Abstract
An outbreak of coronavirus disease 2019 (COVID‐19) occurred in Wuhan and it has rapidly spread to almost all parts of the world. For coronaviruses, RNA‐dependent RNA polymerase (RdRp) is an important polymerase that catalyzes the replication of RNA from RNA template and is an attractive therapeutic target. In this study, we screened these chemical structures from traditional Chinese medicinal compounds proven to show antiviral activity in severe acute respiratory syndrome coronavirus (SARS‐CoV) and the similar chemical structures through a molecular docking study to target RdRp of SARS‐CoV‐2, SARS‐CoV, and Middle East respiratory syndrome coronavirus (MERS‐CoV). We found that theaflavin has a lower idock score in the catalytic pocket of RdRp in SARS‐CoV‐2 (−9.11 kcal/mol), SARS‐CoV (−8.03 kcal/mol), and MERS‐CoV (−8.26 kcal/mol) from idock. To confirm the result, we discovered that theaflavin has lower binding energy of −8.8 kcal/mol when it docks in the catalytic pocket of SARS‐CoV‐2 RdRp by using the Blind Docking server. Regarding contact modes, hydrophobic interactions contribute significantly in binding and additional hydrogen bonds were found between theaflavin and RdRp. Moreover, one π‐cation interaction was formed between theaflavin and Arg553 from the Blind Docking server. Our results suggest that theaflavin could be a potential SARS‐CoV‐2 RdRp inhibitor for further study.
Keywords: RNA‐dependent RNA polymerase, SARS‐CoV‐2, theaflavin, traditional Chinese medicinal compounds
Highlights
Theaflavin has a lower idock score in the catalytic pocket of RdRp in SARS‐CoV‐2, SARS‐CoV and MERS‐CoV from idock.
Theaflavin has a lowest binding energy when it docks in the catalytic pocket of SARS‐CoV‐2 RdRp.
Theaflavin could be potential SARS‐CoV‐2 RdRp inhibitor.
1. INTRODUCTION
An unprecedented outbreak of coronavirus disease 2019 (COVID‐19) occurred in Wuhan, Hubei Province, China, in December 2019. It has since spread rapidly to almost all parts of China and many other countries. As at 3 March 2019, the incidence of COVID‐19 continues to rise. 1
For coronaviruses, RNA‐dependent RNA polymerase (RdRp) is an important enzyme that catalyzes the replication of RNA from RNA templates. Compared the sequence of RdRp in severe acute respiratory syndrome coronavirus (SARS‐CoV), SARS‐CoV‐2 and Middle East respiratory syndrome coronavirus (MERS‐CoV), SARS‐CoV and SARS‐CoV‐2 have remarkably similar sequences, and encode structurally similar structures of RdRp (Figure 1A,B). 2 Moreover, RdRp of the three viruses has a huge and deep groove as an active site for the polymerization of RNA and the variations of residues in SARS‐CoV‐2 and SARS‐CoV are distal to the active site 2 (Figure 1B). Remdesivir (GS‐5734) is a 1′‐cyano‐substituted adenosine nucleotide analog inhibitor of RdRp and shows broad‐spectrum antiviral activity against several RNA viruses, including Ebola virus, SARS‐CoV, and MERS‐CoV. 3 , 4 , 5 More importantly, one report indicated that remdesivir improved the critical condition of one COVID‐19 patient. 6 Therefore, RdRp could be an attractive therapeutic target for SARS‐CoV‐2.
Figure 1.
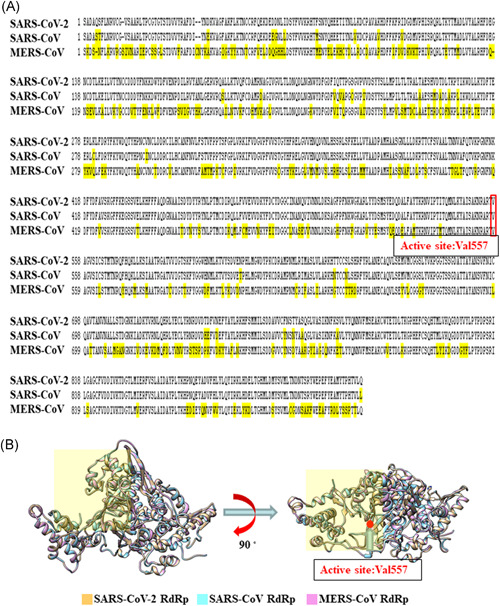
(A) Sequence alignment for the amino acids of RdRp between SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV. (B) A modeled structure of SARS‐CoV‐2 RdRp, SARS‐CoV RdRp, and MERS‐CoV RdRp and Grid box size for binding site using MODELLER. The active site (Val557) and grid box size (light yellow) were showed for binding site. MERS, Middle East respiratory syndrome; RdRp, RNA‐dependent RNA polymerase; SARS‐CoV, severe acute respiratory syndrome coronavirus
In China, many traditional Chinese medicinal compounds have been used to treat SARS‐CoV and SARS‐CoV‐2. 3 However, the mechanisms of activity and the efficiency of these compounds remain unclear. Therefore, we screened the chemical structures of clinically used traditional Chinese medicinal compounds with proven antiviral activity against SARS‐CoV and their similar chemical structures, using a molecular docking method to target RdRp of SARS‐CoV‐2.
2. MATERIALS AND METHODS
2.1. Structure preparation
The three‐dimensional (3D) structure of RdRp of SARS‐CoV‐2 (NCBI Reference Sequence: YP_009725307.1), SARS‐CoV (NCBI Reference Sequence: NP_828869.1), and MERS‐CoV (NCBI Reference Sequence: YP_009047223.1) were generated based on homologous modeling using Modeller 7 incorporated within the UCSF Chimera 8 and SWISS‐MODEL. 9
2.2. Compound dataset collection
Eighty‐three chemical structures from traditional Chinese medicinal compounds and their similar structures were retrieved from ZINC15 database.
2.3. Molecular docking and virtual screening
We used two molecular docking methods for analysis. First, molecular docking and virtual screening was performed using idock download from Github (https://github.com/HongjianLi/idock) in a local Linux machine. Second, the Blind Docking server was used for in silico prediction of the lowest free binding energy. Calculations were carried out with “Achilles” Blind Docking Server, available at: http://bio‐hpc.eu/software/blind‐docking‐server/. The grid box encompassed by the active site (Val557) and surrounding amino acids around the catalytic pocket of SARS‐CoV‐2 RdRp was using for ligand docking and virtual screening (Figure 1B). For each structure, nine docking poses were generated and the scores for the best docking poses of each structure were used for ranking.
3. RESULTS
Screening of these chemical structures revealed that theaflavin (ZINC3978446, Figure 2A) has a lower idock score in the catalytic pocket of RdRp in SARS‐CoV‐2 (−9.11 kcal/mol), SARS‐CoV (−8.03 kcal/mol), and MERS‐CoV (−8.26 kcal/mol) (Figure 2B). The contact modes between theaflavin and RdRp of these viruses with the lowest idocking scores are illustrated in Figure 2C‐E, respectively. Regarding contact modes by idock, hydrophobic interactions contribute significantly in binding and additional hydrogen bonds were found between theaflavin and Asp452, Arg553 and Arg624 of SARS‐CoV‐2 RdRp (Figure 2C), and between theaflavin with Thr440, Ser566, Ala569, and Asp644 of SARS‐CoV RdRp (Figure 2D), and between theaflavin with Arg294, Thr292, Gln291, Leu427, Asn 390, Leu427, and Asp728 of MERS‐CoV RdRp (Figure 2E).
Figure 2.
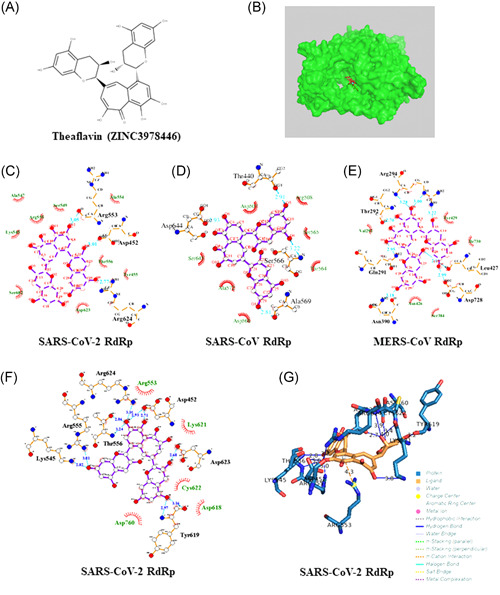
(A) The structure of theaflavin (ZINC3978446). (B) Red and green molecules, respectively, represent crystallographic and predicted pose for theaflavin. (C‐E) The contact model between theaflavin and SARS‐CoV‐2 RdRp (C), SARS‐CoV RdRp (D), and MERS‐CoV RdRp (E) are shown in two‐dimensional (2D) interaction diagram by idock. (F) The contact model between theaflavin and SARS‐CoV‐2 RdRp is shown in the 2D interaction diagram by the Blind Dock server. Their relative distances between amino acid residues and theaflavin are analyzed and illustrated by LigPlot+. Carbon, oxygen, nitrogen, and fluoride molecules are marked as white, red, blue, and green circles, respectively. Covalent bonds in theaflavin and amino acid residues of RdRp are labeled in purple and orange solid lines, respectively. The light blue dot lines label the distance (in Å) of hydrogen bonds formed between the functional moieties of theaflavin and amino acid residues. Hydrophobic interactions between theaflavin and RdRp are depicted by the name of involving amino acid residues, which are labeled with dark green with dark red eyelashes pointing to the involved functional moiety of theaflavin. (G) The hydrogen bonds and π‐cation interaction established by theaflavin with the closest residues are showed through Protein‐Ligand Interaction Profiler (PLIP). MERS, Middle East respiratory syndrome; RdRp, RNA‐dependent RNA polymerase; SARS‐CoV, severe acute respiratory syndrome coronavirus
Because theaflavin has the lowest idock score in the catalytic pocket of SARS‐CoV‐2 RdRp (−9.11 kcal/mol), we used the Blind Docking server to confirm the result. We found that theaflavin has lower binding energy of −8.8 kcal/mol when it docks in the catalytic pocket of SARS‐CoV‐2 RdRp. The 2D and 3D contact modes between RdRp and theaflavin with the lowest binding energy are illustrated in Figure 2F,G. Regarding the contact modes by the Blind Docking server, hydrophobic interactions contribute significantly for binding. We observed additional hydrogen bonds and π‐cation interaction between theaflavin and SARS‐CoV‐2 RdRp (Figure 2F,G). The contact mode showed that hydrogen bonds were formed between theaflavin and Asp452, Lys545, Arg555, Thr556, Tyr619, Lys621, Cys622, Asp623, Arg624, and Asp760 of SARS‐CoV‐2 RdRp, near the active site of RdRp. In addition, one π‐cation interaction was formed between theaflavin and Arg553 (Figure 2F,G).
4. DISCUSSION
Many herbals and compounds used in traditional Chinese medicine have been screened as anti‐SARS‐CoV therapy. 10 , 11 , 12 , 13 , 14 Theaflavin, a polyphenolic compound in black tea, is thought to be responsible for the medicinal value of black tea. Theaflavin and theaflavin gallate derivatives have shown broad‐spectrum antiviral activity against several viruses, including influenza A and B viruses and hepatitis C virus. 15 , 16
Furthermore, it has been shown that extracts from Pu'er tea and black tea, and theaflavin‐3,3′‐digallate and 3‐isotheaflavin‐3‐gallate, in the theaflavins family, have potent inhibitory activity against SARS, by inhibiting SARS‐CoV 3CLpro activity. 17 In this study, we found that theaflavin was able to dock in the catalytic pocket near the active site of RdRp in SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV. Furthermore, theaflavin has the lowest idock score and lower binding energy in the catalytic pocket of SARS‐CoV‐2 RdRp in the two different molecular docking methods. We also found that theaflavin formed additional hydrogen bonds and π‐cation interaction with the catalytic pocket of SARS‐CoV‐2 RdRp. This may explain why theaflavin had lower idock score than the other chemical structures that we screened. It is possible that theaflavin could inhibit RdRp activity through blocking the active site in the groove.
5. CONCLUSION
Our results suggest that theaflavin could be used as a lead compound for developing a SARS‐CoV‐2 inhibitor that targets RdRp. However, the exact in vivo effect is still unclear, and further research is needed to confirm the mechanism whereby theaflavin target SARS‐CoV‐2.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
JL performed the experiments. CYW conceived the idea and designed experiments and wrote the manuscript. YHY, LHS, YCC, HTL, YSL, and JL analyzed the data. YLC revised the English writing of the manuscript. All the authors reviewed and approved the final version.
ACKNOWLEDGMENTS
This study was supported by the Grant CMRPG6H0162 from Chang Gung Memorial Hospital, and MOST 108‐2320‐B‐182‐021 from the Ministry of Science and Technology to Dr CYW. The authors would like to thank the Health Information and Epidemiology Laboratory at the Chiayi Chang Gung Memorial Hospital for the comments and assistance in data analysis.
Lung J, Lin Y‐S, Yang Y‐H, et al. The potential chemical structure of anti‐SARS‐CoV‐2 RNA‐dependent RNA polymerase. J Med Virol. 2020;92:693–697. 10.1002/jmv.25761
DATA AVAILABILITY STATEMENT
All data and materials are contained and described within the manuscript.
REFERENCES
- 1. Dey SK, Rahman MM, Siddiqi UR, Howlader A. Analyzing the epidemiological outbreak of COVID‐19: a visual exploratory data analysis (EDA) approach. J Med Virol. 2020. 10.1002/jmv.25743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu W, Morse JS, Lalonde T, Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. ChemBioChem. 2020;21:730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020. 10.1002/jmv.25707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA‐dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020:pii: jbc.AC120.013056. 10.1074/jbc.AC120.013056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tchesnokov EP, Feng JY, Porter DP, Gotte M. Mechanism of inhibition of Ebola virus RNA‐dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):E326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol Biol. 2008;426:145‐159. [DOI] [PubMed] [Google Scholar]
- 8. Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605‐1612. [DOI] [PubMed] [Google Scholar]
- 9. Waterhouse A, Bertoni M, Bienert S, et al. SWISS‐MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296‐W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang MM, Liu XM, He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World J Gastroenterol. 2004;10(23):3500‐3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet. 2003;361(9374):2045‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryu YB, Jeong HJ, Kim JH, et al. Biflavonoids from Torreya nucifera displaying SARS‐CoV 3CL(pro) inhibition. Bioorg Med Chem. 2010;18(22):7940‐7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JY, Kim JH, Kim YM, et al. Tanshinones as selective and slow‐binding inhibitors for SARS‐CoV cysteine proteases. Bioorg Med Chem. 2012;20(19):5928‐5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi L, Li Z, Yuan K, et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78(20):11334‐11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang ZF, Bai LP, Huang W, et al. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure‐activity relationship analysis. Fitoterapia. 2014;93:47‐53. [DOI] [PubMed] [Google Scholar]
- 16. Chowdhury P, Sahuc ME, Rouillé Y, et al. Theaflavins, polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture. PLoS One. 2018;13(11):e0198226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen CN, Lin CPC, Huang KK, et al. Inhibition of SARS‐CoV 3C‐like protease activity by theaflavin‐3,3′‐digallate (TF3). Evid Based Complement Alternat Med. 2005;2(2):209‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are contained and described within the manuscript.


