Abstract
Infectious bronchitis virus (IBV), an ongoing emergence enveloped virus with a single‐stranded positive‐sense RNA genome, belongs to the Gammacoronavirus genus in the Coronaviridae family. IBV‐associated tracheitis, nephritis, salpingitis, proventriculitis and egg drop have caused devastating economic losses to poultry industry worldwide. Since the end of 2018, a remarkably increasing number of commercial broilers and layers, vaccinated or not, were infected with IBV in China. Here, we described two IB outbreaks with severe respiratory system or kidney injury in IBV‐vaccinated commercial poultry farms in central China. Other possible causative viral pathogens, including avian influenza virus (AIV), Newcastle disease virus (NDV) and Kedah fatal kidney syndrome virus (KFKSV), were excluded by reverse transcription‐polymerase chain reaction (RT‐PCR), and three virulent IBV strains, HeN‐1/China/2019, HeN‐2/China/2019 and HeN‐101/China/2019, were identified. Although the gross pathologic appearance of these two IB outbreaks was different, the newly identified IBV strains were all closely related to the ck/China/I0529/17 strain and grouped into GI‐19 genotype clade based on the sequencing and phylogenetic analysis of the complete S1 genes. Moreover, there are still some evolutionary distance between the newly identified IBV strains, HeN‐101/China/2019 in particular, and other GI‐19 strains, suggesting that Chinese IBV strains constantly emerge and evolve towards different directions. In conclusion, this study provided an insight of the recently emerging IBV outbreaks in IBV‐vaccinated commercial poultry farms and identified the genetic characteristics of three virulent GI‐19 IBV strains, which shows the need to carry out proper preventive measures and control strategies.
Keywords: epidemiology, evolutionary, GI‐19 genotype, infectious bronchitis virus
1. INTRODUCTION
Avian infectious bronchitis (IB), a common avian disease with high morbidity and mortality, has drawn great attention for causing devastating economic losses to poultry industry worldwide (Jackwood & Wit, 2013). Infectious bronchitis virus (IBV), the causative agent, is an ongoing emergence enveloped, single‐stranded, positive‐sense RNA virus, with genome length about 27.6 kb. It is the prototype of the Gammacoronavirus genus in the Coronaviridae family (Bande et al., 2017; Masters & Perlman, 2013). IBV mainly targets the respiratory tract of its natural host— chicken, causing severe respiratory system disease, and spreads via respiratory and feco‐oral routes. Additionally, IBV also infects the uro‐genital tract, digestive system and reproductive system, resulting in nephritis, salpingitis, proventriculitis and egg drop (Bande et al., 2017; Jackwood & Wit, 2013; Sjaak de Wit, Cook, & van der Heijden, 2011).
A great number of IBV serotypes, genotypes and pathotypes have been identified worldwide since its first description in 1931 in America (Bande et al., 2017; Lin & Chen, 2017). To prevent IBV infection, researchers have developed various commercial inactivated and live attenuated vaccines over the past decades, which successfully prevented IBV infections (Jordan, 2017). As a highly mutable coronavirus, however, the continuous emergence of novel IBV strains greatly emasculate vaccines efficacy, and the low cross‐protection rates of IBV vaccines inevitably hamper the prevention and control of the disease (Fan et al., 2018; Jordan, 2017).
Since the end of 2018, a remarkably increasing number of commercial broilers and layers, vaccinated or not, were infected with IBV in China, especially in the intensive poultry raising regions, such as Henan, Hebei and Shandong provinces. Herein, we reported two IB outbreaks in IBV‐vaccinated commercial broiler farms in central China, and the complete S1 genes of the newly identified IBV strains were sequenced, and its genotype, phylogeny and variations were analysed further. This study systematically described the genotype and evolutionary characteristics of the emerging IBV strains and highlighted the importance of continuous extensive surveillance to help choose suitable vaccines and develop control programmes reasonably.
2. MATERIALS AND METHODS
2.1. Broiler farm history and case presentation
Case 1: In April 2019, an acute outbreak of fatal respiratory disease occurred in a commercial broiler farm in Henan Province, Central China. According to the breeder's description, approximately one‐tenth of 20,000 25‐day‐old broilers, vaccinated with commercial live attenuated IBV vaccine H120 at 7‐day‐old, showed severe respiratory symptoms, such as gasping, nasal discharge, tracheal rales, sneezing, and some affected flocks also presented greenish‐yellow diarrhoea, watery eyes and lethargy. The outbreak started from 18 April 2019, and antibiotic‐antimycotic combination therapy did not work. A total of ten dead broilers (5/10) and illness broilers (5/10) were selected randomly to send to the laboratory for diagnosis.
Case 2: In September 2019, an outbreak of fatal nephritis disease occurred in another commercial layer farm in Henan Province. According to the breeder's description, the farm has approximate 9,000 layers with 21‐day‐old, which were vaccinated with commercial live attenuated IBV vaccine H120 at 7‐day‐old. The great majority of the flocks started to show moderate respiratory symptoms from 10 September 2019, and mitigated in a week with the antibiotic and traditional veterinary drugs combination therapies. Since 20 September, about 200 chickens per day died acutely without obvious clinical symptom. Six moribund layers were selected randomly to send to the laboratory for diagnosis.
2.2. Ethical statement
The sick chicken examined in this study was approved by the poultry farm owners. Diagnosis and experimental protocols in this study were approved by the Henan University of Animal Husbandry and Economy Animal Care Committee. The clinical samples used in this study were collected in strict accordance with the guidelines for Animal Ethics Committees. All the chickens were euthanized by cervical dislocation and then examined the anatomical changes, samples of lungs, tracheas and kidneys were collected from sick chicken and stored at −80°C until used for diagnosis and virus isolation.
2.3. Molecular diagnosis
The samples were homogenized and centrifuged to collect supernatants. Total viral RNA was extracted by using TRIzol (Invitrogen, Cat No: 15596026) according to the manufacturer's instructions, and further reversely transcribed to cDNA as described in our previous report (Zhang et al., 2018). IBV, AIV, NDV and KFKSV (Palya et al., 2019), which can cause severe respiratory symptoms, nephritis and acute death, were analysed by RT‐PCR using specific primers as previously described (Nguyen et al., 2013). Moreover, the positive samples were homogenated and then isolated in 9‐day‐old specific pathogen‐free (SPF) eggs via the allantoic cavity, and the allantoic fluid was harvested sterilely and stored at −80°C.
2.4. Gene amplification and sequencing
To identify the genotype of IBV strains identified in this outbreak, the complete S1 genes were amplified by using primers as follows: IBV‐S1 forward: 5′‐GAACAAAAGACCGACTTAGT‐3′ and IBV‐S1 reverse: 5′‐TATGTACTCATCTGTRACAGT‐3′, which were designed based on the conserved regions flanked the S1 gene of IBV strains downloaded from the ViPR database (http://www.viprbrc.org/brc/home.spg?decorator=vipr). The correct size amplicons, with length about 2000 bp, were then cloned into pCloneEZ vector (Clone Smarter) and sequenced.
2.5. Phylogenetic and recombination analysis
The complete S1 genes of the newly identified strains were subjected to online BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and sequences sharing more than 95% nucleotide identify were downloaded for further genetic analysis. Additionally, the S1 genes of representative IBV strains within different genotypes and lineages (Chen et al., 2017; Jiang et al., 2017; Ma et al., 2019; Valastro et al., 2016) were also downloaded for sequence alignment with Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/), and the phylogenetic tree was conducted by MEGA 7.0 with the neighbour‐joining method using 1,000 bootstrap replicates, which were then visualized by iTOL v4 software (Letunic & Bork, 2019). Recombination events among IBV strains were further investigated using the RDP4 software, a widely used tool for analysing individual recombination events and overall recombination patterns, by seven different algorithms recombination detection program (RDP), Bootscan, MaxChi, GENECONV, Chimaera, SiScan and 3Seq (Martin, Murrell, Khoosal, & Muhire, 2017).
2.6. Visualization of mutations within the IBV S1 protein structure
Previous studies have revealed that most of the variable amino acids in S1 region were distributed in the three hypervariable regions (HVRs), including HVR I (38 aa‐67 aa), HVR II (91 aa‐141 aa) and HVR III (274 aa‐387 aa), which were associated with neutralizing epitopes and receptor‐binding domain and usually employed to classify IBV genotypes (Moore, Jackwood, & Hilt, 1997; Valastro et al., 2016). To further investigate the HVRs and mutations in the newly identified IBV strain, the 3D structure of HeN‐2/China/2019 S1 protein was constructed by homology modelling method. From the PDB database, the glycoprotein S1 structure of vaccine M41 strain (Accession number: 6CV0) was downloaded and employed as the template for modelling S1 monomer (Shang et al., 2018). All the HVRs and mutations of HeN‐2/China/2019 and HeN‐101/China/2019 were located on the structure and visualized by PyMOL software (http://www.pymol.org/).
3. RESULTS
3.1. Internal lesions
Among the organs collected from the morbid chicken among two IB outbreaks, the predominant histologic lesions were in the trachea and kidney, respectively. In case 1, a total of ten dead broilers (5/10) and illness broilers (5/10) were selected randomly to examine the anatomical changes, and trachea, especially between bronchus and bronchioles, showed the gross pathologic appearance of typical tracheitis, with serous, catarrhal or caseous exudate in trachea (Figure 1a‐c). Moreover, some of them presented systemic colibacillosis or airsacculitis, and no obvious nephritis and proventriculitis were observed. In case 2, all the six moribund layers exhibited severe lesion in kidneys, which were characterized by pale, swollen and mottled (Figure 1d,e). Some of them showed distended ureters filled with uric acid, but none of them presented tracheitis.
Figure 1.
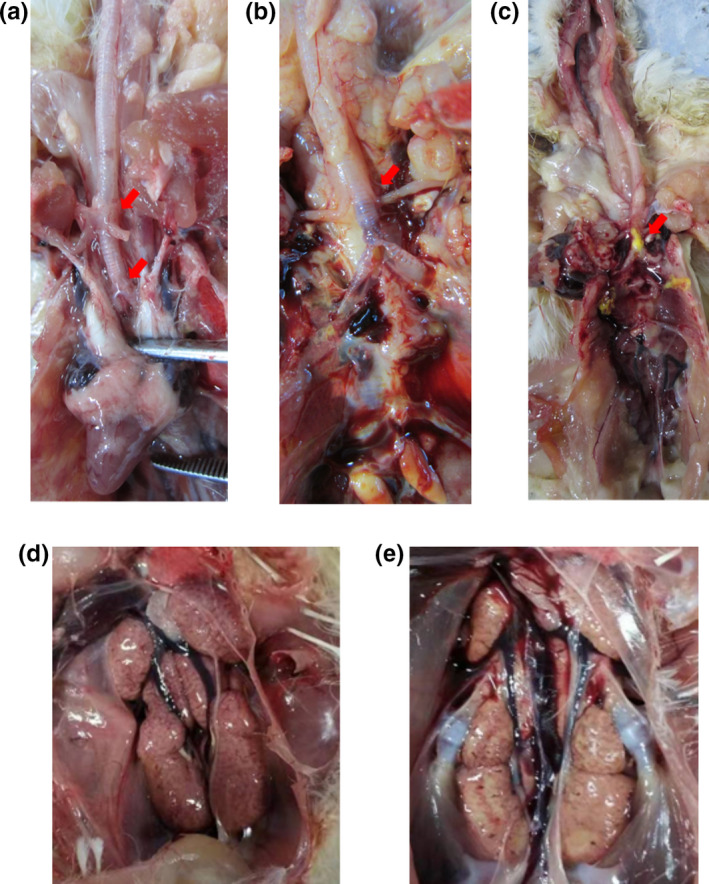
Gross pathologic appearance of tracheitis and nephritis of morbid chickens from the commercial chicken farms in Henan China. Presence of various degrees of tracheitis, including serous (a), catarrhal (b), or caseous exudate (c) in the bronchioles and nephritis of swollen and pale kidneys (d and e) [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Molecular diagnosis
To identify the causative agent of this outbreak, RT‐PCR assays were used to detect the common potential viral pathogens, including IBV, NDV, AIV and KFKSV. As shown in Figure S1a, lung and trachea samples collected from first outbreak were positive for IBV, whereas no corresponding nucleotide fragments were observed for AIV and NDV. Moreover, kidney and spleen samples collected from second outbreak were positive for IBV, and no PCR fragments was observed for AIV, NDV and KFKSV. From both outbreaks, we were able to isolate four IBV strain, HeN‐1/China/2019, HeN‐2/China/2019, HeN‐101/China/2019 and HeN‐102/China/2019.
3.3. Genetic and recombination analysis
To further characterize the biology and ecology of the newly identified IBV strains, we sequenced the complete S1 genes of HeN‐1/China/2019, HeN‐2/China/2019, HeN‐101/China/2019 and HeN‐102/China/2019. Nucleotide identity of the S1 genes between HeN‐1/China/2019 and HeN‐2/China/2019 was 99.9%, and the identity between HeN‐101/China/2019 and HeN‐102/China/2019 was 100%, indicated that the two IB outbreaks were caused by a single IBV strains, HeN‐2/China/2019 and HeN‐101/China/2019, respectively. The sequences were further submitted to NCBI (GenBank accession numbers: MN055627, MN055628 and MN635798). Blast analysis revealed that all isolates were genetically closed to the GI‐19 genotype strain ck/China/I0529/17, isolated from broiler in 2017. Also, genetic analysis based on the complete S1 gene revealed that the HeN‐2/China/2019 and HeN‐101/China/2019 strains shared the high homology with QX (96.0% and 95.4%) and LX4 (97.6% and 96.6%), the representative strains in the GI‐19 genotype. However, these two strains displayed low similarity in gene identity to Chinese available commercial vaccines H120 (74.9% and 74.6%), 4/91 (77.0% and 77.3%) and LDT3‐A (83.5% and 82.9%), respectively.
Phylogenetic relationship among IBV strains has been established on the analysis of complete S1 gene. As shown in Figure 2, the phylogenetic tree further revealed that the HeN‐1/China/2019, HeN‐2/China/2019 and HeN‐101/China/2019 strains were clustered together with ck/China/I0529/17, QX and LX4 strains and grouped into the GI‐19 genotype, which were distantly from the available commercial vaccines H120, 4/91 and LDT3‐A.
Figure 2.
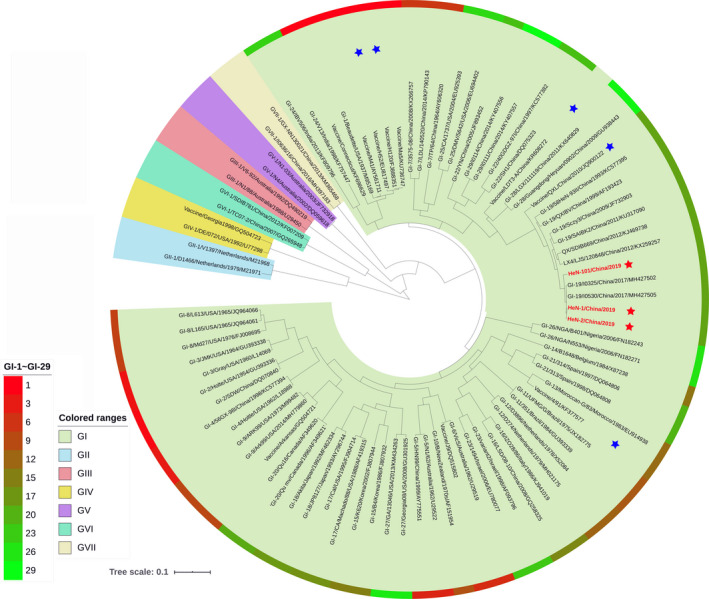
Phylogenetic analysis of the complete S1 genes of newly identified IBV in 2019. Phylogenetic analysis based on the deducted amino acid sequences of complete S1 gene of HeN‐1/China/2019, HeN‐2/China/2019, HeN‐101/China/2019 and other IBV isolates available from GenBank database. Phylogenetic tree was constructed by MEGA 7.0 software using the neighbour‐joining method with 1,000 bootstrap replicates. The seven different IBV genotype, with 35 distinct lineage IBV strains were illustrated by the colour scale with iTOL software. Meanwhile, the available IBV vaccine strains in China were indicated by blue star, and the three IBV isolates in this study were indicated by red star [Colour figure can be viewed at wileyonlinelibrary.com]
As a highly variable coronavirus, numerous IBV variants have been identified and nucleotide substitutions or recombination between field strains and vaccines occurred frequently. Previous study revealed that the recombinant events have already occurred in European strains within the S1 gene (Moreno et al., 2017). We further investigated the recombinant events between GI‐19 and other IBV genotypes using the RDP4 software. The results showed that several recombinant events occurred between GI‐19 and other IBV genotypes with high score (p < .01, recombinant score >0.6), including strains of the newly identified genotype GI‐28, GI‐25 and unassigned strain LDT3 (Figure S2). Interestingly, the GI‐19 genotype plays a two‐faced role in these recombinant events, not only providing fragments for other strains, but also receiving fragments during infection.
3.4. Amino acid polymorphism analysis
To further explore the characteristics of the newly identified IBV strains, amino acid polymorphism of the S1 glycoprotein was compared with other IBV strains of all 35 genotypes (Chen et al., 2017; Jiang et al., 2017; Ma et al., 2019; Valastro et al., 2016). The result revealed that most of the amino acid mutations in S1 region of the newly identified IBV strains were located in the three HVR regions. Meanwhile, several amino acid mutations were only found in HeN‐2/China/2019 (P87L, T300I and H329Y) and HeN‐101/China/2019 (Q89K, S96A, Q97E, S117A, T120R, A130E, R131S, A195S, Q315H and I511M) among GI‐19 strains (Table 1). Compared with the available capsid structure of M41 strain in the PDB database (Shang et al., 2018), the P87L and Q89K mutations are adjacent to HVR I and HVR II region structurally (Figure 3c) and located in the major receptor‐binding domain (19 aa‐253 aa; Promkuntod, van Eijndhoven, de Vrieze, Grone, & Verheije, 2014); the Q97E, S117A, T120R, A130E and R131S are in HVR II region; the T300I and H329Y are in HVR III region (Figure 3d), indicating that these mutations might change virus antigenicity.
Table 1.
Amino acid polymorphism of the S1 protein among HeN‐2/China/2019, HeN‐101/China/2019 and other genotype IBV isolates
| Position | IBV Strains | Location | |||
|---|---|---|---|---|---|
| HeN−2/China/2019 | HeN−101/China/2019 | GI−19 genotype | Other genotypesc | ||
| 87 | La | P | P | P | RBD |
| 89 | Q | Kb | Q | S/N/T | RBD |
| 96 | S | Ab | S | S/N/K | RBD/HVRII |
| 97 | Q | Eb | Q | G/Q/K/E | RBD/HVRII |
| 117 | S | A | S | G/K/A/N | RBD/HVRII |
| 120 | T | Rb | T/S/A | H/A/Q/S | RBD/HVRII |
| 130 | A | E | A/P | P/E/Q/G | RBD/HVRII |
| 131 | R | S | R | Q/E/S/K | RBD/HVRII |
| 195 | A | S | A | A/S | — |
| 300 | Ia | T | T | T/I/S/V | HVRIII |
| 315 | Q | Hb | Q | R/S/D/G/T | HVRIII |
| 329 | Ya | H | H | H | HVRIII |
| 511 | I | Mb | I | I/V | — |
Abbreviations: HVR, respected the hypervariable regions of S1 protein; RBD, respected the receptor‐binding domain of S1 protein.
Specific amino acid mutation only found in HeN‐2/China/2019.
Specific amino acid mutation only found in HeN‐101/China/2019.
The major amino acids in the other genotypes IBV.
Figure 3.
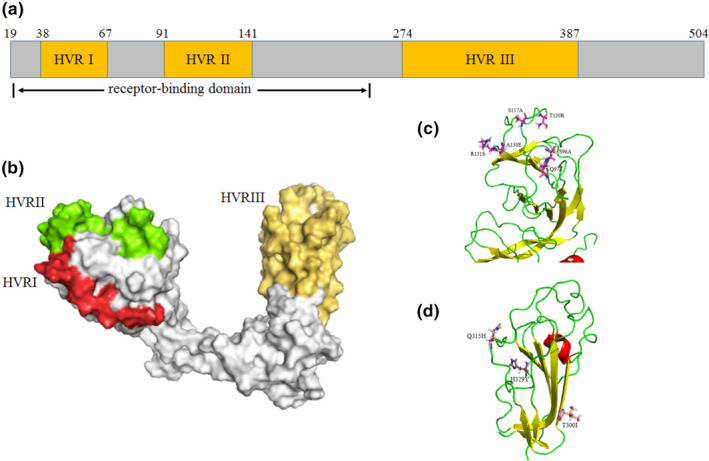
Characterization and localization of specific nonsynonymous mutations in capsid protein of the newly identified IBV strains in comparison with other IBV isolates. The multi‐alignment of S1 glycoprotein of all IBV genotypes was conducted by Clustal‐Omega, and schematic diagram based on the identified protein functional domains of mature S1 protein (without 1aa‐19aa) was illustrated (a); 3D structure template of GI‐1 genotype (M41 strain) was downloaded from PDB database, and the HVR regions of IBVs (b) and the location of mutation sites of HeN‐2/China/2019 (c and d) were visualized by PyMOL software [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSIONS
IB is an economically important avian disease that affects the poultry industries worldwide, particularly in the large poultry‐producing countries, such as USA, China and Brazil (Bande et al., 2017; Jackwood & Wit, 2013). The first case of IB in China dates back to early 1980s, and since then, the outbreaks were constantly emerging and numerous IBV strains were identified, of which strains in genotype GI‐19 (refers to the QX‐like or LX4) became predominant among chickens currently, although the QX strain was initially identified in 1996. Additionally, other genotypes of IBV, such as GI‐1 (Mass), GI‐7 (TW‐I or TW‐like), GI‐13 (4/91‐like), GI‐16 (Q1‐like) and GI‐22 (YN‐like and SAIBK‐like), were also frequently detected in China (Han et al., 2018; Li et al., 2012; Lin & Chen, 2017; Mo et al., 2013). Importantly, several novel IBV genotypes, including GI‐28, GI‐29 and GVII‐1, were identified recently (Chen et al., 2017; Jiang et al., 2017; Ma et al., 2019). All these indicated that the IBV strains circulating in China are genetically diverse, providing a potential platform for recombination and greatly challenging the current biological control measures.
Previous studies have demonstrated that the genotypes of IBV strains circulating in China are complex, and the GI‐19 genotype has predominated in chickens since 2008 and spread all over the world in the next decade (Bande et al., 2017; Han et al., 2011; Li et al., 2012). GI‐1 genotype vaccine H120 has been the most widely used vaccine in China until now, which shared a very low homology of identity with the emerging GI‐19 strain HeN‐1/China/2019 (32.2%) and HeN‐2/China/2019 (32.2%) in glycoprotein S1 gene region, and even the other GI‐19 strains identified recently. These results indicated the H120 vaccine could not provide effective protection against GI‐19 genotype strains infection, which might explain the clinical phenomenon that a number of IBV outbreaks emerged in vaccinated flocks (Lin & Chen, 2017).
As a highly variable coronavirus, numerous IBV variants have been identified and nucleotide substitutions or recombination between field strains and vaccines occurred frequently (Bande et al., 2017; Jordan, 2017; Lin & Chen, 2017; Sjaak de Wit et al., 2011). Similar to the previous studies, we discovered several recombinant events between GI‐19 and other IBV genotypes with great possibilities. All these results indicated the GI‐19 genotype strains were constantly evolving. In China, the genotype GI‐19 vaccine QXL was just approved in the last year (data from the National Veterinary Drug Basic Information Database of China). The continuous emergence of IBV variants undoubtedly facilitates evading immune protection induced by vaccination, as well as adapting to new environment, which would greatly challenge the efficacy of the newly developed vaccine.
Since the end of 2018, a large number of IBV outbreaks emerged in vaccinated commercial broilers and layers in China, indicating that the efficacy of currently used vaccines, including H120, 4/91 and LDT3‐A, was not effective for the circulating GI‐19 genotype. Fortunately, one live attenuated vaccine targeting GI‐19 genotype has been licensed in 2018, which might provide an effective role in controlling the disease in China. The result also highlights the importance of further surveillance to define the variations and distribution of this virulent strain of IBV among the Chinese flocks, which helps to carry out proper preventive measures to limit its devastating effect for poultry industry.
In conclusion, this study identified two virulent GI‐19 IBV strains in IBV‐vaccinated commercial broiler farm during the recent wave of IBV outbreaks in China. Sequencing and phylogenetic analysis revealed that these newly identified IBV strains are closely related to the ck/China/I0529/17 strain. However, there are still some evolutionary distance between the newly identified IBV strains and other GI‐19 strains and contain several special amino acid mutations in the HVR regions of S1 protein, suggesting that Chinese IBV strains constantly emerge and evolve towards different directions. This study provides an insight on recently emerging IBV outbreaks, which shows the need to carry out proper preventive measures and control strategies.
CONFLICT OF INTEREST
The authors declared no potential conflict of interests with respect to the research, authorship and publication of this article.
Supporting information
ACKNOWLEDGEMENTS
We are very grateful to Mr. Jingsheng Xu (Henan Qixiang Biological Engineering Co. Ltd) for kindly providing the epidemic information. This work was supported by the grants from the Scientific and Technological Project of Henan Province (192102110186) and the Youth Science Foundations of Henan University of Animal Husbandry and Economy (906/24030027 and 906/24030090), the Veterinary Discipline Key Construction Project of Henan University of Animal Husbandry and Economy (MXK2016102) and the Science and Technology Innovation Group Project of Henan University of Animal Husbandry and Economy (2018KYTS13).
Zhang X, Deng T, Lu J, et al. Molecular characterization of variant infectious bronchitis virus in China, 2019: Implications for control programmes. Transbound Emerg Dis. 2020;67:1349–1355. 10.1111/tbed.13477
Xiaozhan Zhang and Tongwei Deng contributed equally to this work.
Contributor Information
Zeng Wang, Email: wzzxz20140105@126.com.
Chuanzhou Bian, Email: chuanzhou-bian@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in GenBank at NCBI, reference number [MN055627, MN055628 and MN635798]. These data were derived from the following resources available in the public domain NCBI.
REFERENCES
- Bande, F. , Arshad, S. S. , Omar, A. R. , Hair‐Bejo, M. , Mahmuda, A. , & Nair, V. (2017). Global distributions and strain diversity of avian infectious bronchitis virus: A review. Animal Health Research Reviews, 18(1), 70–83. 10.1017/S1466252317000044 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Jiang, L. , Zhao, W. , Liu, L. , Zhao, Y. , Shao, Y. , … Liu, S. (2017). Identification and molecular characterization of a novel serotype infectious bronchitis virus (GI‐28) in China. Veterinary Microbiology, 198, 108–115. 10.1016/j.vetmic.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, W. S. , Li, H. M. , He, Y. N. , Tang, N. , Zhang, L. H. , Wang, H. Y. , … Wei, P. (2018). Immune protection conferred by three commonly used commercial live attenuated vaccines against the prevalent local strains of avian infectious bronchitis virus in southern China. Journal of Veterinary Medical Science, 80(9), 1438–1444. 10.1292/jvms.18-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Z. , Gao, M. , Chen, Y. , Zhao, W. , Sun, J. , Zhao, Y. , & Liu, S. (2018). Genetics, antigenicity and virulence properties of three infectious bronchitis viruses isolated from a single tracheal sample in a chicken with respiratory problems. Virus Research, 257, 82–93. 10.1016/j.virusres.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Z. , Sun, C. , Yan, B. , Zhang, X. , Wang, Y. , Li, C. , … Liu, S. (2011). A 15‐year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infection, Genetics and Evolution, 11(1), 190–200. 10.1016/j.meegid.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood, M. W. , & Wit, S. D. (2013). Infectious bronchitis. In Swayne D. E. (Ed.), Diseases of poultry, 13th edition (13th edpp. 139–159). Ames, IA: John Wiley & Sons Inc. [Google Scholar]
- Jiang, L. , Zhao, W. , Han, Z. , Chen, Y. , Zhao, Y. , Sun, J. , … Liu, S. (2017). Genome characterization, antigenicity and pathogenicity of a novel infectious bronchitis virus type isolated from south China. Infection, Genetics and Evolution, 54, 437–446. 10.1016/j.meegid.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, B. (2017). Vaccination against infectious bronchitis virus: A continuous challenge. Veterinary Microbiology, 206, 137–143. 10.1016/j.vetmic.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Letunic, I. , & Bork, P. (2019). Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Research, 47(W1), W256–W259. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Wang, X. Y. , Wei, P. , Chen, Q. Y. , Wei, Z. J. , & Mo, M. L. (2012). Serotype and genotype diversity of infectious bronchitis viruses isolated during 1985–2008 in Guangxi, China. Archives of Virology, 157(3), 467–474. 10.1007/s00705-011-1206-6 [DOI] [PubMed] [Google Scholar]
- Lin, S. Y. , & Chen, H. W. (2017). Infectious bronchitis virus variants: molecular analysis and pathogenicity investigation. International Journal of Molecular Sciences, 18(10), 2030. 10.3390/ijms18102030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T. , Xu, L. , Ren, M. , Shen, J. , Han, Z. , Sun, J. , … Liu, S. (2019). Novel genotype of infectious bronchitis virus isolated in China. Veterinary Microbiology, 230, 178–186. 10.1016/j.vetmic.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. P. , Murrell, B. , Khoosal, A. , & Muhire, B. (2017). Detecting and analyzing genetic recombination using RDP4. Methods in Molecular Biology, 1525, 433–460. 10.1007/978-1-4939-6622-6_17 [DOI] [PubMed] [Google Scholar]
- Masters, P. S. , & Perlman, S. (2013). Corornaviridae. In Knipe D., & Howley P. (Eds.), Fields virology (6th ed., Vol. 1, pp. 825–858). Philadelphia, PA: Lippincott Williams and Wilkins. [Google Scholar]
- Mo, M. L. , Li, M. , Huang, B. C. , Fan, W. S. , Wei, P. , Wei, T. C. , … Lang, Y. H. (2013). Molecular characterization of major structural protein genes of avian coronavirus infectious bronchitis virus isolates in southern china. Viruses, 5(12), 3007–3020. 10.3390/v5123007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K. M. , Jackwood, M. W. , & Hilt, D. A. (1997). Identification of amino acids involved in a serotype and neutralization specific epitope within the s1 subunit of avian infectious bronchitis virus. Archives of Virology, 142(11), 2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, A. , Franzo, G. , Massi, P. , Tosi, G. , Blanco, A. , Antilles, N. , … Cecchinato, M. (2017). A novel variant of the infectious bronchitis virus resulting from recombination events in Italy and Spain. Avian Pathology, 46(1), 28–35. 10.1080/03079457.2016.1200011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T. , Kwon, H. J. , Kim, I. H. , Hong, S. M. , Seong, W. J. , Jang, J. W. , & Kim, J. H. (2013). Multiplex nested RT‐PCR for detecting avian influenza virus, infectious bronchitis virus and Newcastle disease virus. Journal of Virological Methods, 188(1–2), 41–46. 10.1016/j.jviromet.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Palya, V. , Kovacs, E. W. , Marton, S. , Tatar‐Kis, T. , Felfoldi, B. , Forro, B. , … Banyai, K. (2019). Novel orthobunyavirus causing severe kidney disease in broiler chickens, Malaysia, 2014–2017. Emerging Infectious Diseases, 25(6), 1110–1117. 10.3201/eid2506.181661 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Promkuntod, N. , van Eijndhoven, R. E. , de Vrieze, G. , Grone, A. , & Verheije, M. H. (2014). Mapping of the receptor‐binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology, 448, 26–32. 10.1016/j.virol.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J. , Zheng, Y. , Yang, Y. , Liu, C. , Geng, Q. , Luo, C. , … Li, F. (2018). Cryo‐EM structure of infectious bronchitis coronavirus spike protein reveals structural and functional evolution of coronavirus spike proteins. PLOS Pathogens, 14(4), e1007009. 10.1371/journal.ppat.1007009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaak de Wit, J. J. , Cook, J. K. , & van der Heijden, H. M. (2011). Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathology, 40(3), 223–235. 10.1080/03079457.2011.566260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastro, V. , Holmes, E. C. , Britton, P. , Fusaro, A. , Jackwood, M. W. , Cattoli, G. , & Monne, I. (2016). S1 gene‐based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infection, Genetics and Evolution, 39, 349–364. 10.1016/j.meegid.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Xiao, J. , Ba, L. , Wang, F. , Gao, D. , Zhang, J. , … Qi, P. (2018). Identification and genomic characterization of the emerging senecavirus A in southeast China, 2017. Transboundary and Emerging Diseases, 65(2), 297–302. 10.1111/tbed.12750 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in GenBank at NCBI, reference number [MN055627, MN055628 and MN635798]. These data were derived from the following resources available in the public domain NCBI.


