Abstract
The COVID-19 pandemic has rapidly evolved and changed our way of life in an unprecedented manner. The emergence of COVID-19 has impacted transplantation worldwide. The impact has not been just restricted to issues pertaining to donors or recipients, but also health-care resource utilization as the intensity of cases in certain jurisdictions exceeds available capacity. Here we provide a personal viewpoint representing different jurisdictions from around the world in order to outline the impact of the current COVID-19 pandemic on organ transplantation. Based on our collective experience, we discuss mitigation strategies such as donor screening, resource planning, and a staged approach to transplant volume considerations as local resource issues demand. We also discuss issues related to transplant-related research during the pandemic, the role of transplant infectious diseases, and the influence of transplant societies for education and disseminating current information.
KEYWORDS: clinical decision-making, clinical research/practice, donors and donation: donor-derived infections, infection and infectious agents – viral, infectious disease, organ transplantation in general
Abbreviations: ACE, angiotensin converting enzyme; BAL, bronchoalveolar lavage; COVID-19, Coronavirus disease 2019; HIV, human immunodeficiency virus; ICU, intensive care unit; MELD, model for end-stage liver disease; MERS, middle east respiratory syndrome; NAT, nucleic acid testing; NP, nasopharyngeal; OPO, organ procurement organization; SARS-CoV, severe acute respiratory syndrome – coronavirus; TID, transplant infectious disease
1. INTRODUCTION
Transplantation has become an established treatment for end-stage organ diseases and is a highly regulated field. There are several threats to transplantation but one particularly important threat is that of an emerging infectious disease. Since the 1980s, there have been several emerging viral diseases including HIV in the late 1980s/early 1990s, SARS-CoV, West Nile Virus, pandemic influenza A/H1N1, Zika, Ebola, and now pandemic COVID-19 caused by SARS-CoV-2. For each of these threats, transplant programs have responded in a coordinated fashion by assessing the risk of donor transmission, assessing the severity of disease in the recipient, and recognizing the potential for transmission to health-care workers.1, 2, 3, 4, 5 This knowledge has then been used to generate algorithms for donor screening, not using organs from potentially infected donors, and recipient management. Many of these emerging viruses have been manageable, sometimes only limited to certain geographic areas, and transplantation/donation has been able to adapt and continue to provide this life-saving therapy in a safe and effective manner. The current COVID-19 pandemic is unique and unprecedented in modern times. It has crossed borders and infected >180 000 persons worldwide that we know of, with likely many more undiagnosed cases. It has been difficult to contain partly due to the contagious nature of the virus and mild illness in a majority of individuals. Nevertheless, the emergence of COVID-19 has impacted transplantation worldwide. The impact has not been just restricted to issues around donors or recipients, but also health-care resource utilization as the intensity of cases in certain jurisdictions exceeds available capacity. Based on our collective experience, we suggest mitigation strategies such as donor screening approaches, resource planning, and a staged approach to transplant volume considerations as local resource issues demand. We also discuss issues related to the management of immunosuppression trials during the pandemic, and the role of transplant infectious diseases and transplant societies for education and disseminating current information. We believe our collective experience will be valuable to the transplant community in the absence of hard published research findings this early in the pandemic.
2. APPROACH TO DONATION
There is a potential for COVID-19 to be transmitted by organ donation although the risk of this is unclear and we are not aware of any reports of transmission. The virus is primarily isolated from the respiratory tract suggesting the lung is a very high-risk for transmission when used from an infected donor. However, virus is also been reported to be isolated from the blood in up to 15% of cases and therefore, all organs may be at risk of acquisition.6 With the SARS epidemic of 2003, autopsy data demonstrated virus in almost all organs including the liver, kidney, and intestines.7 Donor screening from both a clinical and laboratory perspective is therefore an important consideration and has been the subject of much discussion.8 In areas with significant community transmission, if organ donation is to proceed in a safe manner, the authors recommend that both clinical and rapid laboratory screening is required. This approach to donation may differ in countries depending on the degree of community-transmission of COVID-19. However, many areas have noted that due to limitations in test availability the true rate of community penetration may be unknown. During the SARS-CoV outbreak of 2003 in Toronto, a clinical donor screening tool was instituted, incorporating epidemiological and clinical features of the donor, which then allowed deceased-donor transplantation to continue.2 However, unlike in 2003, there has been rapid development of nucleic acid testing (NAT) for SARS-CoV-2 and therefore, testing of nasopharyngeal specimens has been incorporated and is the cornerstone of donor screening algorithms in several jurisdictions. Real-time NP swab donor screening has been successfully deployed in organ procurement organizations (OPOs) within Canada, Italy, Spain, and South Korea. However, many questions remain, including the false negative rates of testing which can be due to inappropriate collection or a patient early in the incubation period. Since SARS-CoV-2 is known to use the ACE2 receptor for viral entry, a bronchoalveolar lavage (BAL) specimen may be more appropriate than naso/oropharyngeal swab. However, bronchoscopy would have the potential risk of aerosolization and may not be logistically feasible. For this approach to be successful, the test result must also be rapidly available. Laboratory-developed or commercial NAT testing needs to be made available to OPOs with results in hours. Ruling out COVID-19 in a donor is also essential for the safety of organ procurement teams. Shortages of testing kits, reagents, and laboratory resources to carry out donor screening in the midst of a pandemic are also a major consideration.
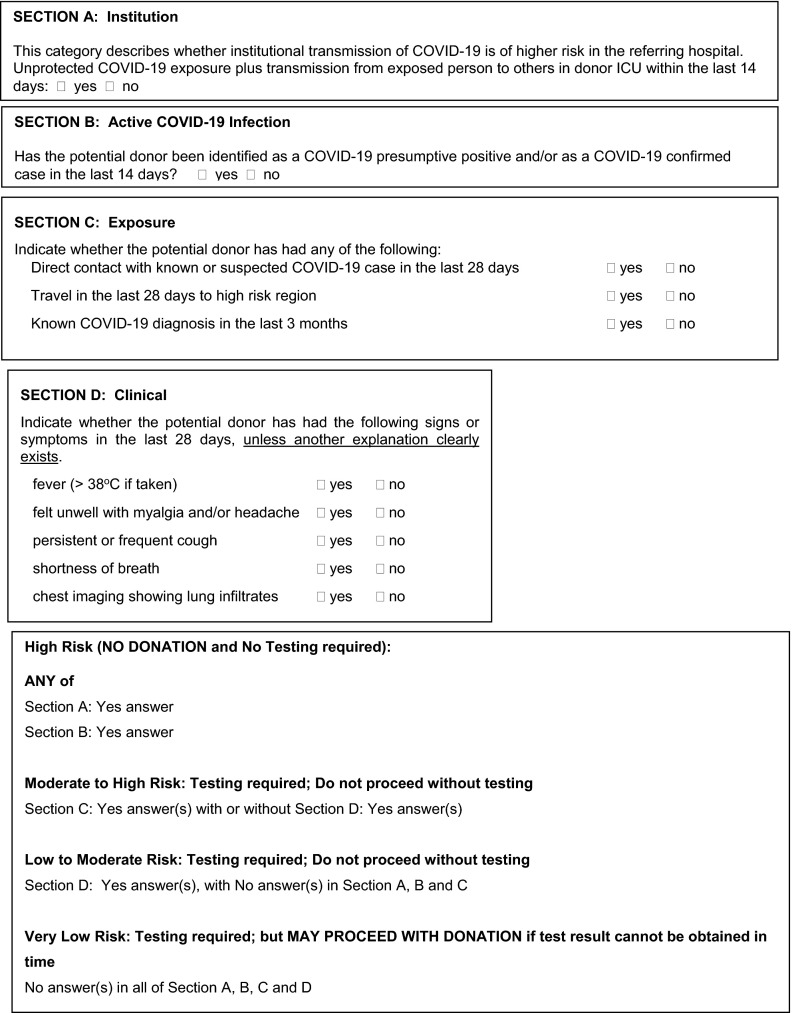
In Canada, we developed a COVID-19 donor clinical screening tool and also started NP swab NAT screening for COVID-19 ( Figure 1). For areas with significant community transmission, the tool could be modified to reduce the importance of travel history. In the latter case, NAT testing would play a much larger role but the tool allows a second layer of redundancy in the screening process. Collectively the authors have had experience with asymptomatic NAT positive donors.
FIGURE 1.
COVID-19 donor screening tool. Donor Screening Tool adapted from Trillium Gift of Life Network Organ Donation Organization, Ontario, Canada
In Switzerland, where virus is currently widely circulating in the community, we established universal screening for all deceased donors by NAT in NP swab or BAL on March 5, 2020 ( Table 1). Given the potential increase in risk for health personnel and current limited resources in the ICU for performing bronchoscopy, we favor NP swab over BAL for screening of SARS-CoV-2. Of note, given the wide clinical presentation of COVID-19, potential donors can be asymptomatic or mildly symptomatic at the time of donation, thus highlighting the need for universal screening.
TABLE 1.
Summary of donor and recipient screening practices
| Countrya | Deceased donor screening | Living donor screening | Pretransplant recipient screening | Specimen type |
|---|---|---|---|---|
| Canadab | Universal NAT | Universal NAT | Clinical | NP or BAL |
| Switzerland | Universal NAT | Universal NAT | Clinical | NP or BAL |
| Italy | Universal NAT | Universal NAT | Clinical | BAL in deceased donor NP in living donor |
| Spain | Universal NAT | Universal NAT; donation postponed 21 d if known exposure | Clinical | NP ± BAL |
| Koreab | Universal NAT | Universal NAT | Universal NAT | NP |
| Japanb | Risk-based NAT due to limited testing capacity | Self-isolation or hospital admission 14 d prior to surgery | Clinical; NAT where testing available | NP (and BAL for intubated patients) |
Abbreviations: BAL, bronchoalveolar lavage; NAT, nucleic acid testing; NP, nasopharyngeal.
May represent the author centers—not necessarily country wide; assumes transplant activity is continuing.
Recommendations of country-specific transplant societies.
In Italy, where there is significant community transmission, deceased donor screening with NAT for SARS-CoV-2 on BAL has become mandatory starting from February 23, 2020. At the time of this manuscript none of the screened donors has been found positive. The main problem in Italy is the huge number of patients requiring mechanical ventilation and that many ICUs that have been transformed to COVID-19 ICUs. For this reason, the number of potential donors is expected to significantly decrease.
In Spain, with high community transmission, universal screening (through at least one NP specimen and, if feasible, one lower respiratory tract sample) is now mandatory for all lung and small bowel donors across the country. In addition, NAT screening is also required for any deceased donor with recent travel to or stay in selected high-risk Spanish regions, contact with a confirmed COVID-19 case, with a positive symptom screen. No donor-derived transmission has been reported to date.
The Japan Society for Transplantation (JST) published their formal statement on March 6, 2020 (http://www.asas.or.jp/jst/pdf/info_20200306.pdf) and recommended to clinically screen donors for significant exposure to COVID-19, travel history to high-risk countries, and symptoms including fever and respiratory symptoms. However, due to limited testing capacity, universal screening has not been adopted. Uniquely, JST recommended for both lung and liver living donors to stay at home or in hospital 14 days prior to avoid COVID-19 exposure, in cases where transplantation can be postponed for 14 days.
The Korean Society for Transplantation (KST) released their recommendation on March 13, 2020. The KST recommended that both living and deceased donors should be tested for SARS-CoV-2 NAT from NP swab prior to procurement. If the living donor and/or recipient visited the highly epidemic domestic regions (Daegu city or Gyeongsangbuk-do Province), or had any exposure, the transplant operation should be postponed for 14 days with close clinical monitoring.
3. APPROACH TO TRANSPLANTATION
In the face of a pandemic that may impact transplant recipients adversely, decisions to continue or cease transplantation need to be made by programs. Although donor transmission is an ominous possibility, many of these decisions are actually independent of donor transmission and have to do with the following considerations: (1) introducing immunosuppression into patients in the midst of a pandemic, (2) the risk vs benefit ratio of postponing transplant vs proceeding, and (3) rationing of healthcare resources including both inpatient and outpatient resources. Lack of ventilator capacity is also an extremely important consideration once widespread activity is present. For example, kidney transplantation reduces the morbidity of dialysis and is cost-saving but is not immediately life saving. The majority of cases of living donor kidney transplantation can be postponed without significant impact on the recipient. Although the clinical outcomes of transplant recipients with COVID-19 are not known, based on previous SARS and MERS publications, the mortality could be high and nasopharyngeal and tissue viral loads may be greater than in immune competence. Therefore, a decision must be made by individual programs whether to newly immunosuppress patients and send them out into the community during the COVID-19 pandemic. The other part of the decision is how much health-care resource is utilized by newly transplanted patients with regard to readmission rates and whether during a period of strained hospital resources, it would be appropriate to perform kidney transplantation. It has been suggested, that the risk of infection in the community for a newly transplanted recipient may be mitigated by either not using induction therapy or using an IL2 receptor antagonist for induction rather than polyclonal globulin induction. This is unknown but is a logical extension of data from other viral infections. Another possibility is to temporarily pause kidney transplantation but continue to transplant highly sensitized patients. This has been done in some jurisdictions.
The decision to pause life-saving transplants such as the liver, heart, and lung is more difficult as waitlist mortality is greater. However, for liver transplantation patients can be stratified based on MELD score and the decision made to only transplant high MELD patients. Similarly, transplanting high status patients only may be a trade-off to completely stopping deceased donor transplantation.
We suggest a phased approach to decreasing transplant activity ( Table 2). Such decisions need to be made collectively and depend on risk tolerance, hospital capacity and degree of virus activity in the jurisdiction. A phased approach can include a 25%, 50%, and 75% trigger for activity reduction. Obviously in a situation where healthcare system is completely overwhelmed a 100% reduction may be unavoidable.
TABLE 2.
Phased approach to new transplant activity during the COVID-19 pandemic
| Transplant activity level | Priority level description | Examples (may include but not limited to) |
|---|---|---|
| 25% reduction in transplant activity | Elective cases. Patients whose conditions is deemed nonlife threatening or can be managed with medication and for whom services can be deferred until the end of a pandemic wave (ie, 6-8 wk) |
|
| 50% reduction in transplant activity | Urgent cases. Patients who are deemed urgent and who need service within 14 d. It may be possible to defer these services for a few days, but not for the length of a pandemic wave. Physicians will determine that these patients are not put at undue risk. If their situation changes they will be changed to emergent |
|
| 75% reduction in transplant activity | Emergent cases: Patients who are deemed critical, whose condition is immediately life threatening. Their immediate need is greatest |
|
| 100% reduction in transplant activity | Health system is overwhelmed with COVID-19; no ICU or other capacity available; severe shortages of health personnel |
|
Abbreviations: ICU intensive care unit; LAS, lung allocation score; MELD, model for end-stage liver disease; PAK, pancreas after kidney; PRA, panel reactive antibody; PTA, pancreas transplant alone.
Another issue to consider is COVID-19 screening of recipients (clinical and/or laboratory) being admitted for transplantation. A standard screening form could be used. Whether asymptomatic patients should have a NP swab for COVID-19 NAT is debatable as this may be negative during the incubation period and may place unnecessary burden on resources. Currently most of the authors’ centers (with the exception of South Korea) are not screening asymptomatic recipients being admitted for transplantation with NAT.
Outpatient transplant clinics also need to be modified as hospitals prepare for increasing numbers of COVID-infected patients ( Table 3). Elective transplant well-visits can be postponed and clinics can be scaled back such that only urgent visits need to be seen. If resources are available, clinic staff may choose to screen all patients by telephone prior to the clinic visit for symptoms compatible with COVID-19. Telehealth/telephone calls can be substituted for such clinic visits. Transplant programs can direct patients to public health websites or transplant-specific websites for information.
TABLE 3.
Ambulatory transplant clinic service reduction during COVID-19 pandemic
| Transplant activity level | Transplant center priority | Description (may include but not limited to) |
|---|---|---|
| 25% reduction in transplant activity | Should be deferred |
|
| 50% reduction in transplant activity | Should be seen |
|
| 75% reduction in transplant activity | Need to be seen |
|
| Near 100% reduction in ambulatory activity | No capacity due to health-care system overwhelmed with COVID; lack of personnel |
|
Manpower issues are equally important. Members of the transplant team should not come to work if experiencing any symptoms compatible with COVID-19 and self-isolate if exposure has occurred. The authors note that rapid testing of transplant team members with even mild symptoms is very important. Transplant teams should have clear back-up plans should a team member become ill or quarantined. The authors centers have either disallowed vacation or allowed non-travel vacations with the understanding that they may be called in to help out—an “all hands on deck” approach.
Although it is difficult to determine the course of the pandemic, the majority of hypotheses are based on peak of disease lasting several months followed by a stable level with either year-round or seasonal circulation of the virus. At some point transplant programs that have decreased activity will need to ramp up. We suggest this could be done in a phased approach where more urgent transplants could proceed first, with more “elective” cases phased in later. This would need to be coupled with safe donation and transplant practices to prevent and treat COVID-19, as well as an understanding of local health resource limitations.
4. ROLE OF TRANSPLANT INFECTIOUS DISEASES
An increasing number of programs worldwide have transplant infectious disease (TID) specialists embedded in programs. During the COVID-19 pandemic, the TID physician can serve as a critical resource in an evolving environment. Although there are no publications on transplant recipients currently, transplant recipients have acquired COVID-19 and anecdotal information suggests that there is a spectrum of illness ranging from mild to severe disease. It is likely that these patients may also have higher viral loads with prolonged shedding. This may require longer quarantine periods until patients can be confirmed swab negative (eg, we use 2 consecutive negative swabs to terminate quarantine). With regard to treatment of an infected transplant recipient, the ID physician can be instrumental in obtaining and suggesting experimental therapies. Currently, there are many clinical trials and experimental therapies for COVID-19 including remdesivir, lopinavir/ritonavir, darunavir-cobicistat, interferon beta and (hydroxy)chloroquine, as well as combinations of these therapies. Passive high-titer immunoglobulin from recovered COVID patients has been used. Blunting the inflammatory response with a trial of corticosteroids is controversial.9 , 10 However, reduction of IL-6 in critically ill patients with tocilizumab has also been attempted. Detailed treatment algorithms are beyond the scope of this viewpoint, and are continually evolving as new data emerge. Numerous centers and groups have developed treatment guidelines for transplant and nontransplant patients. Antimicrobial management of the critically ill transplant patient (including potential drug interaction between antivirals and immunosuppressive drugs), an understanding of experimental therapies and clinical trials, as well as working with the transplant physician in reducing immunosuppression are all roles of a transplant infectious disease physician. The TID physician is also important at a programmatic level. Since surges in hospital capacity are predicted to occur with COVID-19, the TID physician can also determine whether non-COVID-19 patients with infections can be safely discharged home on oral or home IV therapies. Transplant ID physicians can also serve as an educational resource for other transplant physicians and coordinators, assist in answering patient queries regarding COVID-19. They can also be instrumental in building screening strategies for transplant patients, that is decide who can be safely managed as outpatient and who needs hospital admission. Overall, TID physicians can act as a linchpin of the transplant program during the COVID-19 pandemic by establishing safety procedures of donor and recipients, providing up-to-date educational tools, and prioritizing transplant activities according to evolution of the epidemiology of the pandemic.
5. APPROACH TO TRANSPLANT RESEARCH ACTIVITIES
An approach needs to be developed for centers that have ongoing clinical trials, especially those of novel immunosuppressives, in the face of a pandemic. To completely discontinue a trial and withdraw patients would generally not be required. However, modifications could be made such as halting enrollment of new patients. Patients already enrolled in a clinical trial should be advised to practice social distancing and frequent handwashing. Patients may not wish to come to hospital for study visits and this needs to be respected. Research teams should liaise with the sponsor for virtual visits. The hospital can consider a separate area for study blood work and administration or pickup of study drug where study outpatients are separated from patients that may be unwell.
In contrast, research for COVID in transplantation is necessary and needs to continue. Some areas for research include outcomes in transplant patients, predictive diagnostics, and management strategies including optimal approach to immunosuppression adjustment. Vaccines are under development and will likely be in clinical trials. When this occurs a trial in transplant candidates and recipients is also warranted.
6. SAFETY OF THE TRANSPLANT TEAM
We must think about steps to take care of ourselves and ensure we remain healthy so that we can look after our patients. Risks to think about include: (1) teams traveling for donor procurement to areas of high risk, (2) performing high-risk procedures such a bronchoscopies on deceased donors, and (3) team exposures to transplant recipients who may be shedding greater quantities of virus (so-called super-shedders or super-spreaders). Many of these risks can be mitigated by careful use of personal protection measures, and if needed avoidance of high-risk situations and following hospital directives regarding in-person meetings. Any transplant staff who is ill must immediately excuse themselves from work, and have appropriate testing as needed.
Organization of transplant programs with parallel teams (without contact among them) may be appropriate to continue with transplant activity even in case of transmission of cases within health-care workers. A very important but often neglected issue is the mental health of the transplant team. Anxiety and distress related to COVID-19 exposure concerns, transplant program closures, and adverse outcomes in recipients all may contribute. Closely working with the transplant psychiatry team is a proposed strategy for addressing this.
7. ROLE OF TRANSPLANT SOCIETIES
Transplant societies such as AST, ASTS, TTS, ISHLT, and country-specific organizations have an important role as an educational resource in a rapidly evolving pandemic. Experts within societies should disseminate donor screening algorithms, share advice for transplant programs, and management of transplant recipients. The American Society of Transplantation has rapidly created an information sheet for transplant professionals including suggestions for donor screening. In addition, information specific for transplant recipients has also been created. Similarly, The Transplantation Society has also provided information on COVID-19 for transplant professionals. Platforms such as webinars, virtual town halls, chat groups can be used to quickly exchange information. Transplant societies can also act as a conduit for research collaborations on COVID-19. For instance, the Spanish Group for the Study of Infection in Transplantation and the Immunocompromised Host (GESITRA-IC/SEIMC) has drafted a consensus guideline for the therapeutic management of transplant recipients with COVID-19 and has established a prospective multicenter registry to obtain real-time data from clinical experience.
8. SUMMARY
This is an exceptional time for the world full of uncertainty and anxiety. For those of us working in transplantation, it is especially worrisome given the highly vulnerable group of patients we serve. In this context it is of the utmost importance that we come together as a team, to share knowledge and experience that will benefit all of our program and most importantly our patients.
ACKNOWLEDGMENTS
We acknowledge members of the Korean Society for Transplantation as follows: Dr Jongwon Ha, Dr Kyong Ran Peck, Dr Sang Il Kim, Dr Sang-Oh Lee, Dr Won Hyun Cho. The authors also acknowledge Ms Sonika Humar for expert editing of the manuscript.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. DK has received speaker honoraria from AbbVie. The remaining authors have no conflicts of interest as defined by the American Journal of Transplantation.
REFERENCES
- 1.Kumar D, Humar A. Pandemic influenza and its implications for transplantation. Am J Transplant. 2006;6:1512–1517. doi: 10.1111/j.1600-6143.2006.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3:977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi ME, Kumar D, Green M, et al. Considerations for screening live kidney donors for endemic infections: a viewpoint on the UNOS policy. Am J Transplant. 2014;14:1003–1011. doi: 10.1111/ajt.12666. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg EA, Fishman JA. Zika Virus in transplantation: emerging infection and opportunities. Am J Transplant. 2017;17:599–600. doi: 10.1111/ajt.14083. [DOI] [PubMed] [Google Scholar]
- 5.Kaul DR, Mehta AK, Wolfe CR, Blumberg E, Green M. Ebola virus disease: implications for solid organ transplantation. Am J Transplant. 2015;15:5–6. doi: 10.1111/ajt.13093. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar D, Humar A. Emerging viral infections in transplant recipients. Curr Opin Infect Dis. 2005;18:337–341. doi: 10.1097/01.qco.0000172697.44784.ff. [DOI] [PubMed] [Google Scholar]
- 8.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation [published online ahead of print 2020]. Am J Transplant. [DOI] [PMC free article] [PubMed]
- 9.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print 2020]. JAMA Intern Med. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 10.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]