Abstract
Objective
We aim to summarize reliable evidence of evidence‐based medicine for the treatment and prevention of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) by analyzing all the published studies on the clinical characteristics of patients with SARS‐CoV‐2.
Methods
PubMed, Cochrane Library, Embase, and other databases were searched. Several studies on the clinical characteristics of SARS‐CoV‐2 infection were collected for meta‐analysis.
Results
Ten studies were included in Meta‐analysis, including a total number of 50466 patients with SARS‐CoV‐2 infection. Meta‐analysis shows that, among these patients, the incidence of fever was 0.891 (95% CI: 0.818, 0.945), the incidence of cough was 0.722 (95% CI: 0.657, 0.782), and the incidence of muscle soreness or fatigue was 0.425 (95% CI: 0.213, 0.652). The incidence of acute respiratory distress syndrome (ARDS) was 0.148 (95% CI: 0.046, 0.296), the incidence of abnormal chest computer tomography (CT) was 0.966 (95% CI: 0.921, 0.993), the percentage of severe cases in all infected cases was 0.181 (95% CI: 0.127, 0.243), and the case fatality rate of patients with SARS‐CoV‐2 infection was 0.043 (95% CI: 0.027, 0.061).
Conclusion
Fever and cough are the most common symptoms in patients with SARS‐CoV‐2 infection, and most of these patients have abnormal chest CT examination. Several people have muscle soreness or fatigue as well as ARDS. Diarrhea, hemoptysis, headache, sore throat, shock, and other symptoms are rare. The case fatality rate of patients with SARS‐CoV‐2 infection is lower than that of Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). This meta‐analysis also has limitations, so the conclusions of this Meta‐analysis still need to be verified by more relevant studies with more careful design, more rigorous execution, and larger sample size.
Keywords: 2019‐nCoV, clinical symptoms, coronavirus, meta‐analysis, SARS‐CoV‐2
Highlights
A total of 10 articles were included in the Meta‐analysis, including 50466 patients with SARS‐CoV‐2 infection, which was by far the first Meta‐analysis with the largest sample size. The quality of the literature included in this study is high, the analysis performed is rigorous, and the conclusions drawn by this study are highly credible.
1. INTRODUCTION
Since December 2019, the epidemic of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) infectious pneumonia in Wuhan, China. The Chinese government and researchers have taken rapid measures to control the epidemic. 1 On January 30 2020, WHO declared that the epidemic of SARS‐CoV‐2 was a public health emergency of international concern (PHEIC). At present, the number of patients with SARS‐CoV‐2 infection is still rising, and its harm to human beings has exceeded the outbreak of severe acute respiratory syndrome (SARS) in China, 2002. 2 The clinical characteristics and the case fatality rate after SARS‐CoV‐2 infection have always been concerned by people, and it is also the focus of medical workers' research at present. However, due to the different design of different clinical studies and insufficient sample size, the conclusions of the published studies were different. To acquire more accurate conclusions on the clinical characteristics and mortality of patients with SARS‐CoV‐2 infection, we searched the relevant literatures and carried out single‐arm meta‐analysis. 3 Our findings provide important guidance for current clinical work on the prevention and treatment of SARS‐CoV‐2 infection.
2. MATERIAL AND METHODS
2.1. Search strategy
Three popular medical databases including PubMed, Cochrane Library, and Embase databases were searched for related literature, using the following keywords: “2019‐nCoV,” “Coronavirus,” “COVID‐19,” “SARS‐CoV‐2,” and “Wuhan Coronavirus.” Articles reviewed were dated up to February 24, 2020. In this meta‐analysis, there was no language restriction. Only available data from published articles were collected. Data from unpublished papers were not included.
2.2. The inclusive and exclusive criteria
2.2.1. Inclusive criteria
Studies that include randomized controlled trials, nonrandomized controlled trials, case‐control studies, cohort studies, cross‐sectional studies, and also case reports on the clinical characteristics of patients with SARS‐CoV‐2 infection. Clinical characteristics of patients with SARS‐CoV‐2 infection include fever (temperature ≥ 37.3°C), cough, muscle soreness or fatigue, acute respiratory distress syndrome (ARDS), abnormal chest computer tomography (CT) detection, patients in critical condition (severe cases are those who need to be sent to the intensive care unit (ICU) for treatment), as well as death due to SARS‐CoV‐2 infection.
2.2.2. Exclusive criteria
Articles were published repeatedly; studies did not include the research indicators needed for meta‐analysis; research data were missing.
2.3. Data extraction and paper quality evaluation
First of all, we screened the literature according to the literature abstract, excluding the articles that obviously do not meet the inclusive criteria, and then read the full article for rescreening. If any disagreement on the choice of the literature exists, a third evaluator will join to make the decision. All included literature were evaluated using the Newcastle‐Ottawa Scale (NOS). 4 The highest quality of the literature was 9 stars and the lowest 0 stars.
2.4. Statistical analysis
The statistical software Stata version 12.0 was used to carry out the single‐arm meta‐analysis. In order to reduce the influence of heterogeneity between the included studies on final conclusion, we used the random effects model for meta‐analysis. Original data included in the literature were first transformed by double arcsine method to make them conform to the normal distribution and then analyzed in Stata. The initial conclusion obtained by meta‐analysis was then restored using formula (P = (sin(tp/2))2) to reach final conclusion. To objectively evaluate the publication bias of the included literature, the Egger test with P < .05 as the existence of publication bias was performed, the values larger than which were considered as no publication bias.
3. RESULTS
3.1. Literature inclusion
A total of 284 articles were retrieved, among which 39 papers were removed due to repeated retrieval, 212 papers were removed after reading abstracts, and 23 were eliminated after reading the full text. At the end, a total of 10 articles 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 were included in this meta‐analysis, including data from 50 466 patients. The specific operation flow is shown in Figure 1. The characteristics of the literature are shown in Table 1.
Figure 1.
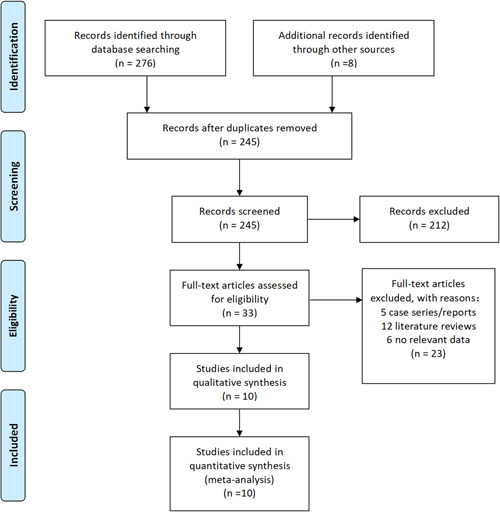
PRISMA flow diagram
Table 1.
The characteristics of the literature
| First author | Year | Country | Follow‐up (days) | No. patients | Sex | Average age | Research type | Literature quality | Clinical symptom |
|---|---|---|---|---|---|---|---|---|---|
| Huang et al 5 | 2020 | China | 18 | 41 | Male: 30 | 49 | Retrospective study | 7 | Fever |
| ARDS | |||||||||
| Muscle soreness or fatigue | |||||||||
| Cough | |||||||||
| Abnormal chest CT | |||||||||
| Female: 11 | Patient in critical condition | ||||||||
| Death of patient | |||||||||
| Wang et al 6 | 2020 | China | 34 | 138 | Male: 75 | 56 | Retrospective study | 7 | Fever |
| ARDS | |||||||||
| Muscle soreness or fatigue | |||||||||
| Cough | |||||||||
| Abnormal chest CT | |||||||||
| Patient in critical condition | |||||||||
| Female: 63 | Death of patient | ||||||||
| Chen et al 7 | 2020 | China | 25 | 99 | Male: 67 | 55.5 | Retrospective study | 6 | Fever |
| ARDS | |||||||||
| Muscle soreness or fatigue | |||||||||
| Female: 32 | Cough | ||||||||
| Abnormal chest CT | |||||||||
| Death of patient | |||||||||
| Guan W‐j 8 | 2020 | China | 28 | 1099 | Male: 640 | 47 | Retrospective study | 8 | Fever |
| ARDS | |||||||||
| Muscle soreness or fatigue | |||||||||
| Female: 459 | Cough | ||||||||
| Abnormal chest CT | |||||||||
| Patient in critical condition | |||||||||
| Death of patient | |||||||||
| Chen et al 9 | 2020 | China | 15 | 29 | Male: 21 | 56 | Retrospective study | 6 | Fever |
| Muscle soreness or fatigue | |||||||||
| Cough | |||||||||
| Patient in critical condition | |||||||||
| Death of patient | |||||||||
| Female: 8 | |||||||||
| Sun et al 10 | 2020 | America | 42 | 288 | Male: 182 | 44 | Retrospective study | 5 | Death of patient |
| Female: 106 | |||||||||
| Yang et al 11 | 2020 | China | 51 | 4021 | Male: 2211 | 49 | Retrospective study | 5 | Patient in critical condition |
| Female:1810 | Death of patient | ||||||||
| Li et al 12 | 2020 | China | 21 | 17 | Male: 9 | 45 | Retrospective study | 6 | Fever |
| Female: 8 | Muscle soreness or fatigue | ||||||||
| Cough | |||||||||
| Abnormal chest CT | |||||||||
| China CDC 13 | 2020 | China | 43 | 44 672 | Male: 22 981 | – | Retrospective study | 6 | Patient in critical condition |
| Female: 21 691 | Death of patient | ||||||||
| Xu 14 | 2020 | China | 16 | 62 | Male: 36 | 41 | Retrospective study | 6 | Fever |
| Muscle soreness or fatigue | |||||||||
| Cough | |||||||||
| Abnormal chest CT | |||||||||
| Patient in critical condition | |||||||||
| Death of patient | |||||||||
| Female: 26 |
Abbreviation: China CDC, Chinese Center for Disease Control and Prevention.
3.2. Meta‐analysis results
Through Meta‐analysis, we found that among all the clinical characteristics of patients with SARS‐CoV‐2 infection, the incidence of fever was 0.891 (95% CI: 0.818, 0.945), the incidence of cough was 0.722 (95% CI: 0.657, 0.782), and the incidence of muscle soreness or fatigue was 0.425 (95% CI: 0.213, 0.652). The incidence of acute respiratory distress syndrome (ARDS) was 0.148 (95% CI: 0.046, 0.296), the incidence of abnormal chest computer tomography (CT) was 0.966 (95% CI: 0.921, 0.993), the percentage of severe cases in all infected cases was 0.181 (95% CI: 0.127, 0.243), and the case fatality rate of patients with SARS‐CoV‐2 infection was 0.043 (95% CI: 0.027, 0.061). Detailed results of Meta‐analysis are shown in Table 2.
Table 2.
Meta‐analysis results
| Clinical symptom | Results of meta‐analysis | Adjusted results |
|---|---|---|
| Fever | 2.47(95% CI: 2.26,2.67) | 0.891(95% CI: 0.818,0.945) |
| Cough | 2.03(95% CI: 1.89,2.17) | 0.722(95% CI: 0.657,0.782) |
| Muscle soreness or fatigue | 1.42(95% CI: 0.96,1.88) | 0.425(95% CI: 0.213,0.652) |
| ARDS | 0.79(95% CI: 0.43,1.15) | 0.148(95% CI: 0.046,0.296) |
| Abnormal chest CT | 2.77(95% CI: 2.57,2.97) | 0.966(95% CI: 0.921,0.993) |
| Patient in critical condition | 0.88(95% CI: 0.73,1.03) | 0.181(95% CI: 0.127,0.243) |
| Death of patient | 0.42(95% CI: 0.33,0.50) | 0.043(95% CI: 0.027,0.061) |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CT, computer tomography.
3.3. Publication bias detection
The results of the Egger test are shown in Table 3. There was a publication bias in the meta‐analysis of the ARDS group (P = .008).
Table 3.
Results of Egger test
| Groups | Fever group | Cough group | Muscle soreness or fatigue group | ARDS group | Abnormal chest CT group | Severe cases group | Death cases group |
|---|---|---|---|---|---|---|---|
| P | .866 | .278 | .090 | .008 | .908 | .826 | .258 |
Abbreviations: ARDS, acute respiratory distress syndrome; CT, computer tomography.
4. DISCUSSION
SARS‐CoV‐2 is one type of coronaviruses that belong to the β‐coronavirus cluster. It causes the third kind of zoonotic coronavirus disease after SARS and Middle East respiratory syndrome (MERS). Wei Ji et al 15 found that SARS‐CoV‐2 seemed to be a recombinant virus between bat coronavirus and coronavirus of another unknown origin. Xiao et al 16 confirmed that Malayan pangolins were the most likely intermediate host of SARS‐CoV‐2. The research of Benvenuto et al 17 found that SARS‐CoV‐2 was only closely related to the coronavirus isolated from the chrysanthemum‐headed bat in 2015. Their research suggests that bats may be the reservoir host for SARS‐CoV‐2. According to the research by Zhou et al 18 and Wu et al, 19 it was found that the sequence homology between SARS‐CoV‐2 and SARS‐CoV was 79.5%. The homology between SARS‐CoV‐2 and bat coronavirus at the genetic level was 96%. Therefore, very likely it can be confirmed that SARS‐CoV‐2 comes from bats.
According to the results of meta‐analysis, we find that the incidence of fever was 89.1%, the incidence of cough was 72.2%, and the incidence of muscle soreness or fatigue was 42.5%. The incidence of ARDS was 14.8%, the incidence of abnormal CT was 96.6%, the percentage of severe cases in all infected cases was 18.1%, and the case fatality rate of patients with SARS‐CoV‐2 infection was 4.3%. Weijie Guan et al. 8 found that the common patterns on chest CT were ground‐glass opacity and bilateral patchy shadowing in patients with SARS‐CoV‐2 infection. Chaolin Huang et al. 5 found that the typical chest CT images of severe cases were bilateral multiple lobular and subsegmental areas of consolidation. In addition, by reading the included literature, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 we found that diarrhea, hemoptysis, headache, sore throat, shock, and other symptoms are rare.
At present, the Chinese government has announced that the case fatality rate of patients with SARS‐CoV‐2 infection in China is 3.8%, which is lower than that of another two widely contagious zoonotic coronavirus diseases, SARS and MERS. Infection mortality rates of SARS and MERS are 9.6% 20 and 35%, 21 respectively. Since the case fatality rate of patients with SARS‐CoV‐2 infection is low, a large number of patients with SARS‐CoV‐2 infection will be cured. Sheng et al 22 have shown that viral infection could increase the risk of pulmonary fibrosis. Xie et al 23 found that 45% of patients showed signs of pulmonary fibrosis within 1 month after being infected with SARS‐CoV. Hui 24 revealed that 36% and 30% of patients infected with SARS‐CoV developed pulmonary fibrosis 3 and 6 months after infection. These studies consistently suggest that pulmonary fibrosis will become one of the serious complications in patients with SARS‐CoV‐2 infection. How to prevent and reduce the occurrence of pulmonary fibrosis in patients with SARS‐CoV‐2 infection are urgent problems for medical workers in the treatment of SARS‐CoV‐2.
Zhao et al 25 found that angiotensin‐converting enzyme 2 (ACE2) was the receptor of SARS‐CoV‐2. In normal lung tissue, ACE2 is mainly expressed by type I and type II alveolar epithelial cells. It was reported that 83% of II type alveolar cells expressed ACE2. Therefore, SARS‐CoV‐2 infection causes damages to most II type alveolar cells. In addition, the use of mechanical ventilation in the treatment of patients with SARS‐CoV‐2 infection can also aggravate the injury of alveolar cells. After alveolar cell injury, transforming growth factor‐β (TGF‐ β) released in tissue promotes lung repair. Virus infection often leads to excessive activation of TGF‐β pathway, which leads to the occurrence of pulmonary fibrosis.
Based on the mechanism of pulmonary fibrosis after virus infection, we can formulate corresponding prevention and treatment proposals. The first is the treatment of SARS‐CoV‐2 infection. At present, a variety of drugs have been found to inhibit SARS‐CoV‐2. Holshue et al 26 achieved remarkable results in the treatment of patients with SARS‐CoV‐2 infection with remdesivir. This drug is currently in clinical trials. Second, it is necessary to inhibit the inflammatory reaction and reduce exudation. In this direction, antibiotics not only have traditional antibacterial effects but also have immunomodulatory and anti‐inflammatory properties. 27 Macrolide antibiotics such as clarithromycin, azithromycin, and erythromycin prevent the production of proinflammatory cytokines and immune mediators. 28 The third is to inhibit the fibrogenic effect of TGF‐β. Luo et al 29 found that arsenic trioxide could inhibit the signal transduction of TGF‐β and play a role in antifibrosis. The fourth is the rational use of ventilator to avoid unnecessary lung damages. In addition, pirfenidone and nintedanib as drugs for the treatment of idiopathic pulmonary fibrosis can also be used to prevent and treat pulmonary fibrosis in patients with SARS‐CoV‐2 infection. 30
In all, a total of 10 articles were covered in the meta‐analysis, including 50 466 patients with SARS‐CoV‐2 infection. By far, it is the first meta‐analysis with the largest sample size. The quality of the literature included in this study is high, the analysis is rigorous, and the conclusions drawn by the study are highly credible. However, this meta‐analysis also has some limitations: (a) all studies included in this meta‐analysis are retrospective studies with large heterogeneity; (b) most patients in our meta‐analysis are Chinese, and we aimed to use the conclusions of this study to predict patients in general, including other countries and races; (c) there was publication bias in the meta‐analysis of the ARDS group. The analytical conclusion of the ARDS group may be influenced by publication bias; and (d) the study subjects were inpatients who have been diagnosed with SARS‐CoV‐2 infection. So, the incidence of severe pneumonia or the fatality rate in the total population infected with SARS‐CoV‐2 would be much lower than the results shown in this study.
Therefore, based on the above‐mentioned limitations, the conclusions of this meta‐analysis still need to be verified by more relevant studies with more careful design, more rigorous execution, and larger sample size.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
JX and PS developed the idea for and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. PS and SQ contributed to the writing of the report. JX contributed to the critical revision of the report. SQ, ZL, JR, and KL contributed to the statistical analysis. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
ACKNOWLEDGMENT
We would like to acknowledge TopEdit LLC for the linguistic editing and proofreading during the preparation of this manuscript.
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 infection: A single arm meta-analysis. J Med Virol. 2020;92:612–617. 10.1002/jmv.25735
[Correction added on 17 March 2020 after first online publication: Manuscript has been revised with author's latest changes]
REFERENCES
- 1. Lancet T. Emerging understandings of SARS‐CoV‐2. Lancet. 2020;395(10221):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006‐1012. [DOI] [PubMed] [Google Scholar]
- 4. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan W‐j, Ni Z‐y, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020. 10.1101/2020.02.06.20020974 [DOI] [Google Scholar]
- 9. Chen J, Liu HG, Liu W. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. [DOI] [PubMed] [Google Scholar]
- 10. Sun K, Chen J, Viboud C. Early epidemiological analysis of the SARS‐CoV‐2 outbreak based on a crowdsourced data. medRxiv. 2020. 10.1101/2020.01.31.20019935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Yang, Lu Qingbin, Liu Mingjin, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020. 10.1101/2020.02.10.20021675 [DOI] [Google Scholar]
- 12. Li Jie, Li Shilin, Cai Yurui, et al. Epidemiological and clinical characteristics of 17 hospitalized patients with 2019 novel coronavirus infections outside Wuhan, China. medRxiv. 2020. 10.1101/2020.02.11.20022053 [DOI] [Google Scholar]
- 13. The Noval Coronavirus Pneumonia Emergency Response Epidemiology Team, Chinese Center for Disease Control and Prevention . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145‐151.32064853 [Google Scholar]
- 14. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji W, Wang W, Zhao X, Zai J, Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross‐species transmission from snake to human. J Med Virol. 2020;92:433‐440. 10.1002/jmv.25682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao Kangpeng, Zhai Junqiong, Feng Yaoyu, et al. Isolation and characterization of SARS‐CoV‐2‐like coronavirus from Malayan Pangolins. medRxiv. 2020. 10.1101/2020.02.17.951335 [DOI] [Google Scholar]
- 17. Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019‐new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92:455‐459. 10.1002/jmv.25688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The Middle East respiratory syndrome (MERS). Infect Dis Clin North Am. 2019;33(4):891‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheng G, Chen P, Wei Y, et al. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta‐analysis. Chest. 2019. 10.1016/j.chest.2019.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie L, Liu Y, Xiao Y, et al. Follow‐up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hui DS. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Y, Zhao Z, Wang Y, et al. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. bioRxiv. 2020. 10.1101/2020.01.26.919985 [DOI] [Google Scholar]
- 26. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buret AG. Immuno‐modulation and anti‐inflammatory benefits of antibiotics: the example of tilmicosin. Can J Vet Res. 2010;74(1):1‐10. [PMC free article] [PubMed] [Google Scholar]
- 28. Wuyts WA, Willems S, Vos R, et al. Azithromycin reduces pulmonary fibrosis in a bleomycin mouse model. Exp Lung Res. 2010;36(10):602‐614. [DOI] [PubMed] [Google Scholar]
- 29. Luo F, Zhuang Y, Sides MD, et al. Arsenic trioxide inhibits transforming growth factor‐β1‐induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir Res. 2014;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chunbin Wang, Shuang Zhang, Qian Zhang, et al. Research progress of two new antifibrotic drugs for idiopathic pulmonary fibrosis. Chin Pharm J. 2019;09:682‐686. [Google Scholar]


