Abstract
The outbreak of 2019 novel coronavirus (COVID‐19) infection emerged in Wuhan, China, in December 2019. Since then the novel coronavirus pneumonia disease has been spreading quickly and many countries and territories have been affected, with major outbreaks in China, South Korea, Italy, and Iran. Influenza virus has been known as a common pathogen in winter and it can cause pneumonia. It was found clinically that very few patients were diagnosed with both COVID‐19 and influenza virus. A total of 5 of the 115 patients confirmed with COVID‐19 were also diagnosed with influenza virus infection, with three cases being influenza A and two cases being influenza B. In this study, we describe the clinical characteristics of those patients who got infected with COVID‐19 as well as influenza virus. Common symptoms at onset of illness included fever (five [100%] patients), cough (five [100%] patients), shortness of breath (five [100%] patients), nasal tampon (three [60%] patients), pharyngalgia (three [60%] patients), myalgia (two [40%] patients), fatigue (two [40%] patients), headache (two [40%] patients), and expectoration (two [40%] patients). The laboratory results showed that compared to the normal values, the patients' lymphocytes were reduced (four [80%] patients), and liver functions alanine aminotransferase and aspartate aminotransferase (two [40%] patients and two [40%] patients) and C‐reactive protein (four [80%] patients) were increased when admitted to hospital. They stayed in the hospital for 14, 30, 17, 12, and 19 days (28.4 ± 7.02), respectively. The main complications for the patients were acute respiratory distress syndrome (one [20%] patients), acute liver injury (three [60%] patients), and diarrhea (two [40%] patients). All patients were given antiviral therapy (including oseltamivir), oxygen inhalation, and antibiotics. Three patients were treated with glucocorticoids including two treated with oral glucocorticoids. One of the five patients had transient hemostatic medication for hemoptysis. Fortunately, all patients did not need intensive care unit and were discharged from the hospital without death. In conclusion, those patients with both COVID‐19 and influenza virus infection did not appear to show a more severe condition because based on the laboratory findings, imaging studies, and patient prognosis, they showed similar clinical characteristics as those patients with COVID‐19 infection only. However, it is worth noting that the symptoms of nasal tampon and pharyngalgia may be more prone to appear for those coinfection patients.
Keywords: clinical characteristics, coinfection, COVID‐19, influenza virus
1. INTRODUCTION
On December 2019, a cluster of cases of novel pneumonia emerged in Wuhan, China. 1 , 2 On 7 January 2020, Chinese health authorities confirmed that this cluster was associated with a novel coronavirus. This novel coronavirus was named as “coronavirus disease 2019” (COVID‐19) by the World Health Organization on February 11. The clinical characteristics of COVID‐19 related pneumonia patients have been shown in recent studies. 1 , 2 , 3 , 4 , 5 Common symptoms at the onset of illness included fever, cough, and fatigue or myalgia. Organ dysfunction included acute respiratory distress syndrome (ARDS), acute cardiac injury, acute liver injury, acute kidney injury, and death could occur in severe cases. 1 , 2 , 3 , 4 Computed tomography (CT) images demonstrated typical radiographic features including ground‐glass opacities (GGOs), multifocal patchy consolidation, and/or interstitial changes with a peripheral distribution. 6 , 7
Influenza virus, a common virus often occurring in winter as well, appears to be similar to COVID‐19 in terms of transmission characteristics. 8 , 9 The common clinical manifestations of patients with influenza virus included fever, cough, rhinitis, sore throat, headache, dyspnea, myalgia, and radiographic evidence of pneumonia, which are similar to those of COVID‐19 patients. 9 , 10 The typical chest CT presentations for influenza virus pneumonia are also similar to those for COVID‐19, including GGO, consolidation, and pleural effusion. 11 , 12
In the clinic, we found that a few patients diagnosed with COVID‐19 were also infected with the influenza virus. However, to the best of our knowledge, the clinical characteristics of patients who were coinfected with COVID‐19 and influenza virus have not been discussed, and that is the focus of this study.
2. METHODS
2.1. Patients
The patients who were both infected with the influenza virus and COVID‐19 were enrolled from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. These case series were approved by the Ethics Commission of Tongji Hospital. Oral consent was obtained from each patient. We always followed the most up‐to‐date version of the guidelines of diagnosis and treatment of pneumonitis caused by the novel coronavirus, which were promulgated by the National Health Commission of China. Therefore, multiple versions including the fourth, the fifth, and the sixth have been used for these 115 patients. 13 The influenza virus diagnosis in this study was built on the serology.
2.2. Data collection
Medical history, clinical symptoms, laboratory findings, chest CT scans, and treatment and outcomes data were obtained with standardized data collection forms. The collected data were also independently reviewed and checked by two reviewers. To minimize the recall and sampling deviations is to communicate with the patients effectively and double‐check with them.
2.3. Statistical analysis
The data were analyzed using the IBM SPSS, version 19 (SPSS Inc, Chicago, IL) and GraphPad Prism version 5.00 (GraphPad Software, La Jolla). All statistical data were presented as meaning ± SEM.
3. RESULTS
3.1. Presenting characteristics when admitted
In this study, we found that 5 patients also got infected with the influenza virus among 115 inpatients confirmed with COVID‐19 infection, an incidence rate of about 4.35%. Three cases infected with influenza A virus (60%), and the other two cases infected with influenza B virus (40%) among the five patients. The clinical characteristics of patients at admission are given in Table 1. The age of these five patients (two males and three females) ranged from 39 to 66 years (50.20 ± 9.83). The past medical history of each patient is also shown in Table 1. Common symptoms at onset of illness included fever (five [100%] patients), cough (five [100%] patients), shortness of breath (five [100%] patients), myalgia (two [40%] patients), fatigue (two [40%] patients), headache (two [40%] patients), chest pain (one [20%] patient), nasal tampon (three [60%] patients), expectoration (two [40%] patients), pharyngalgia (three [60%] patients), and hemoptysis (one [20%] patient) (Table 1). The main laboratory results at the time of admission are shown in Table 2. Compared to the reference range, the patients' lymphocyte levels were reduced (four [80%] patients). Liver alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (two [40%] patients and two [40%] patients), C‐reactive protein (CRP) levels (four [80%] patients), and procalcitonin levels (two [40%] patients) were increased. But the patients' white blood cell count, albumin, renal function indicators (creatinine and blood urea nitrogen), and coagulation function (prothrombin time, activated partial thromboplastin time, and D‐dimer) were normal.
Table 1.
Clinical characteristics of patients infected with COVID‐19 and influenza virus when admitted to hospital
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | n% (Y) | |
|---|---|---|---|---|---|---|
| Age | 47 | 50 | 66 | 39 | 49 | |
| Sex | Female | Male | Female | Male | Female | |
| Comorbidities | ||||||
| Hypertension | NA | Y | Y | NA | NA | 40% |
| Diabetes | NA | NA | NA | NA | NA | 0% |
| Cardiovascular disease | NA | NA | Y | NA | NA | 20% |
| Influenza virus | Influenza A virus | Influenza A virus | Influenza B virus | Influenza B virus | Influenza A virus | 100% |
| Tumor | NA | Y (kidney) | NA | NA | NA | 20% |
| HBV | NA | NA | Y | Y | NA | 40% |
| Signs and symptoms | ||||||
| Fever | Y | Y | Y | Y | Y | 100% |
| Cough | Y | Y | Y | Y | Y | 100% |
| Shortness of breath | Y | Y | Y | Y | Y | 100% |
| Myalgia | Y | NA | NA | NA | Y | 40% |
| Fatigue | Y | Y | NA | NA | NA | 40% |
| Headache | Y | NA | NA | NA | Y | 40% |
| Chest pain | NA | NA | NA | NA | Y | 20% |
| Diarrhea | NA | NA | NA | NA | NA | 0% |
| Nasal tampon | Y | NA | Y | NA | Y | 60% |
| Expectoration | Y | NA | NA | NA | Y | 40% |
| Pharyngalgia | Y | NA | Y | NA | Y | 60% |
| Hemoptysis | NA | NA | NA | NA | Y | 20% |
Abbreviations: COVID‐19, coronavirus disease 2019; NA, not applicable; Y, yes.
Table 2.
Laboratory findings of patients infected with COVID‐19 and influenza virus when admitted to hospital
| Lab findings | Reference range | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | n% (outrange) |
|---|---|---|---|---|---|---|---|
| White blood cell count, ×109/L | 3.5‐9.5 | 3.87 | 4.89 | 4.49 | 3.39 | 9.12 | 0% |
| Neutrophil count, ×109/L | 1.8‐6.3 | 2.62 | 3.67 | 3.05 | 2.03 | 6.4 | 20% |
| Lymphocyte count, ×109/L | 1.1‐3.2 | 0.88 | 0.81 | 0.95 | 0.84 | 1.92 | 80% |
| Prothrombin time, s | 11.5‐14.5 | 13.3 | 12.7 | 13.8 | 13.1 | 13.7 | 0% |
| Activated partial thromboplastin time, s | 29.0‐42.0 | 36.6 | 36.9 | 38.2 | 46.3 | 41.2 | 0% |
| D‐dimer, mg/L | <0.5 | 0.26 | 0.29 | 2.05 | 0.37 | 0.26 | 20% |
| Alanine aminotransferase, U/L | ≤33 | 7 | 35 | 18 | 63 | 14 | 40% |
| Aspartate aminotransferase, U/L | ≤32 | 18 | 35 | 22 | 56 | 15 | 40% |
| Albumin, g/L | 35.0‐52.0 | 43 | 46.3 | 36.4 | 41.5 | 38.4 | 0% |
| Total bilirubin, umol/L | ≤21 | 4.3 | 8 | 5.3 | 5 | 5.4 | 0% |
| Creatinine, μmol/L | 45‐84 (F) and 59‐104 (M) | 50 | 99 | 65 | 81 | 54 | 0% |
| Blood urea nitrogen, mmol/L | 3.6‐9.5 | 3.11 | 5.7 | 3.8 | 3.4 | 3.6 | 0% |
| C‐reactive protein, mg/L | <1 | 1.8 | 21.5 | 0.9 | 6.8 | 9.5 | 80% |
| Procalcitonin, ng/mL | 0.02‐0.05 | 0.03 | 0.07 | 0.04 | 0.06 | 0.02 | 40% |
Note: The reference range for creatinine is different between the female and the man.
Abbreviation: COVID‐19, coronavirus disease 2019.
3.2. The dynamic profile of laboratory findings and CT images in patients coexisting with COVID‐19 and influenza virus infection
To better demonstrate the major clinical features of pneumonia patients with COVID‐19 and influenza virus, the dynamic changes in clinical laboratory parameters and CT scans are presented in Figures 1 and 2. According to the patient's disease progression, the data were presented in three stages, including the admission stage, progression stage, and remission stage (Figure 1). The laboratory parameters included white blood cell count, neutrophil count, lymphocyte count, ALT, albumin, creatinine, abd CRP. We found that only one patient presented a higher white blood cell count during the progression stage than the upper limit of the reference range (one [20%] patient). The neutrophil count of two patients exceeded the upper limit of the reference range during the progression phase (two [40%] patients). Four of five patients showed a lower lymphocyte count than the lower limit of the reference range during progression stages (four [80%] patients). Lymphocytes then increased during the remission phase and even exceeded the lower limit of the reference range. Liver function ALT of three patients (three [60%] patients) rose above the upper limit of the reference range during the progression of pneumonia, which was more significant than those in the admission stage. Four of the five patients had an albumin reduction during the disease progression (four [80%] patients), and three of these four patients even had the albumin count below the lower limit of the reference range. Renal function was normal during the admission and progression stages. The inflammation indicator CRP exceeded the upper limit of the reference range during the entire course of the disease, but the highest value was only 31.9 mg/L.
Figure 1.
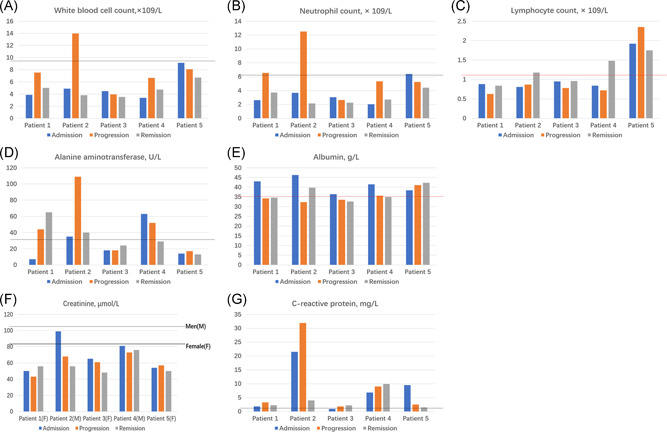
The dynamic profile of laboratory findings in patients coexisting with COVID‐19 and influenza virus infection. Laboratory parameters were presented in three stages, including the admission stage, progression stage, and remission stage. A, White blood cell count. B, Neutrophil count. C, Lymphocyte count. D, Alanine aminotransferase. E, Albumin. F, Creatinine. G, C‐reactive protein. The solid lines in black show the upper normal limit of the laboratory parameter, and the solid line in red shows the lower normal limit of the laboratory parameter. COVID‐19, coronavirus disease 2019
Figure 2.
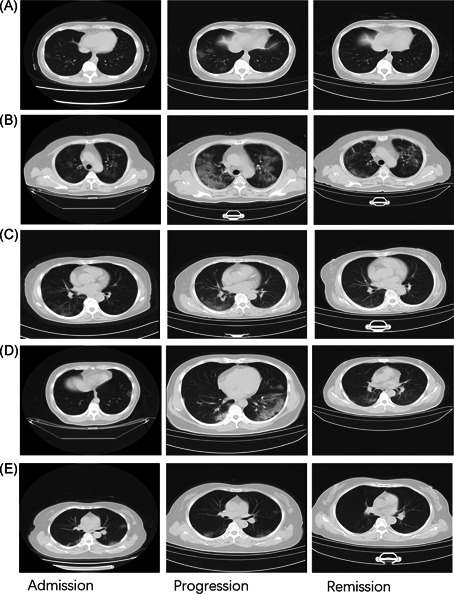
The dynamic profile of CT images in patients coexisting with COVID‐19 and influenza virus infection. CT images were presented in three stages, including the admission stage, progression stage, and remission stage. A, Patient 1. B, Patient 2. C, Patient 3. D, Patient 4. E, Patient 5. COVID‐19, coronavirus disease 2019; CT, computed tomography
CT imaging results demonstrated that patients' lung situation changed during admission, progression, and remission stages. Pulmonary lesions in all the patients gradually relieved after an exacerbation (see Figure 2). According to the CT characteristics, the duration from the onset of the illness to the remission stage in the five patients was 16, 39, 28, 21, and 24 days (25.6 ± 8.68), respectively. The days for the hospital stay were 14, 30, 17, 12, and 19 days (28.4 ± 7.02), respectively.
3.3. The main complications, main interventions, and outcomes for the patients coexisting with COVID‐19 and influenza virus infection
During the pneumonia progression, the main complications are reported in Table 3. We found that only one of five patients had ARDS (one [20%] patient) and the patient's hypoxia gradually recovered by using noninvasive assisted ventilation. In addition, three of the five patients (three [60%] patients) had an abnormal liver function, with the most severe case showing an ALT value of 109 U/L, and all patients had no jaundice. Two of the five patients (two [40%] patients) had diarrhea during the treatment period.
Table 3.
The main complications for the patients infected with COVID‐19 and influenza virus
| Complications | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | n% (Y) |
|---|---|---|---|---|---|---|
| Acute respiratory distress syndrome | NA | Y | NA | NA | NA | 20% |
| Shock | NA | NA | NA | NA | NA | 0% |
| Acute cardiac injury | NA | NA | NA | NA | NA | 0% |
| Acute liver injury | Y | Y | NA | Y | NA | 60% |
| Acute kidney injury | NA | NA | NA | NA | NA | 0% |
| Diarrhea | NA | Y | NA | Y | NA | 40% |
Abbreviations: COVID‐19, coronavirus disease 2019; NA, not applicable; Y, yes.
All patients were treated using antiviral therapy (including oseltamivir), oxygen inhalation, and antibiotics (Table 4). Three of the five patients (three [60%] patients) were treated with glucocorticoids with two being treated with only oral glucocorticoids. One of the five patients (one [20%] patient) had a transient hemostatic medication for hemoptysis. Fortunately, all patients did not receive intensive care unit (ICU) and were discharged from the hospital without death.
Table 4.
The main treatments for the patients infected with COVID‐19 and influenza virus
| Treatment | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | n% (Y) |
|---|---|---|---|---|---|---|
| Oseltamivir | Y | Y | Y | Y | Y | 100% |
| Antiviral therapy (except oseltamivir) | Y | Y | Y | Y | Y | 100% |
| Antibiotic therapy | Y | Y | Y | Y | Y | 100% |
| Glucocorticoid therapy | Y | Y | NA | Y | NA | 60% |
| Oxygen inhalation | Y | Y | Y | Y | Y | 100% |
| Noninvasive ventilation | NA | Y | NA | NA | NA | 20% |
| Invasive mechanical ventilation | NA | NA | NA | NA | NA | 0% |
| Extracorporeal membrane oxygenation | NA | NA | NA | NA | NA | 0% |
| Hemostyptic therapy | NA | NA | NA | NA | Y | 20% |
| Intensive care unit care | NA | NA | NA | NA | NA | 0% |
Abbreviations: COVID‐19, coronavirus disease 2019; NA, not applicable; Y, yes.
4. DISCUSSION
We reported a descriptive study on the clinical characteristics of pneumonia patients with the infection of both COVID‐19 and influenza virus. Some reports have shown that COVID‐19 can cause serious, sometimes fatal, pneumonia. 3 , 4 , 5 The outbreak of COVID‐19 pneumonia in Wuhan began in December 2019, and this is also the time when the influenza virus occurred. Transmission characteristics and onset time of influenza virus appear to be of similar magnitude to COVID‐19. 8 Therefore, the evidence suggested that the patients may have coinfection with COVID‐19 and influenza virus.
In this report, we found that 5 patients coinfected with influenza virus among 115 pneumonia patients confirmed with COVID‐19. Similar to the patients with COVID‐19 infection, the patients coinfected with COVID‐19 and influenza virus also have symptoms such as fever, cough, fatigue, and headache. However, in this report, we found that three of five patients had nasal tampon and pharyngalgia (60%), which are not a frequent symptom of COVID‐19 patients. When these two symptoms appeared, it was generally considered that a common cold or influenza virus infection may occur. Therefore, they may be a reminder to those who had nasal tampon and pharyngalgia symptoms not to ignore the possibility of COVID‐19 infection, if possible, to keep themselves isolated from the rest of the families. Another patient had mild hemoptysis, which was not a common symptom of COVID‐19 infection, and he recovered after taking the hemostatic medicine for three days. It was found that two of five patients developed diarrhea during the disease progression stage, which may be caused by drugs, such as oseltamivir, or COVID‐19 infection, which could be detected in stool samples of patients with abdominal symptoms. 14
In addition, we found that all the patients' leukocytes were in the reference range during the admission phase, while one patient's leukocytes exceeded the upper limit of the reference range during the progression phase. The lymphocytes of patients are below the lower limit of the reference range during the admission and progression stages and can gradually rise during the remission stage, which may be associated with cellular immune deficiency. Liver ALT and AST and CRP may show mild abnormalities. Patients' albumin often declined as the disease progressed, even reaching a point of being below the lower limit of the reference range. No abnormal renal function was noticed throughout the whole process. These laboratory findings above were similar to those previously reported in patients with only COVID‐19 infection. 3 , 4 As for the patient's dynamic CT scan images, one patient had significant bilateral GGO and subsegmental areas of consolidation, which corresponded to the clinical manifestations of the patient. The patient had ARDS, relying on the noninvasive respiratory ventilation, and required the longest period of treatment in the hospital. Through the analysis of the image characteristics of the five patients, it seemed that there was no significant difference from that of pneumonia patients infected with only COVID‐19.
As for the perspective of patient complications, all the patients showed shortness of breath, and only one patient developed ARDS without shock, acute myocardial injury, and abnormal renal function. Severe illness occurred in 15.7% of the patients with COVID‐19 after admission to a hospital, 5 which suggested that patients who coinfected COVID‐19 and influenza virus did not significantly worsen the condition. Until now, no specific treatment has been recommended for coronavirus infection except for meticulous supportive care. 15 As for the therapy methods, the patients did not receive invasive ventilator, extracorporeal membrane oxygenation treatment, and ICU care. All patients were given oxygen inhalation, plus oseltamivir and other antiviral drugs. All the patients in this study received antibacterial agents. All patients recovered and were discharged without death.
On one hand, this study showed that the clinical characteristics of patients with both COVID‐19 and influenza virus infection were similar to those of COVID‐19 infection, but the symptoms of nasal tampon and pharyngalgia may be more prone to appear, which would be more convinced when more cases included. According to the laboratory findings, imaging studies, and patient prognosis, those coinfection patients did not appear to experience a more severe situation. To the best of our knowledge, this is the first time that a comparison of the clinical manifestation between the coinfection patients and the patients infected with COVID‐19 only. It is noted that additional cases and more clinical information will enable making more comprehensive and solid conclusions.
On the other hand, the report reminded us that it is critical to pay attention to the potential coinfection with other respiratory viruses for the COVID‐19 infection patients, which effectively helps us to prevent the aggravation of disease progression and even death for patients. We cannot ignore COVID‐19 infection patients might combine with other respiratory viruses, not just the influenza virus, to make sure that we could provide the best and the most comprehensive treatment to the patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
This study is supported by the National Natural Science Foundation of China (No. 81572422, 81700515).
Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92:1549–1555. 10.1002/jmv.25781
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China [published online ahead of print February 28, 2020]. N Engl J Med. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases [published online ahead of print February 26 2020]. Radiology. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology. 2020;295:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riou J, Althaus CL. Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4):2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caini S, Kroneman M, Wiegers T, El Guerche‐Seblain C, Paget J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza Other Respir Viruses. 2018;12(6):780‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schoen K, Horvat N, Guerreiro NFC, de Castro I, de Giassi KS. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19(1):964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keske S, Gumus T, Koymen T, Sandikci S, Tabak L, Ergonul O. Human metapneumovirus infection: diagnostic impact of radiologic imaging. J Med Virol. 2019;91(6):958‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.General Office of National Health Committee. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019‐nCoV) infected pneumonia (fourth trial version to sixth trial version). http://www.nhc.gov.cn/yzygj/pqt/new_list.shtml
- 14. Zhang H, Kang Z, Gong H. The digestive system is a potential route of 2019‐nCov infection: a bioinformatics analysis based on single‐cell transcriptomes [published online ahead of print January 31, 2020]. bioRxiv. 10.1101/2020.01.30.927806 [DOI] [Google Scholar]
- 15. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]


