Abstract
Objective
Previous meta‐analyses concluded that there was insufficient evidence to determine the effect of N95 respirators. We aimed to assess the effectiveness of N95 respirators versus surgical masks for prevention of influenza by collecting randomized controlled trials (RCTs).
Methods
We searched PubMed, EMbase and The Cochrane Library from the inception to January 27, 2020 to identify relevant systematic reviews. The RCTs included in systematic reviews were identified. Then we searched the latest published RCTs from the above three databases and searched ClinicalTrials.gov for unpublished RCTs. Two reviewers independently extracted the data and assessed risk of bias. Meta‐analyses were conducted to calculate pooled estimates by using RevMan 5.3 software.
Results
A total of six RCTs involving 9 171 participants were included. There were no statistically significant differences in preventing laboratory‐confirmed influenza (RR = 1.09, 95% CI 0.92‐1.28, P > .05), laboratory‐confirmed respiratory viral infections (RR = 0.89, 95% CI 0.70‐1.11), laboratory‐confirmed respiratory infection (RR = 0.74, 95% CI 0.42‐1.29) and influenzalike illness (RR = 0.61, 95% CI 0.33‐1.14) using N95 respirators and surgical masks. Meta‐analysis indicated a protective effect of N95 respirators against laboratory‐confirmed bacterial colonization (RR = 0.58, 95% CI 0.43‐0.78).
Conclusion
The use of N95 respirators compared with surgical masks is not associated with a lower risk of laboratory‐confirmed influenza. It suggests that N95 respirators should not be recommended for general public and nonhigh‐risk medical staff those are not in close contact with influenza patients or suspected patients.
Keywords: influenza, masks, N95 respirator, respiratory protective devices, respiratory tract infections, surgical mask
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV) have mortality rates about 10% and 37%, respectively. 1 Since the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), facemasks have been considered to be vitally important to reduce the risk of infection because vaccination or specific anti‐infective treatments are unavailable. 2 , 3 N95 respirators are used to prevent users from inhaling small airborne particles and must fit tightly to the user's face. Surgical masks are designed to protect wearers from microorganism transmission and fit loosely to the user's face.5,6 Although surgical masks cannot prevent inhalation of small airborne particles, both of them can protect users from large droplets and sprays. 7 , 8
There are conflicting recommendations for severe acute respiratory syndrome (SARS) and pandemic influenza: the World Health Organization (WHO) recommends using masks in low‐risk situations and respirators in high‐risk situations, but the Centers for Disease Control and Prevention (CDC) recommends using respirators in both low and high‐risk situations.9 However, N95 respirators may play a limited role in low‐resource settings, where there are a finite number of N95 respirators, or it may be unaffordable. 9 Also, previous meta‐analyses concluded there was insufficient evidence to determine the effect of N95 respirators due to a small number of studies that is prone to lack of statistical power. 10 , 11 Additionally, these meta‐analyses were limited by the small number of included randomized control trials (RCTs). More rigorous RCTs of comparing N95 respirators with surgical masks against influenza published in recent years were not included in previous meta‐analyses. 12 , 13 , 14
In light of the growing number of RCTs of masks use for protecting against influenza, this systematic review and meta‐analysis aimed to assess the effectiveness of N95 respirators versus surgical masks for prevention of influenza.
2. METHODS
This meta‐analysis was conducted based on the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines. 15
2.1. Inclusion and exclusion criteria
Inclusion criteria were (1) study type: RCT (including cluster‐randomized trial) and nonrandomized controlled study; (2) participants: humans with influenza (including pandemic strains, seasonal influenza A or B viruses and zoonotic viruses such as swine or avian influenza), and other respiratory viral infections (as a proxy for influenza); (3) intervention and comparator: N95 respirators versus surgical masks; (4) primary outcome: laboratory‐confirmed influenza; (5) secondary outcomes: laboratory‐confirmed respiratory viral infections, laboratory‐confirmed bacterial colonization, laboratory‐confirmed respiratory infection, and influenzalike illness; and (6) settings: hospital or community. RCTs were selected due to the potential possibility of high evidence level. Exclusion criteria were (1) theoretical models; (2) human ⁄nonhuman experimental laboratory studies; and (3) conference abstract.
2.2. Search strategy
We searched PubMed, EMBASE, and The Cochrane Library databases from inception to January 27, 2020, to identify published systematic reviews on evaluating the use of masks for preventing influenza. Search strategy in PubMed could be found in Table 1, and the strategy was adequately adjusted to use in other databases. Then, primary RCTs included in the systematic reviews were identified. Additionally, we conducted an additional search to identify RCTs published in the past five years from January 27, 2015, to January 27, 2020, using the databases and search strategies described above. We also searched for ClinicalTrials.gov to obtain unpublished data. There were no publication status and language restrictions on selecting the studies.
TABLE 1.
Search strategy in PubMed
| Number | PubMed |
|---|---|
| #1 | “systematic review”[Text Word] |
| #2 | meta analysis[Publication Type] |
| #3 | #1 OR #2 |
| #4 | masks OR respiratory protective devices[MeSH Terms] |
| #5 | mask* OR facemask* OR N95* OR N‐95*[Text Word] |
| #6 | #4 OR #5 |
| #7 | influenza, human OR severe acute respiratory syndrome[MeSH Terms] |
| #8 | flu OR influenza OR grippe OR SARS OR “severe acute respiratory syndrome”[Text Word] |
| #9 | #7 OR #8 |
| #10 | #3 AND #6 AND #9 |
2.3. Study selection and data extraction
Two reviewers independently screened the articles based on the titles, abstracts and full texts. Then, two reviewers independently exacted the following data from included studies: first author, publication year, country, disease, details of study population and intervention, study design, sample size, settings, and results. All disagreements were resolved by discussion.
2.4. Risk of bias assessment
Two reviewers independently assessed the risk of bias of the selected RCTs using the Cochrane Risk of Bias tool, 16 which includes domains on random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, and selective reporting. For each RCT, every domain was judged among 3 levels: high risk, unclear risk, and low risk. Disagreements were resolved by discussion.
2.5. Data analysis
All statistical analyses were performed using Review Manager (RevMan) version 5.3. Comparable data from studies with similar interventions and outcomes were pooled using forest plots. Relative risk (RR) with 95% confidence intervals (CIs) for dichotomous data was used as the effect measure. Between‐study heterogeneity was assessed using the I 2 for each pooled estimate. 17 We adopted a random‐effects model for heterogeneity P < .10. We performed a subgroup analysis based on the settings (hospital, community) due to the possibility of clinical heterogeneity. A sensitivity analysis was conducted to evaluate the robustness of the results by excluding individual studies for each forest plot. Funnel plots were planned to assessed publication bias. Because of the small number of studies available for each pooled estimate, we failed to assess publication bias.
3. RESULTS
3.1. Search results and study characteristics
The details on the literature search and screening process can be found in Figure 1. Excluded studies and reasons for exclusion were shown in Table 2. In total, we included six RCTs 12 , 18 , 19 , 20 , 21 , 22 and found no unpublished data of RCTs from ClinicalTrials.gov. The characteristics of these RCTs were presented in Table 3. The included studies published between 2009 and 2019. A total of 9171 participants in Canada, Australia, China, or America were included, and the number of participants in each RCT ranged from 435 to 5180 patients. The follow‐up duration varied from 2 to 15 weeks. Five studies included participants in hospitals, 12 , 18 , 20 , 21 , 22 and one in households. 19 Because of different definitions of outcome in included studies, we redefined the laboratory‐confirmed respiratory infection as respiratory influenza, other viruses or bacteria infection.
FIGURE 1.
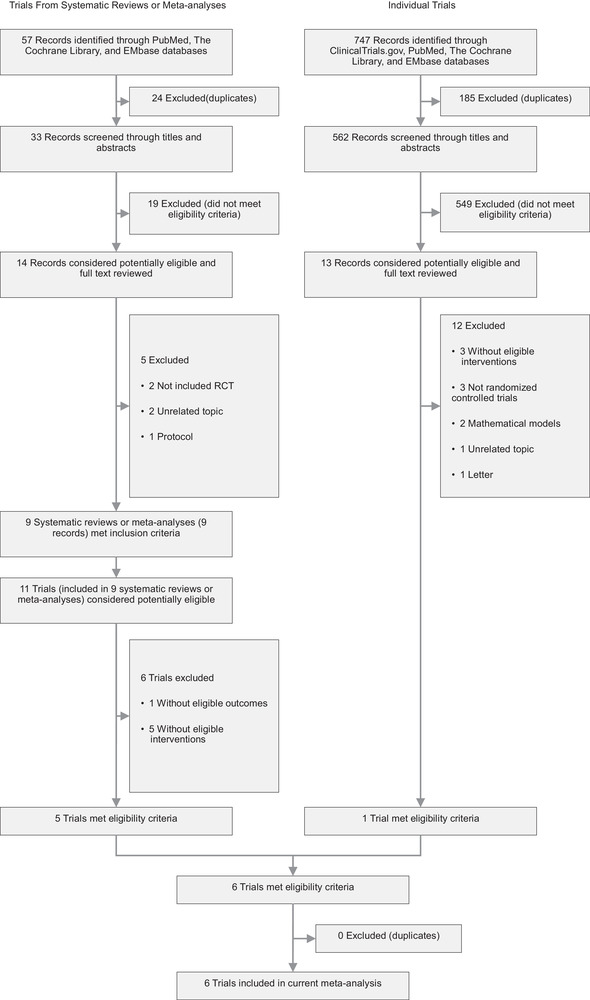
Literature search and screening process
TABLE 2.
Excluded studies and reasons for exclusion
| Excluded studies | Reasons for exclusion |
|---|---|
| Cowling et al 2008 26 | This trial did not have eligible interventions. |
| Jacobs et al 2009 27 | This trial did not have eligible outcomes. |
| Aiello et al 2010 28 | This trial did not have eligible interventions. |
| Barasheed et al 2014 29 | This trial did not have eligible interventions. |
| MacIntyre et al 2015 30 | This trial did not have eligible interventions. |
| Cowling et al 2014 31 | This study developed mathematical models of transmission of influenza and is not a trial in the real world. |
| MacIntyre et al 2015 30 | This trial did not have eligible interventions. |
| Wang et al 2015 32 | This study is a protocol. |
| Ambrosch et al 2016 33 | This is a prospective cohort study. |
| Chughtai et al 2016 34 | This trial focused on compliance with the use of medical and cloth masks. |
| MacIntyre et al 2016 14 | This trial did not have eligible interventions. |
| Sokol et al 2016 35 | This is a retrospective study. |
| MacIntyre et al 2017 36 | This study is a pooled analysis of two trials. |
| Zhang et al 2018 37 | This study developed mathematical models of transmission of influenza, and is not a trial in the real world. |
| Glatt et al 2020 38 | This is a letter. |
| Simmerman et al 2011 39 | This trial did not have eligible interventions. |
| Radonovich et al 2016 13 | This trial is duplicated. |
| Cowling et al 2009 40 | This trial did not have eligible interventions. |
TABLE 3.
Characteristics of studies included in the meta‐analysis
| Study | Setting | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|---|
| Loeb et al 2009 18 | 8 hospitals in Ontario, Canada: emergency departments, acute medical units and pediatric units | 446 nurses; individual‐level randomization |
• Intervention: targeted use, fit‐tested N95 respirator • Control: targeted use, surgical mask |
• Laboratory‐confirmed respiratory infection, influenza‐like illness, workplace absenteeism • 5‐week follow‐up |
• Noninferiority trial • Detection of influenza A and B, respiratory syncytial virus metapneumovirus, parainfluenza virus, rhinovirus‐enterovirus, coronavirus and adenovirus |
| MacIntyre et al 2009 19 | 145 households in Sydney, Australia | 145 index patients and 290 household contacts in 145 households; cluster randomization by household |
• Intervention 1: continual use, surgical mask • Intervention 2: continual use, nonfit‐tested N95 respirator • Control: lifestyle measures |
• Laboratory‐confirmed respiratory virus infection, influenza‐like illness • 2‐week follow‐up |
Detection of influenza A and B, respiratory syncytial virus, parainfluenza virus, rhinovirus‐enterovirus, coronavirus, coronavirus, adenovirus |
| MacIntyre et al 2011 20 /2014 22 | 15 hospitals in Beijing, China: emergency departments and respiratory wards | 1441 nurses, doctors and ward clerks; cluster randomization by hospital |
• Intervention 1: continual use, fit‐tested N95 respirator • Intervention 2: continual use, nonfit‐tested N95 respirator • Control: continual use, surgical mask |
• Laboratory‐confirmed respiratory infection, influenza‐like illness • 5‐week follow‐up |
Detection of influenza A and B, respiratory syncytial virus, metapneumovirus, parainfluenza virus, rhinovirus‐enterovirus, coronavirus, adenovirus, streptococcus pneumoniae, bordetella pertussis, chlamydophila pneumoniae, mycoplasma pneumoniae and haemophilus influenzae type B |
| MacIntyre et al 2013 21 | 19 hospitals in Beijing, China: emergency departments and respiratory wards | 1669 nurses, doctors and ward clerks; cluster randomization by ward |
• Intervention 1: continual use, fit‐tested N95 respirator • Intervention 2: targeted use, fit‐tested N95 respirator • Control: continual use, surgical mask |
• Laboratory‐confirmed respiratory infection, influenza‐like illness • 5‐week follow‐up |
Detection of influenza A and B, respiratory syncytial virus metapneumovirus, parainfluenza virus, rhinovirus‐enterovirus, coronavirus, adenovirus, S. pneumoniae, B. pertussis, C. pneumoniae, M. pneumoniae and H. influenzae type B |
| Radonovich et al 2019 12 | 7 hospitals in US: primary care facilities, dental clinics, adult and pediatric clinics, dialysis units, urgent care facilities and emergency departments, and emergency transport services | 5180 nurses/nursing trainees, clinical care support staff, administrative/clerical staff, physicians/advanced practitioners/physician trainees, registrations/clerical receptions, social workers/pastoral cares and environmental service workers/housekeepers; cluster randomization by outpatient clinic or outpatient setting |
• Intervention: targeted use, fit‐tested N95 respirator • Control: targeted use, medical mask |
• Laboratory‐confirmed respiratory infection, laboratory‐confirmed influenza, laboratory‐detected respiratory illness, influenza‐like illness, acute respiratory illness • 12‐week follow‐up |
• Effectiveness study • Detection of influenza A and B, respiratory syncytial virus, metapneumovirus, parainfluenza virus, rhinovirus‐enterovirus, coronavirus, coxsackie/echovirus |
3.2. Risk of bias
The results of the risk of bias assessment can be found in Figure 2. Five studies reported the computer‐generated random sequences, while only one mentioned randomization. All studies did not mention allocation concealment. Participants and trial staff were not blinded in two studies, and the other two studies failed to mention the blinding of participants and personnel. Four studies did not report whether the outcome assessors were blinded. All studies had complete outcome data or described comparable numbers and reasons for withdrawal across groups and prespecified outcomes.
FIGURE 2.
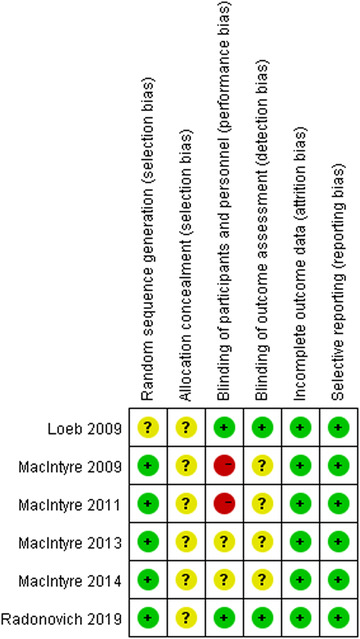
Risk of bias summary
3.3. Effectiveness
Five RCTs involving 8444 participants reported laboratory‐confirmed influenza. 12 , 18 , 19 , 20 , 21 Meta‐analysis with fixed‐effects model revealed that there was no statistically significant differences in preventing influenza using N95 respirators and surgical masks (RR = 1.09, 95% CI 0.92‐1.28, P > .05) (Figure 3). The results of subgroup analyses were consistent with this regardless of the hospital or the community. The results of the sensitivity analysis were not altered after excluding each trial.
FIGURE 3.
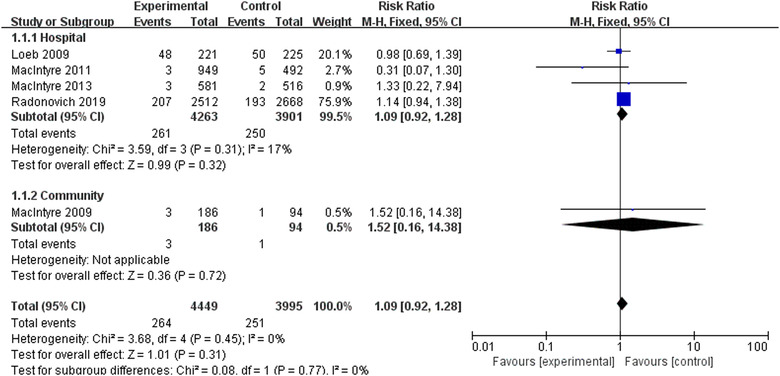
Results of meta‐analysis to determine the effectiveness of N95 respirators versus surgical masks against laboratory‐confirmed influenza
Four RCTs 18 , 19 , 20 , 21 involving 3264 participants reported laboratory‐confirmed respiratory viral infections. Meta‐analysis with fixed‐effects model revealed that there were no statistically significant differences in preventing respiratory viral infections using N95 respirators and surgical masks (RR = 0.89, 95% CI 0.70‐1.11, P > .05) (Figure 4). The results of subgroup analyses were consistent regardless of the hospital or the community. However, the sensitivity analysis after excluding the trial by Loeb et al 18 showed a significant effect of N95 respirators on preventing respiratory viral infections (RR = 0.61, 95% CI 0.39‐0.98, P < .05).
FIGURE 4.
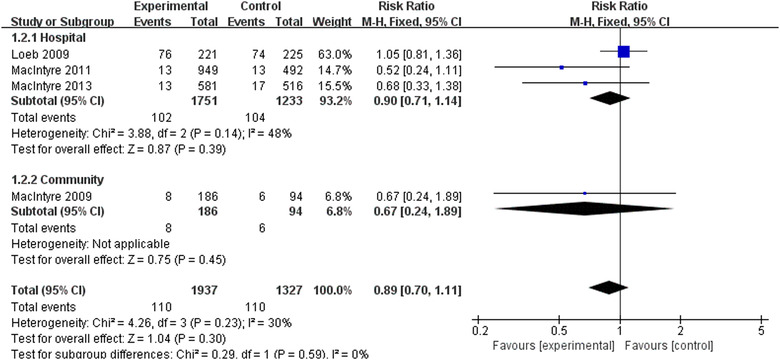
Results of meta‐analysis to determine the effectiveness of N95 respirators versus surgical masks against laboratory‐confirmed respiratory viral infections
Two RCTs 21 , 22 involving 2538 participants reported laboratory‐confirmed bacterial colonization. Meta‐analysis with fixed‐effects model revealed that compared with surgical masks, N95 respirators significantly reduced bacterial colonization in hospitals (RR = 0.58, 95% CI 0.43‐0.78, P < .05) (Figure 5). The sensitivity analysis showed that the results did not change after excluding each trial.
FIGURE 5.
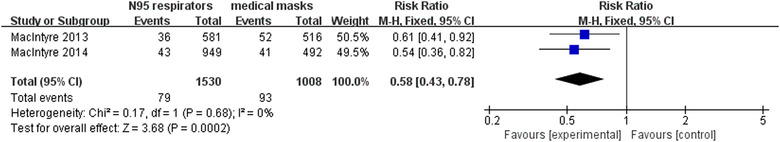
Results of meta‐analysis to determine the effectiveness of N95 respirators versus surgical masks against laboratory‐confirmed bacterial colonization
Two RCTs 12 , 22 involving 6621 participants reported laboratory‐confirmed respiratory infection. Meta‐analysis with random‐effects model revealed that there were no statistically significant differences in preventing respiratory infection using N95 respirators and surgical masks in hospitals (RR = 0.74, 95% CI 0.42‐1.29, P > .05) (Figure 6). However, the sensitivity analysis after excluding the trial by Radonovich et al 12 showed a significant effect of N95 respirators on preventing respiratory infection (RR = 0.53, 95% CI 0.35‐0.82, P < .05).
FIGURE 6.
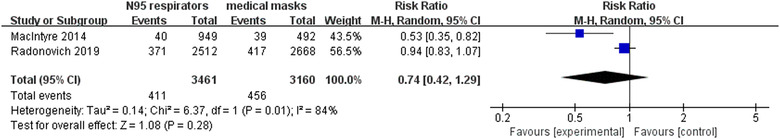
Results of meta‐analysis to determine the effectiveness of N95 respirators versus surgical masks against laboratory‐confirmed respiratory infection
Five RCTs involving 8444 participants reported influenza like illness. 12 , 18 , 19 , 20 , 21 Meta‐analysis with random‐effects model revealed that there were no statistically significant differences in preventing influenza like illness using N95 respirators and surgical masks (RR = 0.61, 95% CI 0.33‐1.14, P > .05) (Figure 7). The results of subgroup analyses indicated that statistically significant superiority of N95 respirators over surgical masks against influenza like illness (RR = 0.37, 95% CI 0.20‐0.71, P < .05) in the community (only one RCT). The sensitivity analysis showed results remained unchanged after excluding each trial.
FIGURE 7.
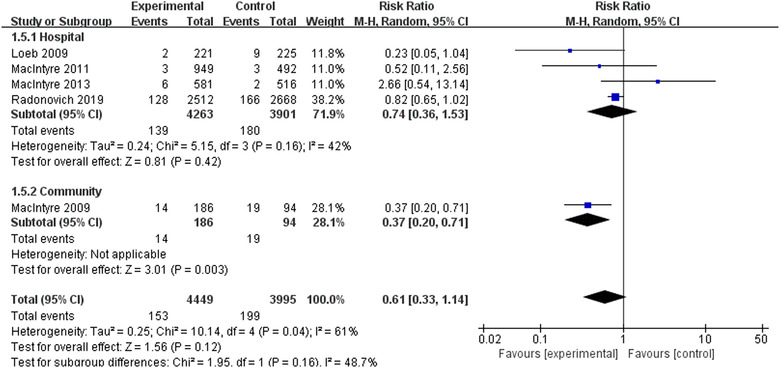
Results of meta‐analysis to determine the effectiveness of N95 respirators versus surgical masks against influenzalike illness
4. DISCUSSION
This meta‐analysis showed that there were no statistically significant differences in preventing laboratory‐confirmed influenza, laboratory‐confirmed respiratory viral infections, laboratory‐confirmed respiratory infection and influenza‐like illness using N95 respirators and surgical masks. N95 respirators provided a protective effect against laboratory‐confirmed bacterial colonization. In subgroup analysis, similar results could be found in the hospital and community for laboratory‐confirmed influenza and laboratory‐confirmed respiratory viral infections. However, sensitivity analysis showed unstable results for the prevention of laboratory‐confirmed respiratory viral infections and laboratory‐confirmed respiratory infection.
Through the course of influenza pandemics, large numbers of facemasks may be required to use in long periods to protect people from infections. 23 Using N95 respirators is likely to result in discomfort, for example, headaches. 23 A previous study 3 reported that there was an inverse relationship between the level of compliance with wearing an N95 respirator and the risk of clinical respiratory illness. It is difficult to ensure high compliance due to this discomfort of N95 respirators in all studies.
The reason for the similar effects on preventing influenza for the use of N95 respirators versus surgical masks may be related to low compliance to N95 respirators wear, 23 which may lead to more frequent doffing compared with surgical masks. 13 Although N95 respirators may confer superior protection in laboratory studies designing to achieve 100% intervention adherence, 24 the routine use of N95 respirators seems to be less acceptable due to more significant discomfort in real‐world practice. 11 Therefore, the benefit of N95 respirators of fitting tightly to faces is offset or subjugated. 13 However, it should be noted that the surgical masks are primarily designed to protect the environment from the wearer, whereas the respirators are supposed to protect the wearer from the environment. 25
There are several limitations to this study. First, some RCTs had a high risk of bias due to lack of allocation concealment and blinding; although it is impractical to blind participants who would know the type of masks they are wearing. Second, the number of included studies focusing on the community was small. Consequently, the results of the subgroup analysis might be unreliable. Third, we identified RCTs from published systematic reviews, which may result in the omission of relative RCTs. Finally, there might be publication bias, and we cannot assess it due to an insufficient number of included RCTs.
In conclusion, the current meta‐analysis shows the use of N95 respirators compared with surgical masks is not associated with a lower risk of laboratory‐confirmed influenza. It suggests that N95 respirators should not be recommended for the general public and nonhigh risk medical staffs those are not in close contact with influenza patients or suspected patients.
CONFLICT OF INTEREST
None.
Long Y, Hu T, Liu L, et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta‐analysis. J Evid Based Med. 2020;13:93–101. 10.1111/jebm.12381
Contributor Information
Jin Huang, Email: michael_huangjin@163.com.
Liang Du, Email: duliang0606@vip.sina.com.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;S0140‐6736(20):30183‐30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011(7):CD006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen X, Chughtai AA, MacIntyre CR. Herd protection effect of N95 respirators in healthcare workers. J Int Med Res. 2017;45(6):1760‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janssen L, Ettinger H, Graham S, Shaffer R, Zhuang Z. The use of respirators to reduce inhalation of airborne biological agents. J Occup Environ Hyg. 2013;10(8):D97‐d103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhiqing L, Yongyun C, Wenxiang C, et al. Surgical masks as source of bacterial contamination during operative procedures. J Orthop Translat. 2018;14:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawrence RB, Duling MG, Calvert CA, Coffey CC. Comparison of performance of three different types of respiratory protection devices. J Occup Environ Hyg. 2006;3(9):465‐474. [DOI] [PubMed] [Google Scholar]
- 7. Sandaradura I, Goeman E, Pontivivo G, et al. A close shave? Performance of P2/N95 respirators in health care workers with facial hair: results of the BEARDS (Adequate Respiratory DefenceS) study. J Hosp Infect. 2020;S0195‐6701(20):30008‐30006. [DOI] [PubMed] [Google Scholar]
- 8. Derrick JL, Gomersall CD. Protecting healthcare staff from severe acute respiratory syndrome: filtration capacity of multiple surgical masks. J Hosp Infect. 2005;59(4):365‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chughtai AA, Seale H, MacIntyre CR. Availability, consistency and evidence‐base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: a global analysis. BMC Res Notes. 2013;6:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta‐analysis. CMAJ. 2016;188(8):567‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta‐analysis. Clin Infecti Dis. 2017;65(11):1934‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radonovich LJ, Simberkoff MS, Bessesen MT, et al. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322(9):824‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radonovich LJ, Bessesen MT, Cummings DA, et al. The Respiratory Protection Effectiveness Clinical Trial (ResPECT): a cluster‐randomized comparison of respirator and medical mask effectiveness against respiratory infections in healthcare personnel. BMC Infect Dis. 2016;16:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacIntyre CR, Zhang Y, Chughtai AA, et al. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open. 2016;6(12):e012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]; 2011.
- 17. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between‐study heterogeneity in meta‐analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302(17):1865‐1871. [DOI] [PubMed] [Google Scholar]
- 19. MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15(2):233‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacIntyre CR, Wang Q, Cauchemez S, et al. A cluster randomized clinical trial comparing fit‐tested and non‐fit‐tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influen Other Respir Viruses. 2011;5(3):170‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacIntyre CR, Wang Q, Seale H, et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187(9):960‐966. [DOI] [PubMed] [Google Scholar]
- 22. MacIntyre CR, Wang Q, Rahman B, et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co‐infection in hospital healthcare workers. Prev Med. 2014;62:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cowling BJ, Zhou Y, Ip DK, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138(4):449‐456. [DOI] [PubMed] [Google Scholar]
- 24. Noti JD, Lindsley WG, Blachere FM, et al. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis. 2012;54(11):1569‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balazy A, Toivola M, Adhikari A, Sivasubramani SK, Reponen T, Grinshpun SA. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks. Am J Infect Control. 2006;34(2):51‐57. [DOI] [PubMed] [Google Scholar]
- 26. Cowling BJ, Fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non‐pharmaceutical interventions to prevent influenza transmission in households. PLoS One. 2008;3(5):e2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacobs JL, Ohde S, Takahashi O, Tokuda Y, Omata F, Fukui T. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am J Infect Control. 2009;37(5):417‐419. [DOI] [PubMed] [Google Scholar]
- 28. Aiello AE, Murray GF, Perez V, et al. Mask use, hand hygiene, and seasonal influenza‐like illness among young adults: a randomized intervention trial. J Infect Dis. 2010;201(4):491‐498. [DOI] [PubMed] [Google Scholar]
- 29. Barasheed O, Almasri N, Badahdah AM, et al. Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza‐like illness transmission among Australian Hajj Pilgrims in 2011. Infect Disord Drug Targets. 2014;14(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 30. MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4):e006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cowling BJ, Ip DKM, Fang VJ, et al. Modes of transmission of influenza B virus in households. PLoS One. 2014;9(9):e108850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M, Barasheed O, Rashid H, et al. A cluster‐randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims. J Epidemiol Glob Health. 2015;5(2):181‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ambrosch A, Rockmann F. Effect of two‐step hygiene management on the prevention of nosocomial influenza in a season with high influenza activity. J Hosp Infect. 2016;94(2):143‐149. [DOI] [PubMed] [Google Scholar]
- 34. Chughtai AA, Seale H, Dung TC, Hayen A, Rahman B, Raina MacIntyre C. Compliance with the use of medical and cloth masks among healthcare workers in Vietnam. Ann Occup Hyg. 2016;60(5):619‐630. [DOI] [PubMed] [Google Scholar]
- 35. Sokol KA, De la Vega‐Diaz I, Edmondson‐Martin K, et al. Masks for prevention of respiratory viruses on the BMT unit: results of a quality initiative. Transpl Infect Dis. 2016;18(6):965‐967. [DOI] [PubMed] [Google Scholar]
- 36. MacIntyre CR, Chughtai AA, Rahman B, et al. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influen Other Respir Viruses. 2017;11(6):511‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang N, Li Y. Transmission of influenza A in a student office based on realistic person‐to‐person contact and surface touch behaviour. Int J Environ Res Public Health. 2018;15(8):E1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glatt AE. Health care worker use of N95 respirators vs medical masks did not differ for workplace‐acquired influenza. Ann Intern Med. 2020;172(2):JC7. [DOI] [PubMed] [Google Scholar]
- 39. Simmerman JM, Suntarattiwong P, Levy J, et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok. Thail Influen Other Respir Viruses. 2011;5(4):256‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7):437‐462. [DOI] [PubMed] [Google Scholar]


