Abstract
Acute respiratory infections (ARIs) are a major cause of morbidity among children. Respiratory viruses are commonly detected in both symptomatic and asymptomatic periods. The rates of infection and community epidemiology of respiratory viruses in healthy children needs further definition to assist interpretation of molecular diagnostic assays in this population. Children otherwise healthy aged 1 to 8 years were prospectively enrolled in the study during two consecutive winters, when ARIs peak in New Zealand. Parents completed a daily symptom diary for 8 weeks, during which time they collected a nasal swab from the child for each clinical ARI episode. A further nasal swab was collected by research staff during a clinic visit at the conclusion of the study. All samples were tested for 15 respiratory viruses commonly causing ARI using molecular multiplex polymerase chain reaction assays. There were 575 ARIs identified from 301 children completing the study, at a rate of 1.04 per child‐month. Swabs collected during an ARI were positive for a respiratory virus in 76.8% (307 of 400), compared with 37.3% (79 of 212) of swabs collected during asymptomatic periods. The most common viruses detected were human rhinovirus, coronavirus, parainfluenza viruses, influenzavirus, respiratory syncytial virus, and human metapneumovirus. All of these were significantly more likely to be detected during ARIs than asymptomatic periods. Parent‐administered surveillance is a useful mechanism for understanding infectious disease in healthy children in the community. Interpretation of molecular diagnostic assays for viruses must be informed by understanding of local rates of asymptomatic infection by such viruses.
Keywords: asymptomatic infection, epidemiology, pediatric, respiratory virus, surveillance
Highlight
During winter, children experienced acute respiratory infections at a rate of 1.04/month.
A virus was detected in 76.8% of acute respiratory infections.
Specific viruses are more likely to be associated with respiratory symptoms.
1. INTRODUCTION
Acute respiratory infections (ARIs) represent a major burden on the health care system. They are the most common cause of illness at all ages, and globally are a leading cause of death in young children less than 5 years of age.1, 2 They consist of upper respiratory tract infections including rhinopharyngitis and sinusitis, and more severe lower respiratory tract infections (LRTIs) such as bronchiolitis and pneumonia.
Viruses cause a large proportion of ARIs,3, 4, 5, 6 presenting alone or as coinfections with other viruses and bacteria in 5% to 10% of cases.7 Influenzaviruses (IFV), human rhinovirus (HRV), respiratory syncytial virus (RSV), human coronavirus (HCoV), parainfluenza viruses, human metapneumovirus (HMPV), human enterovirus (EV), and adenovirus (ADV), are the most common viral aetiological agents associated with ARIs.8 Although most respiratory viral infections are mild, they can potentially cause life‐threatening illness, particularly in high‐risk groups such neonates, the elderly, and those with chronic respiratory disease.
Advances in molecular diagnosis have made it possible to easily and quickly identify viruses in people with ARIs, with quantitative polymerase chain reaction (PCR) now considered by most to be the reference standard for detection of respiratory viruses over traditional methods such as viral culture.9 However, the clinical significance of virus detection is not always clear, as respiratory viruses are also detected in asymptomatic subjects.10, 11 Further understanding of the role of viral detection in ARIs may improve clinical decision‐making around infection control and therapy.
Direct comparisons between studies of respiratory virus epidemiology can be difficult for a number of reasons. Climatic and social factors affect the survival and transmission of respiratory viruses, as well as host mobility and susceptibility.12, 13 This results in many viruses having characteristic seasonal patterns that vary by geographical location. Reported epidemiology is also affected by study design. Only one‐fifth of ARIs result in a health care visit,14 meaning that studies focussed on health care visits tend to overrepresent community‐wide prevalence of viruses causing more severe symptoms.
Population based surveillance is important in assessing morbidity and determining the role of viruses in ARIs. Previously in New Zealand, the Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) project has reported on influenza and other respiratory viruses in hospitalized patients and those seeking health care for acute respiratory illness.15, 16 The community‐wide epidemiology of respiratory viruses is less well established. This study determined rates of virus detection in ARIs, and reports on the epidemiology of respiratory viruses amongst a community cohort of children in this region.
2. METHODS
2.1. Study population and enrolment
As part of a He Kainga Oranga Housing and Health Research Programme funded by the Health Research Council of New Zealand, the Rhinovirus study was conducted to examine the impacts of bedroom temperature on young children's respiratory health in the greater Wellington region (Central Wellington, Porirua, Lower Hutt, Upper Hutt), New Zealand. Ethical approval was received from the Health and Disability Ethics Committee (12/CEN/60). Participants were recruited by recontact via letter from a previous study,17 self‐referral by parents from posters in medical facilities, from popular press articles, referral by friends and family, or by electronic messaging to district health board mailing lists.
A screening questionnaire was used to determine the eligibility of children aged 1 to 8. Of 372 children screened for participation, 305 were recruited for the study. This was made up of 138 children from a previous study,17 while the remaining 167 participants replied to advertising via staff email for the local district health boards, via posters and through referral from family and friends. There were 50 of 372 participants deemed ineligible for various reasons including that they slept in more than one bedroom, the bedroom was already heated, or they were going away for more than 2 weeks during the study period. Before commencement of the study 17 of 372 participants withdrew, while 4 of 305 participants commenced the program but were later excluded after failing to comply with study conditions. The remaining cohort for analysis was 301 children, consisting of 37 aged 1 year, 50 aged 2 years, 72 aged 3 years, 93 aged 4 years, and 49 children aged 5 years or older.
Children were progressively enrolled and commenced the 8 week study during May to October 2013 and April to October 2014. At the beginning of the study period, participants were visited in their homes where researchers obtained written consent and a record of current and previous health using a standard questionnaire. Parents were trained to recognize respiratory symptoms, and were instructed to keep a daily symptom diary for 8 weeks. A modified Jackson (1958) severity score was used to construct the symptom diary. Symptoms tracked included sneezing, sore throat, runny nose, cough, fevers, aches or pains, unwell/off‐color, and wheezing. Each symptom was graded on a 0 to 3 severity scale, with 0 being no symptoms, 1 being mild symptoms, 2 moderate symptoms, and 3 severe symptoms. Researchers also trained parents in the collection of nasal swabs (FLOQ pediatric swabs, COPAN UTM transport media, COPAN Diagnostics Inc., Murrieta, CA) which were to be taken when visible mucus was first produced during episodes of respiratory symptoms, and then stored upright in the participantʼs freezer until collection at the end of the study. At the conclusion of the study period all participants were again visited in their homes, where symptom diaries and nasal swabs were collected, and a final nasal swab was taken by researchers. Swabs were transported frozen and stored at −80°C at the University of Otago, Wellington. Frozen swabs were transported to Virology Research Laboratory, Prince of Wales Hospital, Sydney at the end of each viral season.
2.2. Detection of respiratory viruses
Samples were extracted and tested for 15 respiratory viruses most commonly causing ARI. These were IFV‐A and ‐B, HRV, RSV, PIF1‐4, HCoV‐OC43, HCoV‐229E and HCoV‐NL63, HMPV, EV, human bocavirus (HBoV), and AdV using the EasyScreen Respiratory Virus Detection Kit (Genetic Signatures, Sydney, Australia) according to manufacturerʼs instructions.
The picornaviruses (HRV‐A, HRV‐B, HRV‐C, and EV) were identified by initial multiplex testing followed by specific testing of all samples for the presence of HRV using the SensiFAST OneStep RT‐PCR Mix kit (BioLine, London,UK).18 The HRV were typed using amplification of the VP4/VP2 region of the genome on all samples testing positive by reverse transcription polymerase chain reaction (RT‐PCR). This was performed using the SensiFAST OneStep RT‐PCR Mix kit (BioLine) and the AmpliTaq Gold PCR kit (Thermo Fisher Scientific, Waltham, MA) in the first and second rounds of nested PCR, respectively, as previously described.18 Sequences were compared to the NCBI nonredundant nucleotide (nr/nt) database using the Basic Local Alignment Search Tool (BLAST) on the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and genotype assigned according to the closest identifying sequence.
Details of primers used in detection of respiratory viruses are included in the supplementary material (Table S1).
2.3. Definitions and statistical analysis
Any period in which participants had a multi‐day cumulative symptom score ≥4, with a single day symptom score ≥2 was defined as an ARI episode. The end of an ARI episode was marked by three consecutive symptom‐free days. A symptom score correction (daily baseline score of −1) was applied to participants who were considered to have background rhinitis. This included children who had (i) rhinitis symptoms (runny nose or sneezing) for ≥49 of the 56 study days, (ii) no more than 7 days with no symptoms, or (iii) 7 or more days of only rhinitis symptoms.
In cases where multiple swabs were taken during a single ARI episode, only the first swab taken was included in analysis. The exception to this was if a new virus was detected in subsequent swabs, or if swabs were taken >30 days apart.
Risk ratio (RR) was calculated as the proportion of samples positive for a virus with ARI symptoms divided by the proportion of samples negative for a respiratory virus with ARI symptoms.
3. RESULTS
3.1. Community cohort characteristics
There were 16 856 child‐days of symptom observed and a total of 612 nasal swabs collected from the 301 participants. There were 575 ARIs identified (Table 1). Nasal swabs were received from 400 of 575 of these episodes (69.6%). In addition, 212 swabs were taken when children were asymptomatic, most of which were performed during the clinical visit by researchers at the conclusion of the study.
Table 1.
Participant information and prevalence of viral detections by age
| Age group | ||||||
|---|---|---|---|---|---|---|
| All participants | 1 | 2 | 3 | 4 | ≥5 | |
| Enrolled | 301 | 37 | 50 | 72 | 93 | 49 |
| Sex, M (%) | 173 (57.5) | 21 (56.8) | 28 (56.0) | 51 (70.1) | 49 (52.7) | 24 (49.0) |
| ARI | ||||||
| n (per child‐month) | 575 (1.04) | 72 (1.06) | 102 (1.12) | 143 (1.08) | 173 (1.01) | 85 (0.95) |
| Swabs collected, n | 400 | 54 | 70 | 101 | 112 | 63 |
| Viral detections, n (%) | 307 (76.8) | 48 (88.9) | 57 (81.4) | 81 (80.2) | 77 (68.8) | 44 (69.8) |
| Codetections, n (%) | 37 (9.3) | 7 (13.0) | 8 (11.4) | 6 (5.9) | 15 (13.4) | 1 (1.6) |
| Asymptomatic | ||||||
| Swabs collected, n | 212 | 20 | 33 | 51 | 72 | 36 |
| Viral detections, n (%) | 79 (37.3) | 9 (45.0) | 12 (36.4) | 22 (43.1) | 22 (30.6) | 14 (38.9) |
| Codetections, n (%) | 11 (5.2) | 2 (10.0) | 3 (9.1) | 2 (3.9) | 2 (2.8) | 1 (2.8) |
Abbreviation: ARI, acute respiratory infections.
Due to inclusion of children from a previous study of wheezing, those with asthma were highly represented within our cohort (33.6%). Overall, children with asthma displayed a small increase in the rate of ARIs per child‐month (1.13 vs 1.00) compared with nonasthmatics (Table S2), but this increase was not significant (P = 0.057).
3.2. Virus detection
Of the 400 swabs taken during an ARI, 307 (76.8%) were positive for a virus. During an ARI, detection of respiratory viruses in nasal swabs was highest amongst the youngest age group (1 year old), and decreased to age 4 (Table 1). During ARIs, multiple viruses were codetected in 37 (9.3%) swabs.
The most commonly detected viruses in samples collected during ARI were HRV (52.8%), HCoV (11.0%), parainfluenza virus (PIF) (6.0%), IFV (4.5%), RSV (3.8%), and HMPV (3.5%). These were all significantly more likely to be associated with ARI episodes than asymptomatic detection (Table 2). AdV was detected with similar frequency in both ARI (3.3%) and asymptomatic samples (3.8%), while detection of HBoV was relatively low in both groups. Detection of any virus and codetection of viruses were both significantly associated with swabs collected during ARI episodes.
Table 2.
Acute viral respiratory infections, asymptomatic viral detections, and risk ratios for respiratory viruses
| Swabs taken during ARI n = 400, n (%) | Swabs taken outside of ARI n = 212, n (%) | RR (95% CI), P value | |
|---|---|---|---|
| Any virus detected | 307 (76.8) | 79 (37.3) | 1.22 (1.13‐1.31), <0.0001 |
| All HRVa | 211 (52.8) | 65 (30.7) | 1.17 (1.07‐1.28), 0.0004 |
| HRV‐A | 83 (20.8) | 29 (13.7) | 1.13 (1.00‐1.28), 0.0466 |
| HRV‐B | 13 (3.3) | 4 (1.9) | 1.17 (0.89‐1.53), 0.2543 |
| HRV‐C | 48 (12.0) | 8 (3.8) | 1.31 (1.16‐1.48), <0.0001 |
| IFV | 18 (4.5) | 0 (0.0) | 1.53 (1.44‐1.62), <0.0001 |
| HCoV | 44 (11.0) | 6 (2.8) | 1.35 (1.20‐1.51), <0.0001 |
| RSV | 15 (3.8) | 2 (0.9) | 1.35 (1.12‐1.62), 0.0013 |
| HMPV | 14 (3.5) | 1 (0.5) | 1.43 (1.23‐1.65), <0.0001 |
| PIF | 24 (6.0) | 5 (2.4) | 1.27 (1.06‐1.51), 0.0085 |
| AdV | 13 (3.3) | 8 (3.8) | 0.95 (0.67‐1.33), 0.7545 |
| HBoV | 1 (0.25) | 3 (1.4) | 0.38 (0.07‐2.09), 0.2674 |
| EV | 7 (1.8) | 1 (0.5) | 1.34 (1.02‐1.75), 0.033 |
| Codetection | 37 (9.3) | 10 (4.7) | 1.20 (1.03‐1.41), 0.0222 |
Abbreviations: AdV, adenovirus; CI, confidence interval; EV, human enterovirus; HBoV, human bocavirus; HCoV, human coronavirus; HRV, human rhinovirus; IFV, influenzaviruses; PIF, parainfluenza virus; RSV, respiratory syncytial virus.
Note. Count of individual viruses includes codetections.
Bold values indicate significant < 0.05.
Not all HRV specimens could be genotyped.
Sequencing was performed on 193 of a possible 284 picornavirus positive samples (68.0%). Rhinovirus strains HRV‐A and HRV‐C were significantly associated with ARI episodes (Table 2).
Overall, detection of individual viruses was similar between asthmatic and nonasthmatic children in swabs taken during an ARI (Table S3). HRV‐B (P = 0.042) and RSV (P = 0.034) were significantly over represented in asthmatics during ARIs.
3.3. Monthly distribution of ARIs and viruses
ARIs were most frequent in May (1.39), which was also the month in which the least data was collected. This was followed by June (1.22), August (1.21), and July (1.07). The rate of ARIs steadily decreased following the winter season (Figure 1).
Figure 1.
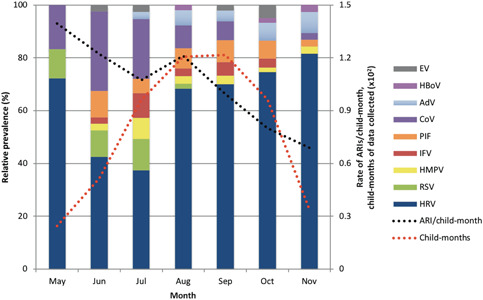
Relative monthly prevalence of respiratory viruses throughout the year in the sampled cohort. Columns represent individual viruses as a proportion of total monthly viral detections. Black line indicates the rate of acute respiratory infections per child‐month, while the red line specifies the amount of data collected for each month. AdV, adenovirus; CoV, coronavirus; EV, human enterovirus; HBoV, human bocavirus; HMPV, human metapneumovirus; HRV, human rhinovirus; IFV, influenzaviruses; PIF, parainfluenza virus; RSV, respiratory syncytial virus
HRV was the predominant virus detected in each month, and was most prevalent in late‐winter and spring (Figure 1). HCoV and RSV had peaks during early winter, while AdV peaked in spring. IFV and HMPV were most prevalent in July, but were also detected in other months. PIF was detected consistently throughout study months, although not in May.
4. DISCUSSION
There is a need for improved population data regarding respiratory viruses in children, particularly in the Southern Hemisphere. This is the first study assessing the epidemiology of an extended range of respiratory viruses in a community cohort of healthy children in New Zealand. Infection with respiratory viruses was common in both symptomatic and asymptomatic children, and this study demonstrated the utility of home‐collected swabs to profile these viral infections in the community.
The frequency of ARIs throughout the study period was 1.04 per child‐month, and when a sample was available, a respiratory virus was detected in 76.8% of illnesses. For studies of community surveillance, virus positive rates for children with ARIs or defined influenza‐like illness range from approximately 39% to 80%.3, 4, 5, 14, 19 A previous analysis of Australian preschool aged children with a comparable design detected a virus in 74% of samples.4 Children in the current study had a relatively high frequency of ARIs over the 7 month winter period of 1.04 per child‐month, compared to the Australian study where a peak of 0.87 was reported for June (winter). These relatively high frequencies were true of children with asthma (1.12) and those without asthma (1.00).
The proportion of ARIs associated with a virus‐positive swab in this population declined with age. Children 1 year of age were positive for a respiratory virus in 88.9% of samples taken during an ARI, compared to 69.8% in participants aged greater than or equal to 5 years age. A similarly designed household surveillance study showed that weekly detections of viruses in young (aged <5) and older (aged 5‐17) children were significantly more frequent than in adults.20 In the current study swabs were taken at the onset of illness, when viral load reportedly peaks for some respiratory viruses.21 It is possible that this method is more likely to detect viruses associated with ARI than other studies obtaining weekly swabs. Sample sizes of each virus detected here were too small to compare individual viruses between age groups. However, a multi‐country community surveillance study has previously shown that with the exception of influenza, prevalence of individual respiratory viruses was highest in younger children.3 The rates of ARI per child‐month were similar between age groups, suggesting that other factors such as development of allergy may play a more significant role than age in acute respiratory health.
The study was conducted during the winter/spring seasons of 2 consecutive years. Consistent with previous reports in the Southern Hemisphere RSV peaked in this pediatric population in early winter.4, 22 Interestingly, we found a similar pattern for detection of HCoV. It has recently been reported in China that while overall HCoV infection is most prevalent in winter, HCoV subtypes display different epidemic patterns.23 As only a small number of HCoVs were detected (n = 50), we did not distinguish between subtypes when reporting our results. Influenza was detected from June to October and peaked in July. A limitation was that no data were collected for the summer and autumn periods, capturing only the times of highest respiratory virus activity. This may account for the low detection of AdV, HBoV, and EV in comparison to other year‐round epidemiological studies.24 A year‐round analyses may also allow for investigation of phenomena such as the February back to school HRV peaks.25
Our study differs from a previous NZ‐based analysis of viruses in hospitalized children with LRTI,22 where relatively high rates of RSV (39%) and HMPV (7%) were detected. In our community‐based study, incidence of these viruses was considerably lower. Previous groups have reported that RSV and HMPV rarely occur in asymptomatic subjects,11, 26, 27 suggesting that detection of these viruses is almost always of clinical relevance. IFV may be present in people with few or no symptoms. However, the duration and copy number of viral shedding is considerably lower in these cases,28 and further research is needed to understand the contribution of asymptomatic detection to community transmission. In the present study, we found these viruses in very few samples of asymptomatic subjects, and demonstrated these three viruses (and others) to be significantly associated with ARIs.
Respiratory viruses were detected in a large proportion of swabs taken during asymptomatic periods (37.3%), with the vast majority of these detections caused by HRV. This is unsurprising as HRVs were the most frequently detected virus overall, and have been commonly detected in asymptomatic children before.11 It has previously been unclear whether subtypes of HRV have varying clinical impact on their hosts, with studies of healthy,5 and asthmatic18 children reporting no differences in symptom risk between HRV subtypes. Our data, along with another recent study19 suggest that HRV‐A and HRV‐C subtypes are significantly more likely to cause symptoms related to ARI. In our study HRV‐B, AdV, and HBoV were detected similarly in samples from both ARI episodes and asymptomatic periods. While their corresponding risk ratios are not considered significant, the number of detections of these viruses is relatively small, and a larger analysis would be required to rule out the clinical significance of detecting HRV‐B, AdV and HBoV in ARIs.
The effect of viral coinfection on respiratory disease severity in children has not been well established. A number of studies have found increased hospitalizations, longer length of stay in hospital, and higher mortality when two or more respiratory viruses were detected.29, 30, 31 However, others report no association between viral coinfection and clinical outcomes32, 33 including a systematic review of 43 such publications.34 In this study we found a codetection was observed in 9.3% of total virus detections during ARIs, which is similar to rates reported previously.4 We also codetected viruses in a small number of swabs collected during asymptomatic periods, and show that codetections are significantly more likely to be associated with ARIs. Our sample size for codetection of viruses is relatively small, and larger studies have demonstrated a more significant association.19
Respiratory viruses cause significant morbidity, particularly for infections with IFV and RSV, and especially in children with underlying chronic respiratory disease. Our parent‐administered surveillance of healthy children provides insight into the community epidemiology of respiratory viruses during the winter and spring seasons in New Zealand. This cohort demonstrates infection with viruses, particularly HRV, is common in symptomatic and asymptomatic children. More than one‐third of swabs taken when children were asymptomatic were positive for a virus, although specific viruses (HRV‐A, HRV‐C, IFV, HCoV, RSV, HMPV, PIF, and EV) are more likely to be associated with symptomatic ARIs. This suggests pathogenesis of infection differs between viruses, and indicates the need for further biomarkers of pathology in these infections.
Supporting information
Supplementary information
Supplementary information
Supplementary information
Walker GJ , Stelzer‐Braid S , Shorter C , et al. Viruses associated with acute respiratory infection in a community‐based cohort of healthy New Zealand children. J Med Virol. 2022;94:454‐460. 10.1002/jmv.25493
References
REFERENCES
- 1. Murray CJL, Vos T, Lozano R, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197‐2223. [DOI] [PubMed] [Google Scholar]
- 2. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta‐analysis. Lancet. 2011;378:1917‐1930. [DOI] [PubMed] [Google Scholar]
- 3. Taylor S, Lopez P, Weckx L, et al. Respiratory viruses and influenza‐like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi‐country population sample. J Infect. 2017;74:29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambert SB, Allen KM, Druce JD, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool‐aged children using parent‐collected specimens. Pediatrics. 2007;120:e929‐e937. [DOI] [PubMed] [Google Scholar]
- 5. Mackay IM, Lambert SB, Faux CE, et al. Community‐wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool‐aged children during a 12‐month period. J Infect Dis. 2013;207:1433‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Conto F, Conversano F, Medici MC, et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3‐year hospital‐based survey in Northern Italy. Diagn Microbiol Infect Dis. 2019. [published online ahead of print January 17, 2019]. 10.1016/j.diagmicrobio.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brealey JC, Sly PD, Young PR, Chappell KJ. Viral bacterial co‐infection of the respiratory tract during early childhood. FEMS Microbiol Lett. 2015;362:fnv062. [DOI] [PubMed] [Google Scholar]
- 8. Debiaggi M, Canducci F, Ceresola ER, Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J. 2012;9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck ET, Henrickson KJ. Molecular diagnosis of respiratory viruses. Future Microbiol. 2010;5:901‐916. [DOI] [PubMed] [Google Scholar]
- 10. Jartti T, Söderlund‐Venermo M, Hedman K, Ruuskanen O, Makela MJ. New molecular virus detection methods and their clinical value in lower respiratory tract infections in children. Paediatr Respir Rev. 2013;14:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howard LM, Johnson M, Williams JV, et al. Respiratory viral detections during symptomatic and asymptomatic periods in young Andean children. Pediatr Infect Dis J. 2015;34:1074‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pica N, Bouvier NM. Environmental factors affecting the transmission of respiratory viruses. Curr Opin Virol. 2012;2:90‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sloan C, Moore ML, Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci. 2011;4:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szilagyi PG, Blumkin A, Treanor JJ, et al. Incidence and viral aetiologies of acute respiratory illnesses (ARIs) in the United States: a population‐based study. Epidemiol Infect. 2016;144:2077‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang QS, Baker M, McArthur C, et al. Implementing hospital‐based surveillance for severe acute respiratory infections caused by influenza and other respiratory pathogens in New Zealand. WPSAR. 2014;5:23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang QS, Turner N, Baker MG, et al. Southern Hemisphere influenza and vaccine effectiveness research and surveillance. Influenza Other Respir Viruses. 2015;9:179‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shorter C, Crane J, Pierse N, et al. Indoor visible mold and mold odor are associated with new‐onset childhood wheeze in a dose‐dependent manner. Indoor Air. 2018;28:6‐15. [DOI] [PubMed] [Google Scholar]
- 18. Tovey ER, Stelzer‐Braid S, Toelle BG, et al. Rhinoviruses significantly affect day‐to‐day respiratory symptoms of children with asthma. J Allergy Clin Immunol. 2015;135:663‐669.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarna M, Lambert SB, Sloots TP, et al. Viruses causing lower respiratory symptoms in young children: findings from the ORChID birth cohort. Thorax. 2018;73:969‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byington CL, Ampofo K, Stockmann C, et al. Community surveillance of respiratory viruses among families in the Utah Better Identification of Germs‐Longitudinal Viral Epidemiology (BIG‐LoVE) Study. Clin Infect Dis. 2015;61:1217‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lau LLH, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201:1509‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trenholme AA, Best EJ, Vogel AM, Stewart JM, Miller CJ, Lennon DR. Respiratory virus detection during hospitalisation for lower respiratory tract infection in children under 2 years in South Auckland, New Zealand. J Paediatr Child Health. 2017;53:551‐555. [DOI] [PubMed] [Google Scholar]
- 23. Zhang S‐f, Tuo J‐l, Huang X‐b, et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV‐OC43 during 2010‐2015 in Guangzhou. PLoS One. 2018;13:e0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O. Viral etiology of common cold in children, Finland. Emerging Infect Dis. 2009;15:344‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stelzer‐Braid S, Tovey ER, Willenborg CM, et al. Absence of back to school peaks in human rhinovirus detections and respiratory symptoms in a cohort of children with asthma. J Med Virol. 2015;88:578‐587. [DOI] [PubMed] [Google Scholar]
- 26. Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631‐2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154:396‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ip DK, Lau LL, Leung NH, et al. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin Infect Dis. 2017;64:736‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guerrier G, Goyet S, Chheng ET, et al. Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr Infect Dis J. 2013;32:e8‐e13. [DOI] [PubMed] [Google Scholar]
- 30. Richard N, Komurian‐Pradel F, Javouhey E, et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27:213‐217. [DOI] [PubMed] [Google Scholar]
- 31. Zhang G, Hu Y, Wang H, Zhang L, Bao Y, Zhou X. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PLOS One. 2012;7:e44568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gagliardi TB, Paula FE, Iwamoto MA, et al. Concurrent detection of other respiratory viruses in children shedding viable human respiratory syncytial virus. J Med Virol. 2013;85:1852‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Costa LF, Queiróz DA, Lopes da Silveira H, et al. Human rhinovirus and disease severity in children. Pediatrics. 2014;133:e312‐e321. [DOI] [PubMed] [Google Scholar]
- 34. Scotta MC, Chakr VC, de Moura A, et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta‐analysis. J Clin Virol. 2016;80:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu X, Holloway B, Dare RK, et al. Real‐time reverse transcription‐pcr assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533‐539. [Supplementary information] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wisdom A, Mc William Leitch EC, Gaunt E, Harvala H, Simmonds P. Screening respiratory samples for detection of human rhinoviruses (hrvs) and enteroviruses: comprehensive Vp4‐Vp2 typing reveals high incidence and genetic diversity of HRV species C. J Clin Microbiol. 2009;47:3958‐3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stelzer‐Braid S, Johal H, Skilbeck K, et al. Detection of viral and bacterial respiratory pathogens in patients with cystic fibrosis. J Virol Methods. 2012;186:109‐112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information


