Abstract
An outbreak of severe acute respiratory syndrome novel coronavirus (SARS‐CoV‐2) epidemic spreads rapidly worldwide. SARS‐CoV‐2 infection caused mildly to seriously and fatally respiratory, enteric, cardiovascular, and neurological diseases. In this study, we detected and analyzed the main laboratory indicators related to heart injury, creatine kinase isoenzyme‐MB (CK‐MB), myohemoglobin (MYO), cardiac troponin I (ultra‐TnI), and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), in 273 patients with COVID‐19 and investigated the correlation between heart injury and severity of the disease. It was found that higher concentration in venous blood of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP were associated with the severity and case fatality rate of COVID‐19. Careful monitoring of the myocardiac enzyme profiles is of great importance in reducing the complications and mortality in patients with COVID‐19.
Keywords: COVID‐19, disease severity, heart injury, laboratory indicators
Highlights
The blood tests of patients on admission showed some patients had higher levels of CK‐MB, MYO, ultra‐TnI and NT‐proBNP.
Increased concentration in venous blood of MYO, ultra‐TnI and NT‐proBNP were associated with the severity of COVID‐19.
All four parameters were significantly higher in the death than in the alive group.
1. INTRODUCTION
Since December 2019, an outbreak of pneumonia caused by a severe acute respiratory syndrome novel coronavirus (SARS‐CoV‐2) has raised intense attention in Wuhan, Hubei province, China. 1 , 2 , 3 This disease, named COVID‐19 by World Health Organization (WHO), spreads rapidly around the country and worldwide.
Coronaviruses can infect a variety of livestock, poultry, and humans, in which they can cause respiratory, enteric, cardiovascular, and neurological diseases. 4 , 5 , 6 , 7 SARS‐CoV‐2 appears to have greater infectivity and a lower case fatality rate (CFR) as compared with SARS and Middle East respiratory syndrome coronavirus (MERS). 8 So far, a few patients with SARS‐CoV‐2 have developed severe pneumonia, pulmonary edema, acute respiratory distress syndrome (ARDS), or multiple organ failure and died. According to a summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, the overall CFR was 2.3% (1023 deaths among 44 672 confirmed cases). 9 No deaths were reported among mild and severe cases. The CFR was 49.0% among critical cases. CFR was elevated among those with pre‐existing comorbid conditions, 10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer. Therefore, to lower the CFR in patients with COVID‐19, much attention have to be paid on heart injury and its clinical performance.
At present, information regarding the clinical features of pneumonia caused by SARS‐CoV‐2 is scarce. 3 , 10 , 11 In this study, we analyzed the main laboratory indicators of heart injury in patients with COVID‐19 and investigated the correlation between heart injury and severity of the disease.
2. METHODS
2.1. Patients
For this retrospective, single‐center study, we recruited 273 patients from January 1 to February 18, 2020, at Renmin Hospital of Wuhan University, China. All patients were diagnosed as having SARS‐CoV‐2 infection according to WHO interim guidance. This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University.
According to the relevant diagnostic criteria, patients were divided into three groups: mild (198 cases), severe (60 cases), and critical (15 cases). The required conditions must be met for mild cases was the positive SARS‐CoV‐2 RNA nucleic acid test by Reverse transcription polymerase chain reaction, fever, or other respiratory symptoms and the typical CT image abnormities of viral pneumonia are optional. 12 Severe cases additionally met at least one of the following conditions: (a) shortness of breath, RR ≥ 30 times/min, (b) oxygen saturation (resting state) ≤93%, or (c) PaO2/FiO2 ≤ 300mm Hg. Critical cases need to met at least one of the extra following conditions: (a) respiratory failure that needs to receive mechanical ventilation; (b) shock; and (c) multiple organ failure that need to be transferred to the intensive care unit (ICU). 11
2.2. Blood test
We collected data of the first detection of heart injury laboratory parameters in these 273 patients with COVID‐19 on admission. All patients were collected 3 mL of venous blood into procoagulant tubes and EDTA‐K2 anticoagulant tubes respectively in the early morning, then centrifuged at 3000 rpm for 10 minutes to separate serum and plasma. The levels of creatine kinase isoenzyme‐MB (CK‐MB), myohemoglobin (MYO), cardiac troponin I (ultra‐TnI) in the serum were measured using standard assay kit and Siemens ADVIA CENTAUR XP automatic chemiluminescence immunoassay analyzer, and the level of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) in the plasma was measured by the CobasE601 immunoanalyzer for all patients according to the manufacturer's instructions.
2.3. Statistical analysis
SPSS 25.0 was used for statistical analysis. Measurement data were expressed as P50 (P25~P75). Count data were analyzed by the χ 2 test or Fisher exact probability method. The measurement data are compared by the Kruskal‐Wallis test. Differences between groups were analyzed. P < .05 was considered statistically significant.
3. RESULTS
A total of 273 patients with COVID‐19 on admission from January 1 to February 18, 2020 were included in this study. According to the relevant diagnostic criteria, patients were divided into three groups: mild (198 cases), severe (60 cases), and critical (15 cases). There was no significant difference in gender and age among three groups in this study (Table 1).
Table 1.
Gender and age characteristics of patients with COVID‐19 in this study
| Mild cases (n = 198) | Severe cases (n = 60) | Critical cases (n = 15) | |
|---|---|---|---|
| Age, y | 58.95 ± 10.80 | 58.97 ± 14.38 | 57.27 ± 17.25 |
| Sex | |||
| Men | 71 | 21 | 5 |
| Women | 127 | 39 | 10 |
Abbreviation: COVID, coronavirus disease.
The blood tests of patients on admission showed most patients had normal levels of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP. Several were above the normal range: CK‐MB were above the normal range (0‐5 ng/mL) in 10 (10/273) patients, MYO were above the normal range (0‐110 μg/L) in 29 (29/273) patients, ultra‐TnI were above the normal range (0‐0.04 ng/mL) in 27 (27/273) patients, and NT‐proBNP were above the normal range (0‐900 pg/mL) in 34 (34/273) patients. These data together indicated that some patients with COVID‐19 developed acute cardiac injury.
Next, the changes of these indicators of the three groups were compared and analyzed, as well as their relationship with the clinical classification of the disease, as shown in Figure 1 and Table 2. The positive rate of CK‐MB had no difference between the mild, severe, and critical groups. While the positive rate of MYO, ultra‐TnI, and NT‐proBNP is higher in severe cases and critical cases as compared with mild cases, the differences among the groups were statistically significant (P < .05). After a pairwise comparison by Bonferroni correction, it was observed that the levels of NT‐proBNP and MYO were significant increased in severe cases and critical cases compared with mild cases (P < .0167), but no difference between severe cases and critical cases (P > .0167). The increase of ultra‐TnI only showed a significant difference between the mild cases and severe cases (P < .0167), while there was no difference between mild and critical cases and severe and critical cases (P > .0167). Thus, the increased concentration in venous blood of MYO, ultra‐TnI, and NT‐proBNP were associated with the severity of COVID‐19.
Figure 1.
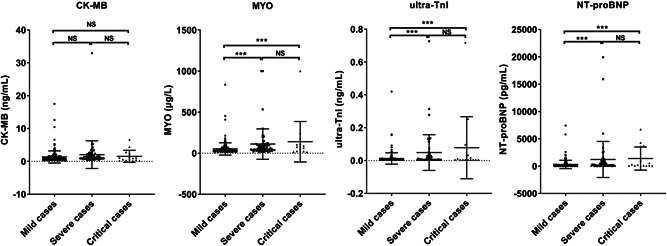
Levels of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP of the three patients with COVID‐19 groups were compared and analyzed. According to the relevant diagnostic criteria, patients were divided into three groups: mild (n = 198), severe (n = 60), and critical (n = 15). CK‐MB, creatine kinase isoenzyme‐MB; MYO, myohemoglobin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; COVID, coronavirus disease. ***P < .001
Table 2.
Levels of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP in patients with COVID‐19 in this study
| Mild cases (n = 198) | Severe cases (n = 60) | Critical cases (n = 15) | |
|---|---|---|---|
| CK‐MB, ng/mL | 0.91 (0.61‐1.41) | 1.10 (0.76‐2.12) | 0.97(0.32‐2.37) |
| ≤5 | 192 (96.97%) | 57 (95.00%) | 14 (93.33%) |
| >5 | 6 (3.03%) | 3 (5.00%)NS | 1 (6.67%)NS |
| MYO, μg/L | 34.66 (26.46‐54.54) | 57.73 (37.43‐100.71) | 75.34 (23.24‐112.47) |
| ≤110 | 187 (94.44%) | 46 (76.67%) | 11 (73.33%) |
| >110 | 11 (5.56%) | 14 (23.33%) a | 4 (26.67%) a |
| ultra‐TnI, ng/mL | 0.01 (0.01‐0.01) | 0.01 (0.01‐0.04) | 0.01 (0.01‐0.03) |
| ≤0.04 | 188 (94.95%) | 46 (76.67%) | 12 (80.00%) |
| >0.04 | 10 (5.05%) | 14 (23.33%) a | 3 (20.00%) |
| NT‐proBNP, pg/mL | 113.65 (45.92‐274.23) | 290.85 (106.13‐958.98) | 224.50 (91.73‐3615) |
| ≤900 | 184 (92.93%) | 45 (75.00%) | 10 (66.67%) |
| >900 | 14 (7.07%) | 15 (25.00%) a | 5 (33.33%) a |
Abbreviations: CK‐MB, creatine kinase isoenzyme‐MB; MYO, myohemoglobin; NS, no significant; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SARS‐CoV, severe acute respiratory syndrome coronavirus.
Compared with wild cases, P < .05.
Till March 23, the overall CFR was 8.79% (24 deaths among 273 cases) (Table 3). The CFR was 22.81% (13 deaths among 57 cases) in the abnormal parameters group, which was much higher than the CFR (5.09% as 11 deaths among 216 cases) in the normal parameters group. Notably, for those severe and critical cases, the CFRs were 42.31% and 33.33%, respectively, in those who had abnormal myocardial parameters.
Table 3.
Case fatality rate of patients with COVID‐19 in this study
| Total | ||||
|---|---|---|---|---|
| Mild | Severe | Critical | Total | |
| Cases | 198 | 60 | 15 | 273 |
| Death | 2 a | 19 | 3 | 24 |
| CFR | 1.01% a | 31.67% | 20.00% | 8.79% |
| Normal parameters group b | ||||
| Cases | 173 | 34 | 9 | 216 |
| Death | 2 | 8 | 1 | 11 |
| CFR | 1.16% | 23.53% | 11.11% | 5.09% |
| Abnormal parameters group c | ||||
| Cases | 25 | 26 | 6 | 57 |
| Death | 0 | 11 | 2 | 13 |
| CFR | 0 | 42.31% | 33.33% | 22.81% |
Abbreviations: CFR, case fatality rate; CK‐MB, creatine kinase isoenzyme‐MB; MYO, myohemoglobin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SARS‐CoV, severe acute respiratory syndrome coronavirus.
Two mild cases on admission progressed to severe and died.
Normal parameters were defined as CK‐MB in the range of 0‐5 ng/mL; MYO, 0‐110 μg/L; ultra‐TnI, 0‐0.04 ng/mL; NT‐proBNP, 0‐900 pg/mL.
Abnormal parameters were defined as parameters above the normal range.
Then, we analyzed these four heart injury parameters between the death (n = 24) and alive (246 recovered and 3 still in hospital at severe situation). All four parameters were significantly higher in the death than in the alive group (P < .001) (Figure 2).
Figure 2.

Levels of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP were compared and analyzed between death and alive patients with COVID‐19. Till 23 March, 24 patients died among 273 cases. Two hundred forty‐six recovered and 3 still in hospital at severe situation (249 alive in total). CK‐MB, creatine kinase isoenzyme‐MB; MYO, myohemoglobin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; COVID, coronavirus disease. ***P < .001
4. DISCUSSION
Viral pathogenicity is a well‐known risk factor in chronic cardiovascular disease, a general consequence of the imbalance between infection‐induced increased metabolic demand and reduced cardiac reserve. In all influenza pandemics other than the 1918 flu, cardiovascular events surpassed all other causes of mortality, including superimposed pneumonia. 13 Both SARS and MERS have been linked to acute myocarditis, acute myocardial infarction, and rapid‐onset heart failure. 14 , 15 Since the emergence of 2019 nCoV, numerous studies have been carried out on the clinical features of the disease. 16 In a recent case report on 138 hospitalized patients with COVID‐19, 7.2% developed acute cardiac injury. 11 In this study, we confirmed the heart injury association with SARS‐CoV‐2 infection and analyzed the correlation between heart injury and severity of COVID‐19.
We divided the patients into three groups: mild, severe, and critical, according to the relevant diagnostic criteria, and detected the concentration in venous blood of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP. We found that a portion of patients showed elevated levels of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP, indicating heart injury caused by SARS‐CoV‐2 infection, or a portion of patients with pre‐existing comorbid conditions, particularly with cardiovascular disease, were sensitive to SARS‐CoV‐2 infection. Moreover, there were statistically significant differences in the level and positive rate of MYO, ultra‐TnI, and NT‐proBNP among the three groups. The increased concentration in venous blood of MYO, ultra‐TnI, and NT‐proBNP is expected to predict the severity of the COVID‐19.
The much higher CFR in the abnormal parameters group than in the normal parameters group indicated a higher fatality risk associated with abnormal myocardial parameters. However, the CFR in this study is higher than the CFR in others' reports, which might due to the irregular period in early February when there was a shortage of medical services for the dramatically increased infection cases.
In conclusion, the higher concentration in venous blood of CK‐MB, MYO, ultra‐TnI, and NT‐proBNP were associated with the severity and CFR of COVID‐19. Therefore, careful monitoring of the myocardiac enzyme profiles is of great importance in reducing the complications and mortality in patients with COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
YF and LC conceived and designed this investigation. LC and WH helped to design the scheme of the investigation. HH and RL collected the original data. LX analyzed the data. JY, FL, and KW contributed to the interpretation of the data. LX, YF, and LC contributed to the writing of the paper. HH and LX contributed equally to this study.
ACKNOWLEDGMENTS
This study was supported by the National Mega Project on Major Infectious Disease Prevention under Grant 2017ZX10103005 (to KLW), the National Natural Science Foundation of China (81672079 to CLZ and 81471940 to YF), and the Open Research Program of the State Key Laboratory of Virology of China (2020KF001 to CLZ).
Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. 10.1002/jmv.25809
Contributor Information
Yong Feng, Email: yongfeng@whu.edu.cn.
Chengliang Zhu, Email: xinchengzhu@163.com.
REFERENCES
- 1. Lu H, Stratton CW, Tang Y‐W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, I Azhar E, Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967‐1976. [DOI] [PubMed] [Google Scholar]
- 6. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 7. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu R, Han H, Liu F, et al. Positive rate of RT‐PCR detection of SARS‐CoV‐2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madjid M, Casscells SW. Of birds and men: cardiologists' role in influenza pandemics. The Lancet. 2004;364(9442):1309. [DOI] [PubMed] [Google Scholar]
- 14. Peiris J, Chu C, Cheng V, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet (London, England). 2003;361(9371):1767‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li LQ, Huang T, Wang YQ, et al. 2019 novel coronavirus patients' clinical characteristics, discharge rate and fatality rate of meta‐analysis [published online ahead of print March 12, 2020]. J Med Virol. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]


