significant reduction of antibiotic treatment or in length of hospital stay. Other parameters had a more significant impact. Therefore, we suggest that standard operation procedures with therapy guidelines are necessary to provide an effective application of POCT systems.
Keywords: acute respiratory tract infections, antibiotic treatment, Biofire® FilmArray®, multiplex RT‐PCR, point‐of‐care‐testing
1. INTRODUCTION
Respiratory tract infections are one of the most common diseases in children and cause high morbidity and mortality worldwide.1
The clinical symptoms of respiratory tract infections often show no or little correlation to the causative pathogen. However, differentiation between bacterial and viral etiology is important for further treatment. Because of the difficulty in identifying the comparatively little number of children suffering from bacterial pathogens, most of the children with fever are admitted to inpatient treatment due to their worsened clinical condition and often treated with antibiotics. This may significantly affect the incidence of multiresistant pathogens.1, 2, 3, 4, 5, 6, 7
Pathogens can be identified by molecular biological systems such as the multiplex RT‐PCR.8 However, this is often underutilized in clinical practice because it remains quite labor‐intensive. Point‐of‐care‐testing (POCT) systems like the Biofire® FilmArray® (FA) provide an opportunity for the analysis and results of respiratory specimens within one hour on the ward.6 Hereby, diagnostic tools are integrated in the clinical environment and support therapy decisions with valid and fact‐based information.9, 10
In our study, the usability of the FA was tested in hospital to address whether the use of antibiotics and the length of hospital stay can be regulated on the rational basis, that is, lead to a reduction in the use of antibiotics, length of hospital stay, and therapy costs.
2. MATERIALS AND METHODS
2.1. Study aims
The primary outcome of the study was the comparison of antibiotic treatment between the study group and the control group. Secondary outcomes were whether antibiotic therapy and duration can be reduced after implementing the FA respiratory panel and if this may lead to a reduction of length of stay in hospital and therapy costs.
2.2. Study design and study population
The study is a mono‐centric, open‐labeled, non‐randomized observational study with a historical control group.
Children admitted to the Infectious Disease Ward of the Children's Department of the Johannes Gutenberg University Mainz with an acute respiratory tract infection were included in the present study. Data of 322 children diagnosed with the use of the FA respiratory panel between February 2017 and April 2018 were analyzed for turnaround time, clinical findings, laboratory parameters, antimicrobial therapy, and length of hospital stay. The data were compared with the retrospectively collected data of 464 children treated for an acute respiratory tract infection between February 2016 and February 2017 with the use of the proprietary multiplex RT‐PCR. Division into subgroups for age older than 12 years and certain viral pathogens (human rhinovirus/enterovirus (hRV/EnterVir), respiratory syncytial virus (RSV), adenovirus (AdenVir), human metapneumovirus (hMPV)) were performed. Children with community‐acquired acute respiratory tract infection aged between 0 and 16 years and with availability of data were included to the study. Children suffering from primary immune deficiency, nosocomial infections, serious chronic diseases of the respiratory tract, age older than 16 years or no consent for the current study group were excluded.
2.3. Clinical specimens and patient data
The patient's data were collected using the clinic internal SAP® and Lauris® programs. By admittance to inpatient treatment, consent was given for the control group to an anonymized scientific analysis of the data acquired during diagnostics and treatment. For the study group, patients’ parents had to sign an informed consent form.
Material for diagnostics was obtained by a nasopharyngeal swab. For the study group, the specimens were directly tested on the ward with the FA by the physician in charge. Results of the testing could be directly accessed on the ward. For diagnosis using the multiplex RT‐PCR, the specimens were transported to the laboratory and processed immediately (during working hours), stored at 4°C for a maximum of 24 hours (after working hours), or frozen at −70°C until they were processed. Collected results of all samples tested with the multiplex RT‐PCR were provided for retrospective data analysis.
2.4. Diagnostic methods
The FA respiratory panel can detect 20 viral and 3 bacterial pathogens simultaneously from nasopharyngeal aspirates: adenovirus, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43, human metapneumovirus, influenza A, influenza A subtype H1, influenza A subtype H3, influenza A subtype H1‐2009, influenza B, parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3, parainfluenza virus 4, human rhinovirus/enterovirus, respiratory syncytial virus, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae.11, 12
The FA is a closed system that performs automated nucleic acid extraction, reverse transcription, nucleic acid amplification from a single nasopharyngeal specimen, and results analysis in approximately 1 hour per specimen. A minimum of 300 µL nasopharyngeal specimen is needed for each run. The user places the specimen into the pouch and loads it into the manual. All other operations are automated.11, 12
The proprietary multiplex RT‐PCR used for the diagnosis of the nasopharyngeal aspirates of the control group was developed and established by Gröndahl et al.8 The upgraded version can detect 19 respiratory pathogens: adenovirus, coronavirus 229E, coronavirus OC43, human metapneumovirus, enterovirus, human rhinovirus, influenza A, influenza A (H1N1) pdm09, influenza B, parainfluenzavirus 1‐4, respiratory syncytial virus, Bordetella pertussis, Bordetella parapertussis, Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella pneumoniae.13 The human bocavirus can be also detected by another developed version.8, 13
The duration of the analysis with the multiplex RT‐PCR is two working days.
2.5. Statistical analysis
The statistical analysis was performed using SPSS® 23.0 and GraphPad Prism. For continuous variables, the mean, median, and the standard deviation were calculated. For categorical variables, the absolute and relative numbers were calculated.
Statistical significance for the overall study collective and children older than 12 months was evaluated with a logistic regression analysis for antibiotic treatment and a linear regression analysis for the duration of antibiotic therapy and the length of hospital stay with a confidence interval of 95% and P‐value <.05. Due to the small number of cases, subgroup analysis for certain pathogens for antibiotic therapy and length of stay in hospital and analysis for prehospital therapy and antiviral treatment were only performed descriptively.
2.6. Ethical committee approval
The present registry was approved by the ethical committee of the Medical Association Rheinland‐Pfalz, Reg. No. 2018‐13640.
3. RESULTS
3.1. Study population
The study group included n = 322 patients treated for an acute respiratory tract infection with the help of the FA from February 2017 to April 2018. The control group included n = 464 patients diagnosed with the use of the proprietary multiplex RT‐PCR from February 2016 to February 2017.
The study collective is depicted in Table 1.
Table 1.
Study population
| Study group | Control group | |
|---|---|---|
| Overall collectivea | 322 | 464 |
| Sexa | ||
| Male | 194 (60.2) | 287 (61.9) |
| Female | 128 (39.8) | 177 (38.1) |
| Age (mo)b | ||
| Minimum | 0 | 0 |
| Maximum | 198 | 203 |
| Mean ± SD | 26.4 ± 34.9 | 31.8 ± 43.0 |
| Median | 15.0 | 16.0 |
| 25%‐percentile | 4.0 | 4.0 |
| 75%‐percentile | 33.2 | 37.0 |
| Admission reasonsa | ||
| 1 | 257 (79.8) | 363 (78.2) |
| ≥2 | 65 (20.2) | 101 (21.8) |
| Lower respiratory tract infection | 199 (61.8) | 276 (59.5) |
| Upper respiratory tract infection | 97 (30.1) | 157 (33.4) |
| Nonrespiratory tract infection | 48 (14.9) | 43 (9.3) |
| Seizure | 29 (9.0) | 61 (13.1) |
| Influenza | 19 (5.9) | 44 (9.5) |
| Past medical historya | 98 (30.4) | 138 (29.7) |
Binary variables: absolute and relative number.
Continuous variables: median, minimum, maximum, mean ± standard deviation (SD).
3.2. Pathogen detection
The mean processing time until the detection of a pathogen was 0.8 days for the study group and 6.6 days for the control group. In 76.1% (245) of children in the study group, one or more pathogens were detected with the FA.
The turnaround time is shown in Table 2.
Table 2.
Processing time, antibiotic treatment, duration of antibiotic therapy, and length of hospital stay
| Study group | Control group | |
|---|---|---|
| Processing time (d)a | ||
| Minimum | 0 | 1 |
| Maximum | 7 | 23 |
| Mean ± SD | 0.8 ± 1.1 | 6.6 ± 3.6 |
| Median | 1.0 | 6.0 |
| Antibiotic treatment prehospitallyb | 46 (16.3) | 57 (12.3) |
| Antibiotic treatmentb | ||
| Overall collective | 145 (45.0) | 197 (42.5) |
| Age > 12 mo | 76 (42.7) | 124 (45.6) |
| EnterVir/hRV | 46 (44.7) | 57 (37.3) |
| RSV | 32 (48.5) | 48 (41.0) |
| AdenVir | 16 (61.5) | 6 (75.0) |
| hMPV | 6 (35.3) | 4 (50.0) |
| Antiviral treatmentb | ||
| Oseltamivir | 6 (1.9) | 4 (0.9) |
| Oseltamivir + antibiotics | 3 (2.1) | 1 (0.5) |
| Oseltamivir ‐ antibiotics | 3 (1.7) | 3 (1.1) |
| Duration of antibiotic therapy (d)a | ||
| Overall collective | ||
| Minimum | 1.0 | 1.0 |
| Maximum | 39.0 | 49.0 |
| Mean ± SD | 9.1 ± 5.4 | 8.6 ± 4.4 |
| Median | 9.0 | 7.0 |
| 25%‐percentile | 7.0 | 7.0 |
| 75%‐percentile | 10.0 | 10.0 |
| Age > 12 mo | ||
| Minimum | 1.0 | 1.0 |
| Maximum | 39.0 | 49.0 |
| Mean ± SD | 9.7 ± 5.4 | 9.0 ± 4.6 |
| Median | 10.0 | 8.0 |
| 25%‐percentile | 7.0 | 7.0 |
| 75%‐percentile | 11.0 | 10.0 |
| Length of hospital stay (d)a | ||
| Overall collective | ||
| Minimum | 1.0 | 0,0 |
| Maximum | 65.0 | 51.0 |
| Mean ± SD | 4.7 ± 5.4 | 4.7 ± 4.4 |
| Median | 3.0 | 4.0 |
| 25%‐percentile | 2.0 | 2.0 |
| 75%‐percentile | 6.0 | 6.0 |
| Age > 12 mo | ||
| Minimum | 1.0 | 0 |
| Maximum | 65.0 | 44.0 |
| Mean ± SD | 4.4 ± 5.6 | 4.3 ± 4.1 |
| Median | 3.0 | 3.0 |
| 25%‐percentile | 2.0 | 2.0 |
| 75%‐percentile | 4.0 | 5.0 |
| EnterVir/hRV | 3.0 | |
| Minimum | 1.0 | 0.0 |
| Maximum | 65.0 | 44.0 |
| Mean ± SD | 4.8 ± 6.8 | 4.3 ± 4.4 |
| Median | 3.0 | 3.0 |
| RSV | ||
| Minimum | 1.0 | 1.0 |
| Maximum | 56.0 | 17.0 |
| Mean ± SD | 6.3 ± 6.8 | 5.1 ± 2.7 |
| Median | 5.0 | 5.0 |
| AdenVir | ||
| Minimum | 2.0 | 2.0 |
| Maximum | 9.0 | 6.0 |
| Mean ± SD | 3.9 ± 1.9 | 4.0 ± 1.3 |
| Median | 3.0 | 4.0 |
| hMPV | ||
| Minimum | 2.0 | 2.0 |
| Maximum | 23.0 | 19.0 |
| Mean ± SD | 5.0 ± 4.8 | 6.0 ± 5.5 |
| Median | 4.0 | 4.0 |
Continuous variables: median, minimum, maximum, mean ± standard deviation (SD).
Binary variables: absolute and relative number.
The following numbers refer to those 244 positive tested children. The most frequently detected pathogens were enterovirus/hRV in 42.2% (103) of cases, RSV in 27.0% (66) of cases, adenovirus in 10.7% (26) of cases, coronavirus and hMPV in 7.0% (17) of cases, influenza A in 6.6% (16) of cases, and influenza B in 4.5% (11) of cases. In 74.8% (347) of children in the control group, one or more pathogens were detected. The most detected pathogens with the multiplex RT‐PCR were enterovirus/hRV in 44.1% (153) of cases, RSV in 33.7% (117) of cases, influenza B in 7.5% (26) of cases, influenza A in 6.9% (24) of cases, and human bocavirus in 5.2% (18) of cases. All other pathogens were markedly less often detected.
Regarding co‐infections detected with the FA or the multiplex RT‐PCR, 16.0% (39) of positive tested children in the study group and 11.2% (39) of cases of the control group two or three pathogens were detected. In the study group, 22 combinations of co‐infections and in the control group, 17 different combinations appeared. The most frequent pathogens for co‐infections were enterovirus/hRV in 22 cases in the study group and 29 cases in the control group and RSV, which appeared in 19 cases in both groups.
Detected pathogens are depicted in Figure 1.
Figure 1.
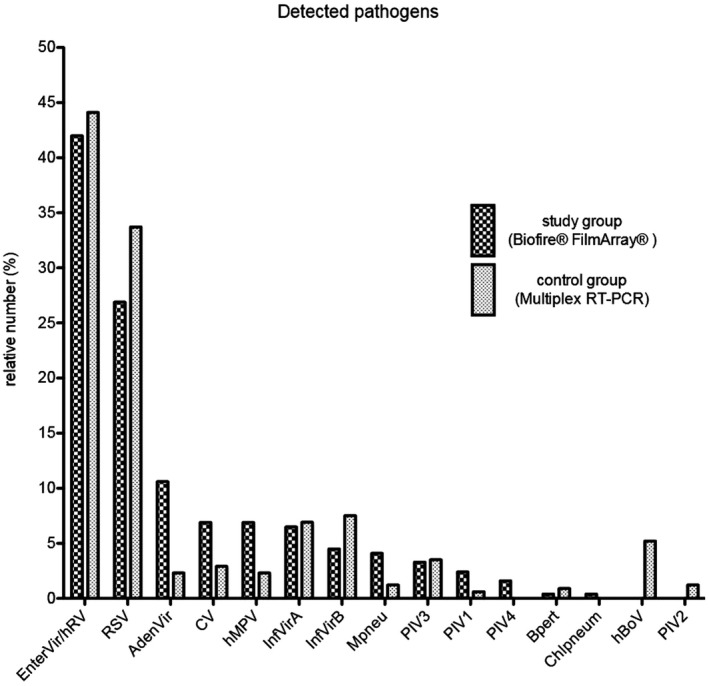
Detected Pathogens. Relative number of the detected pathogens for the study group with the Biofire® FilmArray® and for the control group with the multiplex RT‐PCR
In 10.6% (34) of children in the study group and 10.3% (48) of children in the control group, further pathogens were detected in eye, nose or ear swab, blood culture, cerebrospinal fluid, stool, or urine culture or children had a positive Streptococcus rapid antigen test or a pathogen detection in serology. The pathogens detected in both groups did not differ from each other.
3.3. Antibiotic treatment
A total of 14.3% (46) of children in the study group and 12.3% (57) of children in the control group received prehospital antibiotic treatment. A total of 80.4% (37) of the patients who received prehospital treatment in the study group and 73.7% (42) of the control group were treated with antibiotics during their hospital stay. In most cases, data were unavailable for the exact agent of antibiotics and duration of prehospital treatment. Therefore, this was not included in the analysis.
A total of 45.0% (145) of patients in the study group and 42.5% (197) of patients in the control group were treated with antibiotics during their hospital stay. The binary logistic regression analysis shows no significant (P = .784) impact of the FA or the multiplex RT‐PCR on the antibiotic treatment. The increase in CRP concentration by 1 mg/L has a strong impact on antibiotic treatment and increases the chance of antibiotic therapy by 2.9%. This result is statistically significant (P < .0001). In patients who received prehospital treatment with antibiotics, the chance of receiving antibiotic therapy is 4.65 times higher. This result is also statistically significant (P < .0001). In patients who had further pathogen detection in blood culture, serologic antigen tests or other tests than the multiplex RT‐PCR, the chance of antibiotic treatment is 7.6 times higher. This result is also statistically significant (P < .0001). 6.9% (10) patients in the study group and 8.6% (17) in the control group received a combined therapy of antibiotic treatment with acyclovir, probably triggered by signs of meningeal affection. Changes in antibiotic treatment based on the results could not be identified.
A total of 1.7% (6) of children of the study group and 0.9% (4) of the control group received antiviral treatment with oseltamivir for influenza infection.
Because of differing antibiotic treatment guidelines in infants, we performed additional statistical analysis for children older than 12 months. In children older than 12 months, 42.7% (76) of the study group and 45.6% (124) of the control group were treated with antibiotics during their hospital stay. The binary logistic regression analysis shows no significant (P = .189) impact of the FA or the multiplex RT‐PCR on the antibiotic treatment for children older than 12 months. The increase in the CRP by one unit has a strong impact on the antibiotic treatment and increases the chance of antibiotic therapy by 2.6%. The result is statistically significant (P < .0001). In patients that were treated prehospitally with antibiotics, the chance of receiving antibiotic therapy in hospital is 6.37 times higher. This result is also statistically significant (P < .0001). In patients who had further pathogen detection, the chance of receiving antibiotic therapy is 5.19 times higher. The result is also statistically significant (P < .0001).
Patients with the detection of adenovirus received 61.5% of antibiotic treatment in the study group compared to the control group with 75.0% of antibiotic treatment. Patients with the detection of hMPV received 35.5% of antibiotic treatment compared to the control group with 50% antibiotic treatment.
Antibiotic treatment is depicted in Figure 2 and Table 2.
Figure 2.
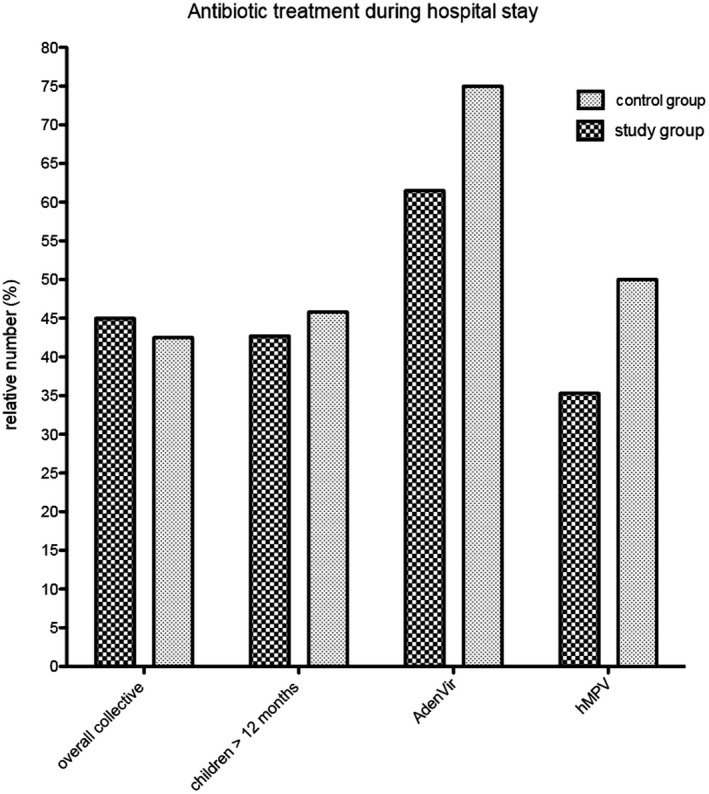
Antibiotic treatment during hospital stay. Relative number of antibiotic therapies for the overall collective, children older than 12 mo, children with adenovirus infection, and children with hMPV infection during hospital stay
3.4. Duration of antibiotic therapy
The mean duration of antibiotic therapy was 9.1 days for the study group and 8.6 days for the control group. The median was 9.0 days (IQR 7‐10) for the study group and 7.0 days (IQR 7‐10) for the control group. The diagnostic method, FA or multiplex RT‐PCR, showed no significant (P = .592) impact on the duration of antibiotic treatment in the linear logistic regression analysis. The CRP at admission (P = .002), further pathogen detection (P = .0005), and past medical history (P = .026) showed a significant impact on the duration of antibiotic therapy.
For children older than 12 months, the mean duration of antibiotic treatment was 9.7 days for the study group and 9.0 days for the control group. The median was 10.0 (IQR 7‐11) days for the study group and 8.0 days (IQR 7‐10) for the control group. The linear regression analysis for the duration of antibiotic treatment for children older than 12 months also showed no significant difference between the FA and the multiplex RT‐PCR. A significant impact on the duration of antibiotic therapy exists for CRP (P = .0002) and further pathogen detection (P = .027).
Duration of antibiotic therapy is depicted in Table 2.
3.5. Length of hospital stay
The mean hospital length of stay for both study groups was 4.7 days. The median was 3.0 days (IQR 2‐6) for the study group and 4.0 days (IQR 2.6) for the control group. The diagnostic method, FA or multiplex RT‐PCR, showed no significant impact on the length of hospital stay in the linear regression analysis. A statistically significant impact on the length of stay was found for the antibiotic treatment (P < .0001), further pathogen detection (P < .0001), and past medical history (P < .0001). Furthermore, younger patients had a longer hospital length of stay (P = .001).
We analyzed the age ranges for antibiotic treatment for children older than 12 months separately and observed that antibiotic treatment has an impact on the length of hospital stay. The duration of hospital stay was analyzed separately. The mean length of stay in hospital for children older than 12 months was 4.4 days for the study group and 4.3 days for the control group. The median was 3.0 days (IQR 2‐4) for both study groups. The linear regression analysis for the length of stay in children older than 12 months also showed no significant impact of the FA or multiplex RT‐PCR. Antibiotic treatment (P < .0001), further pathogen detection (P < .0001) and past medical history of the patients (P = .035) have an effective impact on the length of hospital stay.
Patients with the detection of Adenovirus had a median length of stay of 3.0 days in the study group compared to the length of stay of 4.0 days in the control group. Patients with the detection of hMPV had a median hospital length of stay of 4.0 days compared to the length of stay of 4.5 days in the control group.
The length of hospital stay is depicted in Figure 3 and Table 2.
Figure 3.
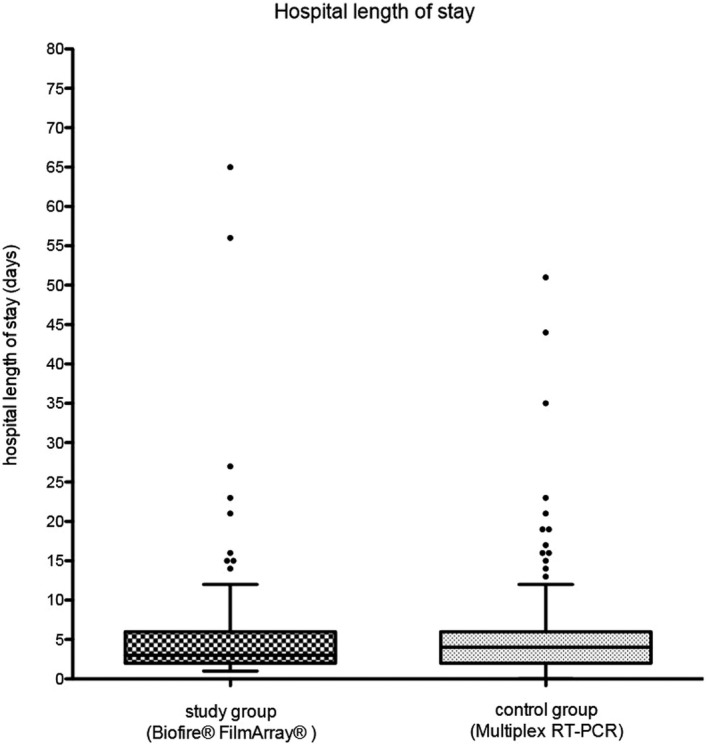
Length of hospital stay. Length of hospital stay in days for the study and the control group
4. DISCUSSION
4.1. Antibiotic treatment and length of hospital stay
In our study, we could reduce the turnaround time from a mean processing duration of 6.6 days for the control group to 0.8 days for the study group. The decrease in turnaround time did not show any significant effect on the frequency of inpatient antibiotic treatment. In the present study, we also found no evidence of reduction of length of stay in hospital using the FA compared with the proprietary multiplex RT‐PCR overall. The binary logistic regression analysis and linear regression analysis did not show an impact of the method used on the antibiotic prescription, duration of antibiotic therapy, and length of hospital stay. However, a decrease in frequency of antibiotic therapy and reduction of length of hospital stay by half a day to one day could be shown for children with adenovirus and hMPV infections in a descriptive analysis. Furthermore, timely oseltamivir therapy for 6 out of 27 patients with Influenza detection in the study group in contrast to 4 out of 50 patients with influenza detection in the control group could be enabled. Andrews et al also did not show any significant decrease in length of hospital stay using the FA respiratory panel as POCT for adults with respiratory tract infections.10 However, in our study, CRP, prehospital antibiotic treatment, past medical history, age, and further pathogen detection have a statistically significant impact on antibiotic treatment and length of hospital stay. Branche et al and Gelfer et al also showed that a significant reduction in antibiotic treatment could be shown for patients with viral pathogen detection and low procalcitonin values among adult patients with ambulant acquired pneumonia.14, 15
Visseaux et al showed that higher viral pathogen detection in children due to the diagnostic use of multiplex RT‐PCR may decrease antibiotic treatment.16 This claim could not be proved in the present study.
In contrast to our study, Rogers et al demonstrated that the implementation of the FA resulted in a reduction of antibiotic treatment of half a day and length of hospital stay of a quarter of a day, but only if the analysis was performed within the first four hours after admission to the hospital. Thus, the hospital costs were reduced by US$231 and the antibiotic costs by US$17 per patient.9
Marinari et al showed a reduction of antibiotic prescription and a decrease in the duration of antibiotic treatment in adults due to the use of the FA.17 Brendish et al also showed a decrease in length of hospital stay due to the use of POCT for respiratory infections in adult patients.18 Furthermore, Barenfanger et al calculated an increase in processing costs of US$1000 due to the use of a multiplex RT‐PCR but also a decrease in the length of hospital stay of 5.3 days in the second year after implementation, meaning that the hospital costs decreased by US$154 332 whereby US$144 332 could be saved per year.19 Due to the rapid reporting of viral respiratory infections, patients could be discharged sooner and costs that are normally spent for the stay in hospital and antibiotic treatment could be reduced. McFall et al demonstrated a significantly decreased length of hospital stay and reduced antibiotic treatment for children younger than three months using the FA respiratory panel for sepsis workup within 48 hours of admission, which may suggest reduced health care costs despite high acquisition costs of the FA.20
Keske et al showed a reduction of antibiotic treatment due to the implementation of an antibiotic stewardship program. They used the FA for some years but could show an effect on the antibiotic treatment only after staff training.21 A Cochrane meta‐analysis of Davey et al showed a reduction of antibiotic treatment of 1.95 days due to antibiotic stewardship programs without increasing the patients’ mortality.22 Furthermore, feedback from the physician in charge on the performed intervention increases the effect of those programs.
An antibiotic stewardship program with coordinated interventions based on the mentioned impact factors for the appropriate use of antimicrobials may decrease the antibiotic prescription rate and thus length of hospital stay. Furthermore, the frequency of antibiotic therapy and length of hospital stay was decreased with the detection of Adenovirus and hMPV. The fast pathogen detection may be particularly useful for respiratory tract infections with certain pathogens. Adenovirus infection often cause leucocytosis and increase in CRP23 and hMPV infection clinically presents as pneumonia24 and resembles bacterial infection. In RSV, for which skilled handling already exists due to rapid antigen‐based tests, no decrease in antibiotic prescription and hospital length of stay was seen. The development of standard operation procedures (SOP) with therapy guidelines for the handling of viral pathogen detection considering clinical parameters could lead to a decrease in antibiotic treatment, duration of stay in hospital and thus costs.9, 18, 19, 21, 25 Furthermore, the patients’ past medical history and prehospital antimicrobial therapy support specific diagnostics. The development of an algorithm and teaching of the physicians how to use the FA as a POCT system in the clinical context is therefore required. In addition, the use of POCT systems for certain viral pathogens and the specific effects of a shorter turnaround time on isolation, prevention of nosocomial infections, and cost savings must be further explored.
4.2. Limitations
This study has potential limitations. As we used a historic control group from the previous season, there is a lack of comparison between the study group and the control group due to a different number of patients and the seasonal differences in pathogen detection. The detection of pathogens with the FA and the multiplex RT‐PCR from the previous season does not match exactly that may influence antibiotic treatment and length of hospital stay. RSV and hRV were detected more frequently among the control group that may affect the result of our study.
The FA and the multiplex RT‐PCR detect the same pathogens with almost the same sensitivity and specificity, except for Human Bocavirus and Legionella pneumoniae.8, 11, 12, 13 Therefore, we did not perform an additional RT‐PCR analysis of the POC samples.
In addition, we did not exclude children with bacterial detection with the FA or the multiplex RT‐PCR from the study collective. In both groups, bacteria were detected, and some children had further pathogen detection which could not be analyzed exactly. For more specific analysis of antibiotic prescription in children with viral pathogen detection in POCT systems, analysis should be performed with the exclusion of bacterial detection. Moreover, the effect of POCT systems on the precise prescription of antibiotic for children with bacterial detection could be evaluated.
Furthermore, the numbers of patients in both groups were not identical and the median age of children from the control group is 16 months compared to children from the study group who had a median age of 15 months. In linear and logistic regression analysis, the children's age had a significant impact of antibiotic treatment and length of hospital stay. Further limitation is the small number of certain pathogen detection such as adenovirus and hMPV. Therefore, subgroup analysis was performed descriptively, and we could only show a tendency, but no significant result. To perform a statistically significant analysis, larger subgroups are required.
An approach testing for either multiplex RT‐PCR or FA would have been an option for better comparison. But we only performed the study within one season and a division would have reduced the already low study size and may have made further subgroup analysis impossible.
5. CONCLUSIONS
We found no significant reduction in the frequency of antibiotic treatment, duration of antibiotic therapy, and length of hospital stay overall using the FA but did for children older than 12 months and Adenovirus and hMPV infection. CRP, prehospital antibiotic treatment, antibiotic treatment, past medical history, age, and further pathogen detection showed a significant impact on antibiotic therapy and duration of hospital stay.
Further studies are therefore required that focus on implementing therapy guidelines including pathogen detection and clinical parameters for the handling of POCT systems. Specific studies that focus on a larger number of cases and the effect of POCT systems for certain pathogens and the effect of a shorter turnaround time should be performed. We currently perform a follow‐up study at the Children's Hospital of the Johannes Gutenberg University in Mainz based on the findings of this study and an accordingly adjusted SOP for the treatment of respiratory tract infections in children in order to examine the impact on antibiotic treatment and hospital length of stay.
Reischl AT, Schreiner D, Poplawska K, et al. The clinical impact of PCR‐based point‐of‐care diagnostic in respiratory tract infections in children. J Clin Lab Anal. 2020;34:e23203 10.1002/jcla.23203
Contributor Information
Anna Theresa Reischl, Email: areischl@students.uni-mainz.de.
Stephan Gehring, Email: stephan.gehring@uni-mainz.de.
REFERENCES
- 1. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23(1):74‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(suppl. 4):S284‐S289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heikkinen T, Järvinen A. The common cold. Lancet. 2003;361(9351):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byington CL, Castillo H, Gerber K, et al. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med. 2002;156(12):1230‐1234. [DOI] [PubMed] [Google Scholar]
- 5. Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21(4):716‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pérez‐Ruiz M, Pedrosa‐Corral I, Sanbonmatsu‐Gámez S, Navarro‐Marí M. Laboratory detection of respiratory viruses by automated techniques. Open Virol J. 2012;5(1):151‐159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Popow‐Kraupp T, Aberle JH. Diagnosis of respiratory syncytial virus infection. Open Microbiol J. 2011;5(1):128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grondahl B, Puppe W, Hoppe A, Kuhne I, Weigl JA, Schmitt HJ. Rapid identification of nine microorganisms causing acute respiratory tract infections by single‐tube multiplex reverse transcription‐PCR: feasibility study. J Clin Microbiol. 1999;37(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers BB, Shankar P, Jerris RC, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2014;139(5):636‐641. [DOI] [PubMed] [Google Scholar]
- 10. Andrews D, Chetty Y, Cooper BS, et al. Multiplex PCR point of care testing versus routine, laboratory‐based testing in the treatment of adults with respiratory tract infections: a quasi‐randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis. 2017;17(1):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. BioFire . FilmArray® Respiratory Panel (RP) Instruction Booklet. Salt Lake City, UT: BioFire Diagnostics; 2015. [Google Scholar]
- 12. Leber AL, Everhart K, Daly JA, et al. Multicenter evaluation of the BioFire FilmArray respiratory panel 2 for the detection of viruses and bacteria in nasopharyngeal swab samples. J Clin Microbiol. 2018; 56(6). pii: e01945‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puppe W, Weigl J, Gröndahl B, et al. Validation of a multiplex reverse transcriptase PCR ELISA for the detection of 19 respiratory tract pathogens. Infection. 2013;41(1):77‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelfer G, Leggett J, Myers J, Wang L, Gilbert DN. The clinical impact of the detection of potential etiologic pathogens of community‐acquired pneumonia. Diagn Microbiol Infect Dis. 2015;83(4):400‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Branche AR, Walsh EE, Vargas R, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015;212(11):1692‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Visseaux B, Collin G, Ichou H, et al. Usefulness of multiplex PCR methods and respiratory viruses’ distribution in children below 15 years old according to age, seasons and clinical units in France: A 3 years retrospective study. PLoS ONE ONE. 2017;12(2):e0172809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marinari L, Danny M. Impact of Viral Respiratory Panel Testing on AntibioticTherapy and Outcomes. A107 Clinical and Translational Investigations into Acute Respiratory Infections: American Thoracic Society; 2018; A2628‐A.
- 18. Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point‐of‐care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open‐label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barenfanger J, Drake C, Leon N, Mueller T, Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol. 2000;38(8):2824‐2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McFall C, Salimnia H, Lephart P, Thomas R, McGrath E. Impact of early multiplex FilmArray respiratory pathogen panel (RPP) assay on hospital length of stay in pediatric patients younger than 3 months admitted for fever or sepsis workup. Clin Pediatr. 2017;57(10):1224‐1226. [DOI] [PubMed] [Google Scholar]
- 21. Keske Ş, Ergönül Ö, Tutucu F, Karaaslan D, Palaoğlu E, Can F. The rapid diagnosis of viral respiratory tract infections and its impact on antimicrobial stewardship programs. Eur J Clin Microbiol Infect Dis. 2018;37(4):779‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients . Cochrane Database Syst Rev. 2017;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berce V, Unuk S, Duh D, Homšak M, Vičič M. Clinical and laboratory characteristics of viral lower respiratory tract infections in preschool children. Wien Klin Wochenschr. 2015;127(5):255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Don M, Korppi M, Valent F, Vainionpaa R, Canciani M. Human metapneumovirus pneumonia in children: results of an Italian study and mini‐review AU ‐ Don. Massimiliano. Scand J Infect Dis. 2008;40(10):821‐826. [DOI] [PubMed] [Google Scholar]
- 25. Blaschke AJ, Korgenski EK, Wilkes J, et al. Rhinovirus in febrile infants and risk of bacterial infection. Pediatrics. 2018;141(2):e20172384. [DOI] [PMC free article] [PubMed] [Google Scholar]


