Infectious diseases remain one of the top 10 global health threats as announced by the World Health Organization [1]. The emergence of new pathogens for which there is no effective treatment has redrawn the attention to the usefulness of convalescent plasma. Indeed, convalescent plasma can be an alternate and fast therapeutic option in outbreaks of infectious diseases such as Middle East Respiratory Syndrome, Severe Acute Respiratory Syndrome (SARS), Chikungunya, Ebola and Zika [2, 3, 4]. The recent Coronavirus Disease 2019 (COVID‐19) pandemic caused by SARS‐CoV‐2 has prompted not only a search for effective antiviral treatment and spread control measures, but also a reconsideration of the use of convalescent plasma for COVID‐19 treatment [5, 6].
Role and experience of convalescent plasma in infectious diseases
Passive immunization for prevention and treatment of human infectious diseases can be traced back to the 20th century when it was observed that plasma from patients recovered from the infection were able to neutralize the pathogen and lead to its eradication from the blood circulation. Although antibiotics have largely supplanted their use in bacterial infections, convalescent plasma can be an important option in the treatment of many viral infections when specific antiviral treatments are largely unavailable and the infection carries serious morbidities and mortalities [3].
Experience and preparedness
Hong Kong, a densely populated city, has been hit by a few novel infectious diseases in the last two decades: avian influenza in 1997, SARS in 2003, influenza A (H1N1) pandemic in 2009 [A (H1N1)] and recently COVID‐19. To equip with the ability to well respond to the novel infectious disease threats so as to reduce mortality and morbidity, it is now timely and of utmost importance to revisit the preparedness of convalescent plasma production and usage.
In response to A (H1N1), a randomized double‐blinded controlled study was conducted in Hong Kong to investigate the outcome of additional hyperimmune intravenous immunoglobulin (H‐IVIG) in severely affected patients [7]. Hong Kong Red Cross Blood Transfusion Service (BTS), as part of the investigating team, was responsible for harvesting the convalescent plasma in accordance to prevailing blood donation standard [8]. A total of 9101 people who had confirmed to have recovered from influenza A (H1N1) were contacted. Screening appointments were made for 1309 potential donors and 786 attended. About 493 potential donors were found to be eligible for plasma donation, but only 301 attended the apheresis plasma donation appointment. Another 379 donors who satisfied the criteria donated one unit of whole blood each. A total of 276 litres of convalescent plasma were eventually sent to fractionation to produce H‐IVIG (Fig. 1) [8]. Because of the need of larger volume and the time lead required for fractionation, the clinical team also made use of some of the convalescent plasma collected to treat some of the seriously affected patients [9]. The use of convalescent plasma or H‐IVIG was significantly associated with lower respiratory tract viral load and mortality in the treatment group [7, 9].
Fig. 1.
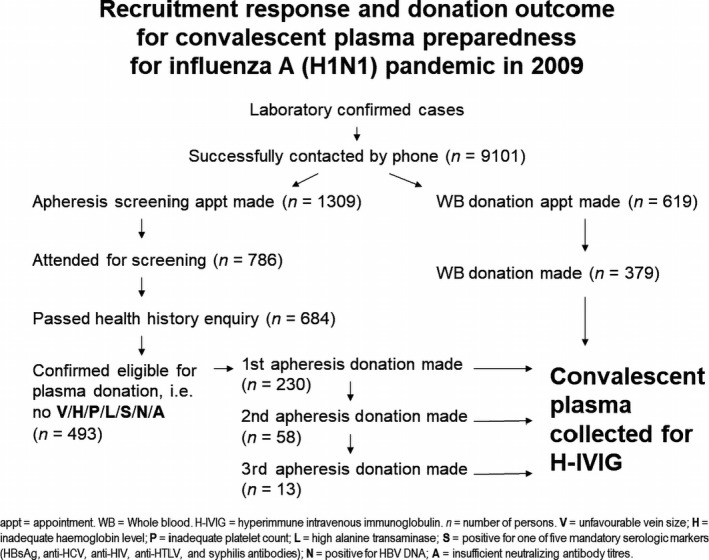
Recruitment response and donation outcome for convalescent plasma preparedness for influenza A (H1N1) pandemic in 2009.
Hurdle in convalescent plasma collection
Like blood donation programme around the world, identification, selection and recruitment of potential donors are not simple tasks. Besides, there were organizational and technological challenges in the collection, production and use of the products [3]. Nevertheless, the BTS was able to collect the volume of plasma needed for the production of H‐IVIG despite various limiting factors (e.g. difficult or failure to contact) as well as the impending urgency to accomplish the recruitment and collection within the shortest possible time. It provided an excellent opportunity to the BTS in the mobilization of existing resources and expertise within a short period of time to cope with the recruitment of large number of recovered patients. Further refinement of local factors for recruitment strategy and operational logistics would be beneficial in the event that large‐scale plasma collection is needed [8].
Measures in mitigating the risk to blood safety and prospective donor deferral
Though convalescent plasma was obtained from patients who had confirmed recovery from infection and developed humoral immunity, for the safety, those willing to participate must meet donor selection criteria as well as in compliance with existing policies and routine procedures. Per our experience, as many as 291 of 784 potential donors (37·1%) were screened out because of failure in health history screening, unfavourable vein size, inadequate haemoglobin level and platelet count for plasmapheresis, failed laboratory screening for infectious diseases such as hepatitis B, syphilis and insufficient neutralizing antibody titres (Fig. 1) [8]. As a responsible blood service, protection of donors’ welfare, blood safety and quality were always important and should not be compromised.
Conclusion
With the recent rapid evolution of COVID‐19 pandemic around the world and the currently observed mortalities, it is about time to consider the role of convalescent plasma in addition to various existing measures to limit and control the infection. Following the reports of beneficial responses to convalescent plasma, the Ministry of Health of China updated recently their draft treatment protocol to second edition [10]. With the previous experience of convalescent plasma collection and the various clinical results for its use in different respiratory tract infectious diseases [7, 9, 11, 12], it is conceivable that in the battle of COVID‐19 pandemic, preparation of collecting convalescent plasma should be planned now. On one hand, it is important to stand ready to provide adequate safe and effective products that could potentially save many lives in this pandemic. On the other hand, it is necessary to ensure that its collection, production and use take place in accordance to all necessary ethical considerations so as to produce an evidence base for its role in managing the severely COVID‐19 affected patients.
References
- 1. WHO : Ten threats to global health in 2019. https://www.who.int/emergencies/ten‐threats‐to‐global‐health‐in‐2019 (Last accessed 13/01/2020).
- 2. Bozzo J, Jorquera JI: Use of human immunoglobulins as an anti‐infective treatment: the experience so far and their possible re‐emerging role. Expert Rev Anti Infect Ther 2017; 15:585–604 [DOI] [PubMed] [Google Scholar]
- 3. Marano G, Vaglio S, Pupella S, et al.: Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus 2016; 14:152–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO : Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. Interim guidance for national health authorities and blood transfusion services. 2014. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1 (Last accessed.
- 5. Chen L, Xiong J, Bao L, et al.: Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis 2020. https://10.1016/S1473‐3099(20)30141‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casadevall A, Pirofski LA: The convalescent sera option for containing COVID‐19. J Clin Invest 2020. https://10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung IFN, To KKW, Lee CK, et al.: Hyperimmune IV immunoglobulin treatment: a multicenter double‐blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013; 144:464–73 [DOI] [PubMed] [Google Scholar]
- 8. Wong HK, Lee CK, Hung IF, et al.: Practical limitations of convalescent plasma collection: a case scenario in pandemic preparation for influenza A (H1N1) infection. Transfusion 2010; 50:1967–71 [DOI] [PubMed] [Google Scholar]
- 9. Hung IF, To KK, Lee CK, et al.: Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52:447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. 《新冠肺炎康复者恢复期血浆临床治疗方案(试行第二版)》 . 2020. http://www.gov.cn/zhengce/zhengceku/2020‐03/05/5487145/files/b1c12354cf404d629fee44738543627f.pdf (Last accessed 16/03/2020.
- 11. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al.: The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis 2015; 211:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou B, Zhong N, Guan Y: Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007; 357:1450–1 [DOI] [PubMed] [Google Scholar]


