Abstract
In Africa, bat‐borne zoonoses emerged in the past few decades resulting in large outbreaks or just sporadic spillovers. In addition, hundreds of more viruses are described without any information on zoonotic potential. We discuss important characteristics of bats including bat biology, evolution, distribution and ecology that not only make them unique among most mammals but also contribute to their potential as viral reservoirs. The detection of a virus in bats does not imply that spillover will occur and several biological, ecological and anthropogenic factors play a role in such an event. We summarize and critically analyse the current knowledge on African bats as reservoirs for corona‐, filo‐, paramyxo‐ and lyssaviruses. We highlight that important information on epidemiology, bat biology and ecology is often not available to make informed decisions on zoonotic spillover potential. Even if knowledge gaps exist, it is still important to recognize the role of bats in zoonotic disease outbreaks and implement mitigation strategies to prevent exposure to infectious agents including working safely with bats. Equally important is the crucial role of bats in various ecosystem services. This necessitates a multidisciplinary One Health approach to close knowledge gaps and ensure the development of responsible mitigation strategies to not only minimize risk of infection but also ensure conservation of the species.
Keywords: Africa, bats, henipavirus, paramyxovirus, coronavirus, filovirus, Ebola, rabies
Hundreds of viruses have been described from bats in Africa, some with proven spillover and causing human deaths, however for the majority there is no information on spillover potential. We summarize and critically analyze the current knowledge on African bats as reservoirs for corona, filo, paramyxo and lyssaviruses, including important characteristics of bats like biology, evolution, distribution and ecology that not only make them unique amongst most mammals but also contribute to their potential as viral reservoirs. We highlight the gaps in knowledge that is often not available to make informed decisions on zoonotic spillover potential. Despite this it is still important to recognize the role of bats in zoonotic disease outbreaks and implement mitigation strategies to prevent exposure to infectious agents, including working safely with bats. Equally important is the crucial role of bats in various ecosystem services.

Introduction
It is estimated that around 75% of human emerging infectious diseases (EIDs) are zoonotic (Jones et al., 2008; Allen et al., 2017; Table 1) and in large part associated with wildlife hosts including bats (Order Chiroptera). Examples of bat‐borne zoonotic viruses include rabies and rabies‐related lyssaviruses, paramyxoviruses such as Hendra (HEV) and Nipah virus (NiV), filoviruses including Ebolavirus (EBOV) and Marburg virus (MARV), and coronaviruses such as severe acute respiratory syndrome (SARS) coronavirus. The recent intensification of viral surveillance (Table 1) in bats and advancements in pathogen detection techniques have enabled the identification of more than 200 bat‐borne viruses (Moratelli & Calisher, 2015), with this number actively increasing (Chen et al., 2014). Despite the growing diversity described, only a small percentage of these viruses have spilt over into other animals and humans, resulting in disease.
Table 1.
Definitions of key terminology
| Term | Definition |
|---|---|
| Zoonoses/Zoonosis | An infection or disease resulting from the transmission of a pathogenic agent that is transmissible from animals to humans under natural conditions. |
| Reservoir | An ecological system in which an infectious agent survives indefinitely. It may or may not show signs of disease. |
| Spillover host | An individual that comes into contact with the reservoir and is infected. The infection may or may not be transmitted within the new host population. |
| Intermediate host | A secondary host or intermediate host is a host that harbours the pathogen only for a short transition period, and transmits the pathogen to another host. It can be considered the second host in a three host transmission system (reservoir> intermediate host> host). These hosts are capable of retaining the spillover viral agent and disseminate it between members of its population. The viral agents may accumulate necessary mutations, recombination events or concentrations to make it capable of transmission to other hosts such as people. |
| Surveillance | The ongoing, systematic collection, analysis and interpretation of data. |
In Africa, several bat‐borne zoonoses have emerged in the past few decades, resulting in either large scale outbreaks (e.g. filoviruses) or sporadic spillovers (e.g. lyssa‐ and paramyxoviruses). Several environmental and anthropogenic factors have been considered as potential drivers of zoonotic disease emergence from wildlife species, including bats (Plowright et al., 2015). A key example of this is the rapid human population growth observed globally, consequently leading to large scale agricultural intensification, deforestation and bat bushmeat hunting (Pernet et al., 2014; Allen et al., 2017). These factors not only lead to habitat destruction and species decline but also to allow for increased contact between humans and wildlife species. As such, additional opportunities arise for spillover, leading to a higher probability that more of the bat‐borne viral diversity could spillover in the future.
Bats are the second most speciated mammal with a near worldwide distribution. More than 70% of bat species are insectivorous, while others are frugivorous, carnivorous or sanguivorous. Of more than 1300 bat species documented globally (Simmons et al., 2008; Fenton & Simmons, 2015), at least 20% occur on the African continent, predominantly in the sub‐Saharan regions, and a few on the northern African coast (ACR, 2018). A large number of these African bat species have been the focus of several research studies focused on bat‐borne zoonoses as well as viral surveillance. The Chiropteran order was divided into two suborders: the Megachiroptera and Microchiroptera (Dobson, 1875) with the Megachiroptera including the family Pteropodidae (frugivorous bats) and the Microchiroptera comprising of all the other families of bats (predominantly insectivorous bats) (Koopman, 1994). This taxonomic classification has changed to incorporate the recognition of two separate lineages: Yinpterochiroptera (Pteropodiformes) which includes the Rhinolophidae (horseshoe insectivorous bats) and Pteropodidae, and Yangochiroptera (Vespertilioniformes) which is comprised of all the other families (Springer, Teeling & Stanhope, 2001; Van den Bussche & Hoofer, 2004; Hutcheon & Kirsch, 2006). For the purpose of this review, bats will loosely be referred to as frugivorous bats (Pteropodidae) or insectivorous bats (other bat families on the African continent).
This review discusses characteristics of bat biology, evolution, distribution and ecology related to the viral host status and propensity as reservoir hosts (Table 1). The focus will be on important RNA virus families implicated in zoonotic events, and the current knowledge on bat species implicated. A critical assessment of the risks and factors important for spillover (Table 1) is reviewed.
Bats as virus reservoirs
Viruses are found within nearly all living species on earth, including bats. Except for rabies and rabies‐related lyssaviruses, bats generally harbour viruses with no clinical signs of disease, which may be suggestive of a long history of co‐evolution (Moratelli & Calisher, 2015). Luis et al. (2013) and Olival et al. (2017) investigated whether bats harbour a higher viral diversity than other animal species. After controlling for sampling bias and reporting, a higher proportion of zoonotic viruses per species were still evident for bats. Several contributing factors have been suggested, including certain unique life traits:
Longevity
Bats are characterized by an exceptionally long life span, up to ten times higher in comparison with other mammals of the same size (Salmon et al., 2009). Longevity varies significantly between species, with the longest life span recorded in a Brandt’s bat (Myotis brandtii) from Siberia of approximately 41 years based on recapture data (Podlutsky et al., 2005). With increased longevity, bats provide a stable environment for sustainable persistent virus replication and survival, eliminating the need for a rapid viral replication strategy. Additionally, prolonged survival allows for multiple horizontal and vertical transmission events through several generations, thus permitting virus maintenance within populations over time (Olival et al., 2017).
Reproduction
Bat reproduction is similar to that of other mammals. Gestation varies from 1.5 to 7 months, but averages around two months with most species producing a single pup per litter (Wilson, 1997). Bats distributed in areas with less seasonal variation and year‐round food availability generally reproduce biannually or more (polyestrous), as is typical in equatorial areas, compared with only once a year (monoestrous) in more temperate regions. A prime example is the Egyptian rousette bat (Rousettus aegyptiacus) in Africa, where a single birthing pulse is noted in South Africa (Jacobsen & Du Plessis, 1976) compared with biannual pulses in Uganda (Bernard & Cumming, 1997). Bat maternity colonies have also been reported to act as viral amplifiers (Dietrich et al., 2015). Bat species that roost at high densities undergo patterns of seasonal breeding resulting in a large influx of susceptible individuals, initially protected by maternal antibodies with waning of protection (3‐6 months) resulting in increased viral transmission and circulation among susceptible hosts (Plowright et al., 2008; Amman et al., 2012).
Roosting
Roost selection varies greatly between bat species and can be natural structures, such as caves, crevices, burrows, trees and other plants, or man‐made structures such as buildings, culverts or mines. Roost choice is influenced by species, season and sex of individuals. The use of man‐made structures results in increased opportunities for contact with humans, whereby exposure to bat‐borne viruses through contaminated excreta is likely. Caves, in particular, offer the opportunity for a large number of bats to co‐roost and occupy the same caves throughout a year (Chege, Schepers & Wolfaardt, 2015). The gregarious nature of some bat species leads to the formation of assemblages of individuals within roosts that may number in the tens to millions, with anything in between. These highly dense populations result in constant close contact between individuals due to social behaviours such as mating, grooming or fighting. This increases contact rates with body fluids including saliva, blood, faeces and urine. As such, dense roosting aggregations facilitate high rates of intra‐ and interspecies transmission of viruses (Luis et al., 2015). Viral maintenance within species that roost in smaller, less‐dense populations is not as clearly defined and not well investigated.
Self‐powered flight
Bats are the only mammals adapted to true self‐powered flight allowing them to move over vast distances either during foraging or during migration. The African straw‐coloured fruit bat (Eidolon helvum) is associated with long‐distance migrations, crossing country boundaries as they migrate from southern and sub‐Saharan Africa and aggregate in numbers of more than 5 million at Kasanka National Park in Zambia to give birth (Richter & Cumming, 2006). Similarly, the Egyptian rousette has been recorded to migrate as far as 500 km in South Africa (Jacobsen & Du Plessis, 1976). Increased contact with other animal species during these movements enhances the potential for interspecies virus transmission (Wang, Walker & Poon, 2011) in addition to long‐distance dissemination of viruses across large geographical regions. Global warming has also been hypothesized to result in future changes in bat species distribution and introduction of diseases into new areas (Sherwin, Montgomery & Lundy, 2013; Hayes & Piaggio, 2018).
Immunology
The topic of bat immunology has been explored in recent years, with current conclusions indicating that a number of interacting features are involved in combating viral infections. Bats have both an innate immune response and adaptive immune response, with similar immune organs, tissues, cells and immunoglobulin types as compared to other mammals (Schountz et al., 2017), with some unique differences. Bat genome data indicate evolution of the mitochondrial DNA damage and repair pathways coinciding with the timing of the evolution of flight (and increase metabolic cost) in bats (Zhang et al., 2013; O’Shea et al., 2014). This results in significantly higher metabolic rates in bats, as compared to other hyperactive mammals, and associated higher body temperature. This increase in body temperature, similar to a typical immune‐mediated febrile response (O’Shea et al., 2014), was suggested as a viral control mechanism. The ‘flight as fever’ hypothesis proposes that bat‐borne viruses may be more tolerant of fever responses, and therefore appear to be more virulent when encountered in a non‐natural host, such as humans. This has not been substantiated by any experimental evidence and has been contradicted for filoviruses (Miller et al., 2016). In addition, studies indicated that the type I interferon (IFN) system is continually expressed resulting in upregulation of additional immune genes that control viral replication (Zhou et al., 2016). Bats also have a combinatorial diversity of immunoglobulin segments, up to five times more segments than reported for humans (giving rise to many more different antibody molecules). This leads to a higher naïve immunoglobulin repertoire (combination of all antibody specificities in an individual) that allows for a more effective response to infection (Schountz et al., 2017). In contrast, a recent study concluded that the genome analysis of the Egyptian rousette bat indicated crucial differences between the antiviral mechanisms of bats and other mammals (Pavlovich et al., 2018) and that the ability to control viral infection is less due to enhanced potency of the antiviral defences and more associated with inhibitory immune states (tolerance). Studies are usually focused on a single bat species that complicates interpretation of results and extrapolating findings to all members of the Chiroptera order. The lack of bat immunological reagents and defined bat models are a major limitation in studies with several aspects of the bat immune system not being investigated at all. Other differences not related to the immune system can also contribute towards reservoir status, including physiological, behavioural and metabolic characteristics.
Echolocation and hibernation
Lastly, a few other characteristics such as echolocation, hibernation and torpor have been suggested as contributory factors, though not all bats share these characteristics. Insectivorous bats are known to echolocate, while pteropodids do not. However, certain exceptions have been reported in fruit bat species (Rousettus spp.), who produce ultrasounds by tongue clicking (Nowak et al., 1994). Echolocation has been postulated as a mechanism for potential viral spread (Calisher et al., 2006), representing a sneeze and expulsion of droplets of oropharyngeal fluids, mucus or saliva that could act as a transmission route. Hibernation and torpor are hypothesized to aid in viral persistence and subsequent viral reactivation due to decreased metabolic and immunological activity (Gerow et al., 2019). There are currently few reports of torpor in African bat species; however, it may be more common than currently documented (Geiser & Stawski, 2011).
Although there are several arguments to support the above‐mentioned characteristics as contributory to a successful reservoir status, it is important to consider that rodents also harbour a characteristically high number of viral pathogens (Luis et al., 2013; Han et al., 2016). However, many of the aforementioned factors do not apply to rodents, as there is rapid population turnover, and their life spans are much reduced, non‐volant, etc. Thus, although these bat life traits might contribute to some extent, they should not be considered as mutually exclusive and may play intricate roles along with yet unknown factors in supporting the high viral diversity observed in bats.
Viral detection and considerations
Active surveillance for bat‐borne viruses is becoming increasingly important to gain insight into viral diversity, ecology and epidemiology. However, the interpretation of viral discoveries varies greatly depending on the surveillance and detection strategies employed. Strategies include serological (detecting antibodies) and nucleic acid detection (PCR assays and next‐generation sequencing technologies) as well as viral isolations.
Serological techniques do not usually distinguish between bat immunoglobulins, IgM versus IgG, and are only an indication of exposure to the agent – be it an active or previous infection. This does not provide information on the maintenance or pathogenicity of the agent within the host population. A major concern is also the potential for cross‐reactivity with other related viral species. For most viral families, nucleic acid detection tests (PCR assays), that are based on degenerate consensus broadly reactive primers, targeting conserved partial viral genome regions, are available. Such assays, however, require that the sequence information of newly detected viruses share a certain degree of similarity to the previously characterized viruses in the genus or family, and will therefore be less likely to detect highly divergent sequences that may represent novel viruses. Unbiased sequence‐independent methods such as next‐generation sequencing (NGS) technologies have also gained more popularity and are excellent tools for novel pathogen discovery. Viruses identified might be part of the natural microflora present in bats, be a bat pathogen, or even as coincidental spillovers from other species – thus their detection should not immediately be considered as evidence for the designation of bats as reservoirs to those viruses.
Sample selection for surveillance will depend on the chosen analysis strategy, though is also dependent on the targeted virus, due to differences in tissue tropism. Nondestructive sampling, such as urine and faecal collection, is preferred but may not always give an accurate reflection of viral prevalence. Specific surveillance strategies (e.g. cross‐sectional) may fail to detect diverse pathogens since viral presence may be influenced by seasonality and/or migration of a particular host species.
Prominent virus families associated with bats in Africa
Coronaviruses
Coronaviruses are pathogens of humans, other mammalian species and bird hosts, and capable of causing respiratory, gastrointestinal, hepatic and neurological diseases. These viruses have positive‐sense RNA genomes and belong to four genera, namely Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus (Wong et al., 2019). Human coronaviruses (HCoV) such as HCoV229E, HCoVNL63, HCoVOC43 and HCoVHKU1, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus are respiratory pathogens transmitted primarily through aerosols. A number of animal‐infecting coronaviruses such as feline, bovine and porcine coronaviruses result in gastroenteritis and are thus transmitted via a faecal–oral route. Coronaviruses are known to frequently undergo recombination and mutation events, conferring fitness advantages to new variants (Tao et al., 2017). Global surveillance for coronavirus diversity in bats has rapidly expanded in the last 17 years and can be mostly linked to emerging coronavirus disease outbreaks. SARS coronavirus emerged in China in 2002 and MERS coronavirus in 2012 on the Arabian Peninsula. Both pandemics resulted in significant morbidity and mortality (10% and 35%, respectively) among human populations, which were exacerbated by global travel (Berry, Gamieldien & Fielding, 2015).
Severe acute respiratory syndrome coronavirus sparked a large pandemic that spread from China to 25 other countries, infecting over 8000 people (Berry et al., 2015). The rapid onset of a novel viral agent, with potential animal exposures of nearly all index patients, leads to suspicions of an emerging zoonosis (Cui, Li & Shi, 2019). Masked palm civets (Paguma larvata) and Raccoon dogs (Nyctereutes procyonoides) were the first animal hosts from which SARS coronavirus was detected in the live animal markets of China (Guan et al., 2003). These hosts were likely infected by another animal in the market (Cui et al., 2019), which was suggested to be the horseshoe bat (Rhinolophus) genus, due to the detection of viruses sharing genetic similarity to SARS from these bats (referred to as SARS‐related viruses) (Lau et al., 2005; Li et al., 2005). Horseshoe bats were further shown to host a large diversity of SARS‐related viruses (Hu et al., 2017), though these viruses shared low similarity to SARS coronavirus within the spike gene region – which is essential for recognition and attachment to host receptors (Lau et al., 2005; Li et al., 2005). Eleven years of continued surveillance within Chinese horseshoe bat species to better understand the emergence of SARS and genetic diversity of SARS‐related viruses allowed for the identification of viruses from horseshoe bat species sharing near‐identical similarity to human SARS (Ge et al., 2013; Yang et al., 2016). These similar viruses were even capable of using the same binding receptor as human SARS coronavirus, a feature the majority of bat‐borne SARS‐related viruses were lacking (Ge et al., 2013) suggesting direct infection of humans and other hosts during the outbreak in 2002 was possible (Ge et al., 2013; Yang et al., 2016; Hu et al., 2017). No new cases of SARS have been reported since 2004, though the continued presence of various recombinant SARS‐related strains in horseshoe bat populations makes re‐emergence possible.
The emergence of MERS coronavirus is somewhat more straightforward. The virus was first identified from a man in Saudi Arabia (Zaki et al., 2012), through retrospective investigations of infections in Jordan confirmed an even earlier emergence of MERS (de Groot et al., 2013). International global travel activities have since resulted in the spread of MERS to several other countries, though the virus requires sustained contact to transmit between people ((Berry et al., 2015). Many of the index cases of MERS had direct contact with dromedary camels, from which near‐identical viruses were detected (Memish et al., 2014). MERS prevalence has since been shown to be widespread among dromedary camel populations of the Middle East and Africa (Dighe et al., 2019), and MERS antibodies have been detected in camel sera collected even as far back as the 1980s (Reusken et al., 2014). Zoonotic MERS from camels has only been reported from the Arabian Peninsula (Chu et al., 2018). The association of bats with the emergence of MERS has been less well described. During the initial search for the zoonotic origins of MERS, bats were investigated due to shared genome similarities of MERS with previously identified Asian bat coronaviruses (Woo et al., 2006). A short coronavirus sequence (190 nt) sharing 100% identify within a conserved gene region of human MERS coronaviruses was identified from an Egyptian tomb bat (Taphozous perforatus), collected in Saudi Arabia (Memish et al., 2013a). No additional genome regions could be recovered, and the absence of additional genetic information makes further conclusions challenging. A number of coronaviruses more distantly related to MERS have been reported from various bat species, and this is further discussed below.
The implication of bats acting as possible progenitors of emerging coronaviruses prompted global surveillance activities from which large diversities of bat coronaviruses have been identified (from diverse bat species). These viruses display host genus specificity, though cross‐species transmissions are also frequently observed (Anthony et al., 2017b; Leopardi et al., 2018). The vast amount of data has led to the hypothesis that bats host the genetic diversity of the Alpha‐ and Betacoronavirus mammalian infecting genera (Woo et al., 2012). Furthermore, the emergence of novel coronaviruses is strongly linked to high diversity present in host populations, frequent mutation and recombination events that may lead to novel viral strains which are capable of adaptation to new hosts (Hu et al., 2017; Anthony et al., 2017b). Bat coronaviruses are predominantly excreted in faecal material, which creates opportunities for exposure to other individuals and species, and facilitates easy transmission throughout bat populations. Bat coronaviruses have also been reported at low frequencies in oral swabs, urine (Mendenhall et al., 2017) and tissues such as rectum and intestine (Geldenhuys et al, 2018), lung (Shehata et al., 2016), spleen and brain (Anindita et al., 2015).
Coronavirus surveillance studies in African countries have been mostly opportunistic and focused on the detection of coronavirus RNA (Tables 2 and 3). These studies reported highly diverse and novel coronavirus species, as well as a number of coronaviruses related to known human coronaviruses. Viral nucleic acids related to HCoV229E (Duvinacovirus subgenus) have been reported from several species within the roundleaf bat (Hipposideros) genus (Fig. 1a) (Pfefferle et al., 2009; Maganga et al., 2014; Corman et al., 2015; Waruhiu et al., 2017; Anthony et al., 2017b; Bourgarel et al., 2018). Genetic comparisons of similar coronaviruses of bats, alpacas and camels to HCoV229E have suggested that camelids may historically have acted as intermediate (Table 1) hosts during the emergence of HCoV229E (Corman et al., 2015). Relatives of HCoVNL63 (Setracovirus subgenus) have thus far only been identified from Trident bat species (Triaenops) (Fig. 1b) (Tao et al., 2017; Anthony et al., 2017b). Interestingly, it has been suggested that HCoVNL63 may have risen from a recombinant progenitor, created by recombination of separate clades of bat‐borne coronaviruses, similar to both 229E‐related and NL63‐related viruses (Tao et al., 2017).
Table 2.
Nucleic acid evidence of potential zoonotic viruses in bats in Africa
| Genus | Virus | Bat species (diet/roost)a | Geographical location of detections in bats | |
|---|---|---|---|---|
| Coronaviridae | Alphacoronavirus | Duvinacovirus subgenus | Hipposideros sp. (I/C‐RC‐AS), Hipposideros abae (I/C‐RC‐AS), Hipposideros caffer (I/C‐RC), Hipposideros cf. ruber (I/C‐RC‐AS), Hipposideros vittatus c (I/C‐AS) | Cameroon, Gabon, Ghana, Kenya, Republic of Congo, Uganda, Zimbabwe |
| Setracovirus subgenus | Triaenops sp.(I/T), Triaenops afer (I/T) | Kenya, Republic of Congo, Tanzania | ||
| Betacoronavirus | Sarbecovirus subgenus | Chaerephon sp. (I/RC‐AS), Rhinolophus sp. (I/C‐RC), Rhinolophus clivosus (I/C‐RC), Rhinolophus hildebrandtii (I/C‐HT) | Kenya, Rwanda, Uganda | |
| Merbecovirus subgenus | Neoromicia capensis (I/RC‐LB‐AS), Pipistrellus hesperidus (I/HT‐LB) | South Africa, Uganda | ||
| Filoviridae | Ebolavirus | Bombali ebolavirus | Chaerephon pumillus (I/RC‐AS), Mops condylurus (I/HT‐RC‐AS) | Sierra Leone |
| Zaire ebolavirusb | Epomops franqueti (F/T), Hypsignathus monstrosus (F/T), Miniopterus cf inflatus d (I/C), Myonycteris torquata (F/T) | Gabon, Liberia, Republic of Congo | ||
| Marburgvirus | Marburg marburgvirusb | Hipposideros spp. (I/C‐RC‐AS), H. monstrosus (F/T), M. cf inflatus d (I/C), Rhinolophus eloquens (I/C), Rousettus aegyptiacus (F/C) | Democratic Republic of Congo, Gabon, Kenya, South Africa, Uganda | |
| Paramyxoviridae | Henipavirus | Henipavirus‐related | Eidolon helvum (F/T), Epomophorus gambianus (F/T), H. monstrosus (F/T), M. torquata (F/T), R. aegyptiacus (F/C), | Central African Republic, Democratic Republic of Congo, Gabon, Ghana, Kenya, Rwanda, South Africa, Tanzania, Uganda, Zambia |
| Orthorubulavirus | Bat mumps orthorubulavirus | Epomophorus sp. (F/T) | Democratic Republic of Congo | |
| Mumps virus‐related viruses | E. helvum (F/T), Megaloglossus woermanni (F/T), R. aegyptiacus (F/C) | Gabon, Ghana, Republic of Congo, South Africa | ||
| Human orthorubulavirus‐related viruses | H. caffer (I/C‐AS), Hipposideros gigas c (I/C), M. cf inflatus d (I/C), R. aegyptiacus (F/C) | Gabon, Kenya, South Africa | ||
| Pararubulavirus | Sosuga pararubulavirusb | R. aegyptiacus (F/C) | Uganda | |
| Achimota pararubulaviruses | E. helvum (F/T) | Ghana | ||
| Rhabdoviridae | Lyssavirus | Duvenhage virusb | Nycteris thebaica (I/C‐RC‐AS) | Kenya, South Africa, Zimbabwe |
| Lagos bat virus | E. helvum (F/T), Epomophorus wahlbergi (F/T), Micropteropus pusillus (F/T), Nycteris gambiensis (I/C), R. aegyptiacus (F/C) | Central African Republic, Ghana, Kenya, Nigeria, Senegal, South Africa; Imported to France from an unknown location in Africa | ||
| Shimoni bat virus | H. vittatus c (I/C‐AS) | Kenya |
Coronaviridae: (Pfefferle et al., 2009; Tong et al., 2009; Maganga et al., 2014; Corman et al., 2015; Tao et al., 2017; Waruhiu et al., 2017; Anthony et al., 2017a; Bourgarel et al., 2018; Markotter et al., 2019); Filoviridae: (Leroy et al., 2005; Swanepoel et al., 2007; Pourrut et al., 2009; Towner et al., 2009; Kuzmin et al., 2010; Hayman et al., 2010, 2012a; Amman et al., 2012; Goldstein et al., 2018; Paweska et al., 2018); Paramyxoviridae: (Hayman et al., 2008a, 2011; Drexler et al., 2009, 2012; Baker et al., 2012, 2013; Weiss et al., 2012; Peel et al., 2013; Conrardy et al., 2014; Muleya et al., 2014; Amman et al., 2015a; Mortlock et al., 2015; Markotter et al., 2019, Mortlock, 2019); Rhabdoviridae: (Boulger & Porterfield, 1958; Sureau et al., 1977; Foggin, 1988; Swanepoel et al., 1993; Aubert, 1999; Kuzmin et al., 2008, 2010, 2011; Markotter et al., 2008; Hayman et al., 2008b, 2010, 2012b;Freuling et al., 2015).
Diet presented as F, frugivorous or I, insectivorous; roost presented as C, cave; T, tree; AS, artificial structures; LB, behind loose bark on trees; HT, hollow trees; CW, cliff walls; RC, rock crevices.
Viruses associated with human disease.
Hipposideros vittatus classification has changed to Macronycteris vittatus; Hipposideros gigas classification has changed to Macronycteris gigas (Foley et al., 2017).
Evidence exists that Miniopterus inflatus is not one species throughout its distribution, and this distribution map will change with more scientific evidence published.
Table 3.
Serological evidence of potential zoonotic viruses in bats in Africa
| Genus | Virus | Bat species (diet/roost)a | Geographical location | |
|---|---|---|---|---|
| Coronaviridae | Alphacoronavirus | SARS‐CoV | Hypsignathus monstrosus (F/T), Lyssonycteris angolensis (F/C‐HT), Miniopterus cf inflatus c (I/C), Mops condylurus (I/HT‐RC‐AS), Myonycteris torquata (F/T), Rhinolophus fumigatus (I/C), Rousettus aegyptiacus (F/C) | Democratic Republic of Congo, South Africa |
| Betacoronavirus | MERS‐CoV | None investigated | ‐ | |
| Filoviridae | Ebolavirus | Zaire ebolavirusb | Eidolon helvum (F/T), Epomophorus gambianus (F/T), Epomops franqueti (F/T), H. monstrosus (F/T), Micropteropus pusillus (F/T), M. condylurus (I/RC‐T‐AS), M. torquata (F/T), R. aegyptiacus (F/C) | Democratic Republic of Congo, Gabon, Ghana |
| Marburgvirus | Marburg marburgvirusb | E. franqueti (F/T), H. monstrosus (F/T), M. cf inflatus c (I/C), Rhinolophus eloquens (I/C), R. aegyptiacus (F/C) | Democratic Republic of Congo, Gabon, Kenya, South Africa, Uganda | |
| Paramyxoviridae | Henipavirus | Henipavirus‐related | E. helvum (F/T) | Cameroon, Equatorial Guinea, Ghana, Malawi, Tanzania, Uganda, Zambia |
| Pararubulavirus | Achimota pararubulaviruses | E. helvum (F/T) | Ghana, Tanzania | |
| Rhabdoviridae | Lyssavirus | Duvenhage virusb | Nycteris thebaica (I/C) | Swaziland |
| Lagos bat virus | E. helvum (F/T), E. gambianus (F/T), Epomophorus wahlbergi (F/T), Epomops buettikoferi (F/T), H. monstrosus (F/T), Hipposideros vittatus d (I/C‐AS), Miniopterus sp. (I/C), M. torquata (F/T), R. aegyptiacus (F/C) | Democratic Republic of Congo, Ghana, Kenya, Nigeria | ||
| Mokola viruse | E. helvum (F/T) | Nigeria | ||
| Shimoni bat virus | Chaerephon pumila (I/HT‐RC‐AS), E. helvum (F/T), E. wahlbergi (F/T), Miniopterus sp. (I/C), Pipistrellus sp. (I/HT‐LB), R. aegyptiacus (F/C) | Democratic Republic of Congo, Kenya, Nigeria | ||
| West Caucasian bat virus | Miniopterus sp. (I/C) | Kenya |
Coronaviridae : (Müller et al., 2007); Filoviridae: (Leroy et al., 2005; Swanepoel et al., 2007; Pourrut et al., 2009; Hayman et al., 2011; Amman et al., 2012; Hayman et al., 2011); Pa ramyxoviridae : (Hayman et al., 2010; Baker et al., 2012; Pernet et al., 2014); Rhabdoviridae : (Hayman et al., 2010, 2011, Kuzmin et al., 2008, 2011; Dzikwi et al., 2010; Wright et al., 2010; Markotter, Monadjem & Nel, 2013; Kia et al., 2014; Freuling et al., 2015; Kalemba et al., 2017).
Diet presented as F, frugivorous or I, insectivorous; roost presented as C, cave; T, tree; AS, artificial structures; LB, behind loose bark on trees; HT, hollow trees; CW, cliff walls; RC, rock crevices.
Viruses associated with human disease.
Evidence exists that Miniopterus inflatus is not one species throughout its distribution, and this distribution map will change with more scientific evidence published.
Hipposideros vittatus classification has changed to Macronycteris vittatus (Foley et al., 2017).
Cross neutralization reported for Mokola and Lagos bat virus (Kuzmin et al., 2008).
Figure 1.
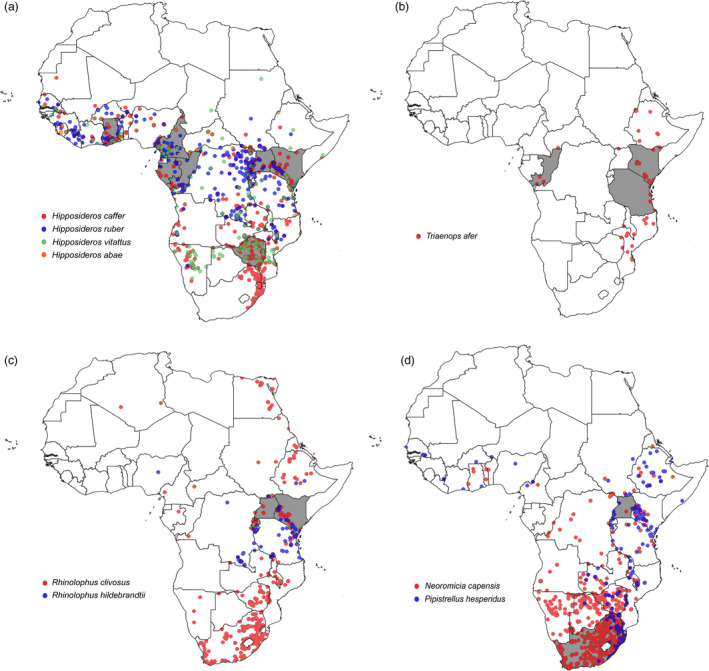
Distribution of bat species (ACR, 2018) associated with coronaviruses. (a) Species distribution of hosts associated with coronaviruses related to human alphacoronavirus 229E (Duvinacovirus subgenus) is indicated by coloured circles: Hipposideros caffer (red), Hipposideros ruber (blue), Hipposideros abae (purple) and Hipposideros vitattus that was recently changed to Myonycteris vitattus (green). Detection of coronavirus RNA related to HCoV229E was reported from countries shown by grey shading (Ghana, Gabon, Uganda, Cameroon, Kenya, Republic of Congo and Zimbabwe). (b) Distribution of bat species (ACR, 2018) associated with coronaviruses related to human alphacoronavirus NL63 (Setracovirus subgenus). The species distribution of Triaenops afer is indicated by red coloured circles. Detection of coronavirus RNA related to HCoVNL63 was reported from countries shown by grey shading (Kenya, Republic of Congo and Tanzania). (c) Distribution of bat species (ACR, 2018) associated with SARS‐related coronaviruses (Sarbecovirus subgenus). Species distribution is indicated by coloured circles: Rhinolophus clivosus (red) and Rhinolophus hildebrandtii (blue). Detection of SARS‐related coronavirus RNA was reported from countries shown by grey shading (Rwanda, Uganda and Kenya). (d) Distribution of bat species (ACR, 2018) associated with MERS‐related coronaviruses (Merbecovirus subgenus). Species distribution is indicated by coloured circles; Neoromicia capensis (red) and Pipistrellus hesperidus (blue). Detection of MERS‐related coronavirus RNA has been reported from countries shown by grey shading (South Africa and Uganda) (QGIS 3. 6. 3‐Noosa).
Viral sequences sharing similarity to SARS (Sarbecovirus subgenus) have been reported from horseshoe bats collected in Rwanda and Uganda (Anthony et al., 2017b; Markotter et al., 2019) (Fig. 1c), as well as from the free‐tailed bat (Chaerephon) genus in Kenya (Tong et al., 2009). Due to the strong genus specificity of SARS‐related viruses to horseshoe bats, detection from a free‐tailed bat likely represents a transient spillover event. Viruses from the Hibecovirus subgenus are genetically similar to the Sarbecovirus subgenus (SARS and related viruses), though are sufficiently divergent to constitute an assignment to a separate subgenus. Hibecoviruses have predominantly been detected in roundleaf bats (Hipposideros) from Nigeria, Kenya, Ghana and Zimbabwe (Pfefferle et al., 2009; Quan et al., 2010; Waruhiu et al., 2017; Bourgarel et al., 2018).
MERS‐related coronaviruses (Merbecovirus subgenus) have been found in a larger number of diverse bat genera, including even genera from different bat families (Annan et al., 2013; Memish et al., 2013b). Studies from Africa have reported bat‐borne MERS‐related viruses from the Vespertilionidae family that are currently the most closely related to human and camel MERS viruses. The viruses have been identified from the Cape serotine bat (Neoromicia capensis) in South Africa and the African pipistrelle bat (Pipistrellus hesperidus) in Uganda (Fig. 1d) (Ithete et al., 2013; Anthony et al., 2017a; Geldenhuys et al., 2018). Though surveillance for MERS has not been extensively performed in other species of these genera, host genus specificity would suggest that similar viruses may be circulating (Geldenhuys et al., 2018). Indeed, Pipistrellus (P. pipistrellus, P. nathusii and P. pygmaeus) populations in various European countries (Netherlands, Romania and Ukraine) confirm circulation of MERS‐related viruses within this genus even in distant geographical regions (Annan et al., 2013). These studies represent initial investigations identifying hosts of MERS‐related viruses, but significant further research is required to improve the understanding of the emergence of MERS coronaviruses.
Coronavirus surveillance of African bats has made progress in identifying both viral diversity and host species, in addition to better understanding the evolution and possible historical emergence of human coronaviruses such as HCoV229E and HCoVNL63. However, African bat coronavirus surveillance is severely limited in aspects necessary to understanding the epidemiology and ecology of bat coronaviruses and also the potential of spillover, as no outbreaks directly linked to bats have been reported on the African continent.
Filoviruses
Viruses in the Filoviridae family are non‐segmented, negative‐sense, single‐stranded RNA viruses represented by five genera of which three infect mammals: Ebolavirus, Marburgvirus and Cuevavirus (Kuhn et al., 2019). Lloviu virus (LLOV), Cuevavirus genus, has only been detected in common bent‐wing bats in Spain and Hungary (Kemenesi et al., 2018) and has not been associated with human disease, but members of the Ebolavirus and Marburgvirus genera cause life‐threatening haemorrhagic fevers. Recently, the diversity of filoviruses has expanded with new unclassified viruses being described from bats namely Mengla virus in rousette bats (Rousettus spp.) and cave nectar bats (Eonycteris spp.) from China (Yang et al., 2019), as well as Bombali virus from Sierra Leone and Kenya associated with the Angolan free‐tailed bat (Mops condylurus) and the little free‐tailed bat (Chaerephon pumillus) (Goldstein et al., 2018; Forbes et al., 2019).
The first haemorrhagic fever outbreak was reported in the late summer of 1967 in laboratory workers in Germany and Yugoslavia (now Serbia) transmitted from green monkeys (Chlorocebus sabaeus) consigned from Uganda to Europe. This new virus, Marburg virus (MARV), has since been documented in various parts of Africa (Fig. 2a) (Olival & Hayman, 2014) and causes disease in both humans and nonhuman primates. The two most significant human outbreaks were reported from the Democratic Republic of Congo (DRC) in 1998–2000 and Angola in 2004–2005 with 128 and 227 human fatalities (Brauburger et al., 2012). Although circumstantial evidence linked human outbreaks to bats, the reservoir and epidemiology of MARV remained undetermined for more than two decades after its discovery. In 1999, MARV RNA was detected in bats for the first time, including the Egyptian rousette bats, eloquent horseshoe bats (Rhinolophus eloquens) and a greater long‐fingered bat (Miniopterus cf. inflatus) captured in DRC (Swanepoel et al., 2007). In the following years, MARV was detected and isolated from Egyptian rousette bats in Eastern Africa (Towner et al., 2009) with more recent detections also from the northern regions of South Africa (Paweska et al., 2018). The Egyptian rousette bat has been confirmed as a reservoir of the Marburgvirus genus and does not show signs of disease (Fig. 2a; Tables 2 and 3) (Towner et al., 2009; Amman et al., 2012). Birthing periods are reported to be a driver for MARV infection (Amman et al, 2012) with distinct viral pulses in juvenile bats of approximately six months old, coinciding with biannual bat birthing seasons in Uganda as well as the timing of human infections. It is not clear how the virus first transmits from bats to humans, but outbreaks have been linked to gold mining activities and entering of caves with potential contact with faecal excretions or aerosols.
Figure 2.
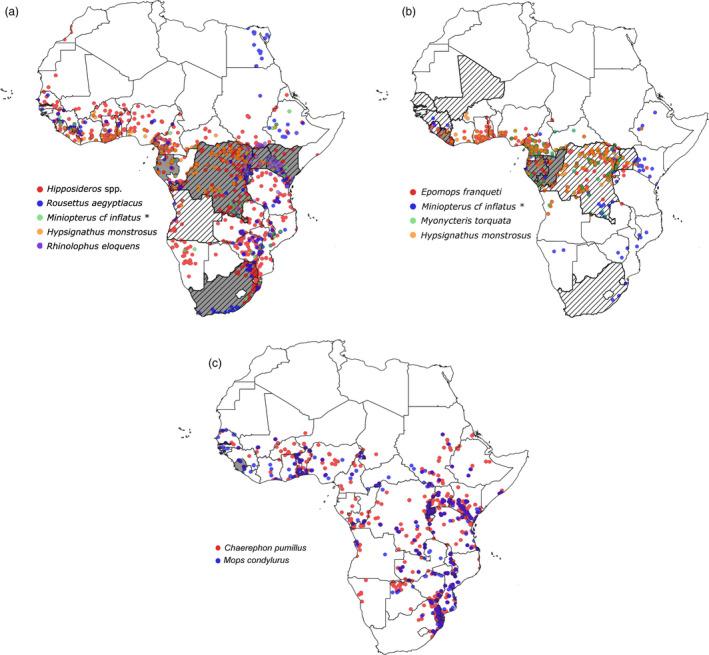
Distribution of bat species associated with filovirus species in Africa (ACR, 2018). (a) Marburg virus; (b) Ebolavirus; and (c) Bombali virus. Coloured dots represent the geographical distribution of bat species in which a filovirus RNA was detected. Countries where viral RNA was detected in bats are indicated in grey, and where human cases were identified, countries are indicated by diagonal lines. *Evidence exists that Miniopterus inflatus is not one species throughout its distribution, and this distribution map will change with more scientific evidence published (QGIS 3. 6. 3‐Noosa).
Ebolavirus was discovered in 1976 when two consecutive outbreaks of a fatal haemorrhagic fever occurred in the Democratic Republic of Congo (previously Zaire) near the Ebola river and South Sudan (Olival & Hayman, 2014). The Ebolavirus genus now consists of five species representing Ebolavirus (EBOV) (previously Ebola Zaire), Bundibugyo virus (BDBV), Sudan virus (SUDV), Taï Forest virus (TAFV) and Reston virus (RESTV) (Kuhn et al., 2019). Three of the ebolavirus species, EBOV, BDBV and SUDV, are highly lethal in people with more than 25 human outbreaks reported since 1976 and case fatality rates of 25‐90%. Most outbreaks are caused by EBOV (Tables 2 and 3, Fig. 2b) with the most significant one during 2013‐2016 in Guinea, Sierra Leone and Liberia with more than 11 000 fatalities (Spengler et al., 2016). Index cases were most often in direct contact with ebolavirus‐infected primates or other large vertebrates, either killed or found dead in forested areas. However, these animals also succumb to disease and are therefore not considered the reservoir species. Investigations into possible reservoir hosts for EBOV (including birds and small terrestrial vertebrates) indicated the presence of EBOV RNA only in three fruit bat species: hammer‐headed bat (Hypsignathus monstrosus), Franquet's epauletted fruit bat (Epomops franqueti) and the little collared fruit bat (Myonycteris torquata) in Gabon and DRC (Leroy et al., 2005) (Tables 2 and 3, Fig. 2b). Other studies reported circumstantial evidence, for example, the proposed index case in the 2016 Ebolavirus outbreak (a 2‐year‐old boy from the small village of Meliandou, Guinea) may have been infected while playing in a hollow tree used as a roost by a colony of Angolan free‐tailed bats (Marí Saéz et al., 2015). However, this was not substantiated by subsequent field and virological investigations. Experimental infection studies of EBOV in plants and animals revealed that three bat species, that is the Angolan free‐tail (Tadarida condylura, now M. condylurus), the little free‐tail (T. pumila, now C. pumila) and the epauletted fruit bats, support EBOV replication with seroconversion and no disease manifestation (Swanepoel et al., 1996). Numerous other studies have reported on the presence of anti‐ebolavirus antibodies in several bat species in Africa as well as other continents, including Asia and Europe (Olival & Hayman, 2014) (Tables 2 and 3). In search of the reservoir species, a recent detection of EBOV RNA in the greater long‐fingered bat (Miniopterus cf. inflatus) in Liberia (Epstein personal communication) was reported.
Although filovirus outbreaks are rare, with transmission likely an uncommon event due to restricted contact occurring between susceptible humans and reservoir hosts, it has been estimated that more than 40% of Ebolavirus spillover events may remain undetected (Glennon et al., 2019). Transmission may occur from bats to humans by direct contact between mucous membranes or open wounds with excreta, bites and consumption of or contact with fruit and other objects contaminated with bat excreta (Swanepoel et al., 1996; Leroy et al., 2005; Amman et al., 2015b). Human‐to‐human transmission chains then follow and generally occur through direct contact with the body fluids of infected patients or remain during traditional burial practices (Roddy et al., 2010) or contaminated objects. The distribution of filoviruses in bats is more widespread than the occurrence of human outbreaks (Tables 2 and 3, Fig. 2a–c), and the epidemiology, reservoir species and factors leading to spillover are poorly understood.
Paramyxoviruses
Viruses in the family Paramyxoviridae have single‐stranded negative‐sense RNA genomes, which are non‐segmented and capable of infecting a wide host range including humans, nonhuman primates, avians, reptiles and fish (Virtue, Marsh & Wang, 2009). Well‐known human and animal paramyxoviruses include measles, mumps, Newcastle disease and canine distemper virus, and they are mostly associated with central nervous system and respiratory infections. The taxonomy of this viral family has seen several changes over the past decade (Afonso et al., 2016). More recently, as a result of numerous surveillance studies in a several hosts including bats, the viral family was divided into four subfamilies (Avulavirinae, Rubulavirinae, Orthoparamyxovirinae and Metaparamyxovirinae) now with 14 genera to accommodate the growing diversity (ICTV, 2019). However, a large number of putative paramyxoviruses recently described still require full genome characterization and could potentially represent novel genera within the family.
It was not until the 1990s that paramyxoviruses were recognized as important zoonotic pathogens. Two novel henipaviruses, Hendra and Nipah virus (Henipavirus genus, Orthoparamyxovirinae subfamily), emerged from bats in the genus Pteropus, that is flying foxes from Australia and South‐East Asia, respectively (Murray et al., 1995; Chua et al., 1999, 2002; Halpin et al., 2000). These viruses were associated with neurological and respiratory disease in their intermediate spillover hosts (horses and pigs, respectively) and subsequently humans. The henipaviruses are of considerable public and veterinary health importance given the associated high morbidity and mortality rates in humans and their animal hosts, as well as the lack of effective post‐exposure treatment or availability of a human vaccine as a preventative measure. Nipah virus, in particular, has a case fatality rate ranging from 38% to 100% in particular outbreaks (Ang, Lim & Wang, 2018). In addition, this virus has reportedly been transmitted directly from bats to humans with subsequent human‐to‐human transmission in near‐annual Bangladesh outbreaks (Islam et al., 2016). The geographical distribution of Nipah virus outbreaks has expanded in the last few years with reports from South India where an 88.9% mortality rate was reported for the 2018 outbreak (Sadanadan et al., 2018). These henipaviruses are excreted in bat urine (Smith et al., 2011), which is believed to be the route of transmission to humans and other susceptible animals. Additionally, viral detection and isolation have been reported from excreted uterine fluid during the bat reproductive season (Halpin et al., 2000), as well as from partially eaten fruit dropped to the ground presenting additional routes of potential virus transmission (Chua et al., 2002).
Initially, the distribution of henipaviruses was believed to be restricted to the geographical distribution of the flying foxes (Pteropus spp.) in Australia and South‐East Asia. However, serological surveillance for henipaviruses in the African straw‐coloured fruit bat from Ghana (Tables 2 and 3) provided the first evidence of Henipavirus‐related viruses on the African continent (Hayman et al., 2010). This was supported by the detection of viral RNA in faecal material from the same species (Drexler et al., 2009). Since these reports, numerous other surveillance studies were conducted on a number of African bat species resulting in the detection of a diversity of paramyxovirus sequences in both insectivorous and frugivorous bats. In addition, potential zoonotic paramyxoviruses closely related to known human pathogens have been reported mainly from frugivorous bat species (Drexler et al., 2009, 2012; Baker et al., 2012; Weiss et al., 2012; Conrardy et al., 2014; Muleya et al., 2014; Mortlock et al., 2015, 2019) including Henipavirus‐related viruses (Fig. 3a). Since viruses within the Henipavirus genus are of zoonotic importance, initial surveillance studies were focused on the African straw‐coloured fruit bat. Subsequently, other fruit bat species including the Egyptian rousette bat, little collared fruit bat (Myonycteris torquata), Gambian epauletted fruit bat (Epomophorus gambianus) and hammer‐headed bat (Hypsignathus monstrosus) also tested positive for henipavirus‐related RNA (Tables 2 and 3).
Figure 3.
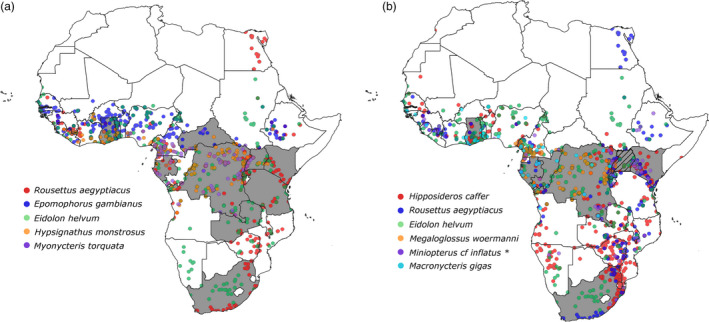
Distribution of bat species (ACR, 2018) identified as natural hosts of (a) Henipavirus and related viruses and (b) Orthorubula- and Pararubulavirus and related viruses. An Epomophorus bat not classified to species level was also linked to the Orthorubulavirus genus – distribution not shown. Host species represented were selected based on nucleic acid detection of paramyxovirus RNA. Coloured dots represent the geographical distribution of bat species across the continent. Countries where viral RNA was detected in bats are indicated in grey, and where human cases were identified, countries are indicated by diagonal lines. *Evidence exists that Miniopterus inflatus is not one species throughout its distribution, and this distribution map will change with more scientific evidence published (QGIS 3. 6. 3‐Noosa).
Detection of viruses related to human‐specific paramyxoviruses species has also been reported. These include viral sequences related to Mumps orthorubulavirus (human mumps virus) as well as Human orthorubulaviruses 2 and 4 (human parainfluenza viruses 2 and 4) (Orthorubulavirus genus, Rubulavirinae subfamily) (Drexler et al., 2012; Conrardy et al., 2014; Mortlock et al., 2019). A bat‐borne virus conspecific to human mumps virus was detected through surveillance in an epauletted fruit bat belonging to the Epomophorus genus sampled in the Democratic Republic of Congo (Drexler et al., 2012), that is bat mumps orthorubulavirus (Amarasinghe et al., 2017). Viral sequences in this bat mumps viral clade have since been detected in other fruit bat species (Tables 2 and 3) (Baker et al., 2012; Drexler et al., 2012; Mortlock et al., 2019). Concurrently, the Egyptian rousette bat, the giant roundleaf bat (Macronycteris gigas, previously Hipposideros gigas), Sundevall's roundleaf bat (Hipposideros caffer) and the greater long‐fingered bat (M. cf inflatus) have been associated with viral sequences closely related to the human parainfluenza viruses. These four bat species are cave‐dwelling, and global analysis indicated a higher likelihood for viral sharing when compared to non‐cave roosting bats (Willoughby et al., 2017).
To date, there has only been a single confirmed human infection with a zoonotic paramyxovirus reported on the African continent (Fig. 3b). Sosuga virus (Pararubulavirus genus, Rubulavirinae subfamily) was found to be the aetiological agent responsible for a case of a severe non‐fatal febrile disease in a field biologist (Albariño et al., 2014) conducting sampling in bats and rodents in South Sudan and Uganda in the weeks prior to disease onset. Although several bat species with which the field biologist came into contact with during the expedition (including Egyptian rousette bats, the Ethiopian epauletted fruit bat (Epomophorus labiatus), Angolan fruit bat (Lissonycteris angolensis) and hipposiderid bats) were tested for Sosuga virus RNA, it was only detected in the Ugandan Egyptian rousette bats (Amman et al., 2015a). Viral RNA was subsequently detected in archival samples collected from this species and country as far back as 2009, indicating that the virus had been present in these populations for several years prior to emergence. As such, it is believed that the Egyptian rousette bat is the most likely source from which spillover occurred.
Although the zoonotic potential of these African bat‐borne paramyxoviruses is unknown, henipavirus‐related antibodies have been detected in pigs (Hayman et al., 2011) and Cameroonian locals (Pernet et al., 2014), highlighting the potential of livestock as intermediate hosts and spillover into animal and human populations. The study reporting on serological evidence of henipaviruses in the Cameroonian locals found that contact with bats, butchering of bats and deforestation were significant risk factors for exposure to these viruses (Pernet et al., 2014). Various research studies, mostly targeted towards Hendra and Nipah virus, have reported virus dynamics and possible drivers of disease emergence. These factors and viral dynamics, however, may be different for African bat populations.
Zoonotic paramyxoviruses of considerable public health importance have to date only been associated with frugivorous bat species in Australia and South‐East Asia. The only zoonotic association in Africa has been the isolated case of Sosuga virus for which the Egyptian rousette bat has been implicated. However, although there has not yet been an association between insectivorous bat‐borne paramyxoviruses and zoonotic events, there is still a vast diversity of paramyxoviruses associated with these bat hosts on the African continent (Drexler et al., 2012; Conrardy et al., 2014; Mortlock et al., 2015; Bourgarel et al., 2018). In the pool of this vast diversity of viruses, there may be species that have the necessary mutations to be able to infect other hosts and lead to the emergence of zoonotic viruses (Willoughby et al., 2017). Nonetheless, when considering the zoonotic henipaviruses and related viral sequences, they have exclusively been detected in frugivorous bat species warranting caution when encountering these bats.
Lyssaviruses
These negative‐sense, single‐stranded RNA viruses have been classified in the virus order Mononegavirales, family Rhabdoviridae and genus Lyssavirus, are adapted to replicate in the mammalian central nervous system and cause the disease rabies. Transmission occurs through direct contact with infected saliva on open wounds or mucosal membranes with a bite from an infected animal as the most common introduction (Banyard & Fooks, 2017). Currently, the genus consists of 16 officially recognized viral species: the type species Rabies lyssavirus and the rabies‐related lyssaviruses [Aravan lyssavirus, Australian bat lyssavirus, Bokeloh bat lyssavirus, Duvenhage lyssavirus, European bat 1 lyssavirus, European bat 2 lyssavirus, Gannoruwa bat lyssavirus, Ikoma lyssavirus, Irkut lyssavirus, Khujand lyssavirus, Lagos bat lyssavirus, Lleida bat lyssavirus, Mokola lyssavirus, Shimoni bat lyssavirus and West Caucasian bat lyssavirus with one additional putative virus; Taiwan bat lyssavirus (Hu et al., 2018; Amarasinghe et al., 2019)]. Rabies virus (RABV) occurs worldwide and has the broadest reported host range of all the lyssaviruses infecting multiple mammalian orders including carnivores and bats. Most human rabies deaths, estimated to be ca. 59 000/annum, are due to exposure to rabid dogs and rabies virus infection. The predominant reservoir of all the other lyssaviruses (rabies‐related lyssaviruses) is Old World bats with RABV only associated with bats in the New World (Markotter & Coertse, 2018). Six lyssavirus species have been described from Africa: RABV that is only associated with terrestrial mammals, Lagos bat lyssavirus (LBV), Duvenhage lyssavirus (DUVV) and Shimoni bat lyssavirus (SHIBV) that are associated with various bat species (Fig. 4) and Mokola lyssavirus (MOKV) and Ikoma lyssavirus (IKOV) with an unknown reservoir host that has only been reported in spillover hosts (Markotter & Coertse, 2018).
Figure 4.
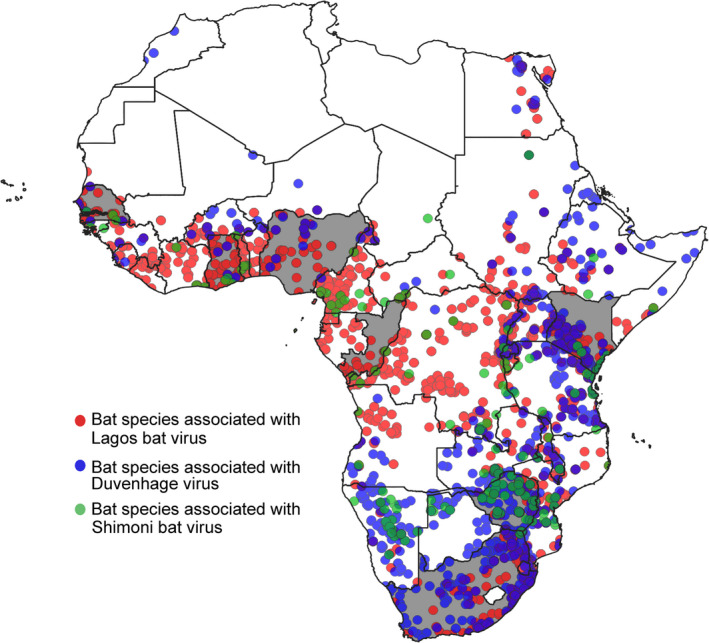
Geographical distribution of bat species (ACR, 2018), associated with lyssaviruses in Africa. Bat species associated with different lyssaviruses are shaded in different colours, that is Lagos bat lyssavirus (Eidolon helvum, Epomophorus wahlbergi and Rousettus aegyptiacus) are indicated in red, Duvenhage lyssavirus (Nycteris thebaica) indicated in green and Shimoni bat lyssavirus (Macronycteris vittatus previously Hipposideros vittatus) indicated in blue. Countries where viral RNA was detected in bats are indicated in grey, and where human cases were identified, countries are indicated by diagonal lines (QGIS 3. 6. 3‐Noosa).
The first rabies‐related lyssavirus, LBV, was described in 1956 from African straw‐coloured fruit bats on Lagos Island in Nigeria (Boulger & Porterfield, 1958; Shope et al., 1970). Since then, LBV has been reported sporadically in various African countries (Fig. 4) including South Africa, Nigeria, Central African Republic, Guinea, Senegal, Zimbabwe, Ethiopia, Kenya and Ghana (Kuzmin et al., 2008, 2010; Markotter et al., 2008; Freuling et al., 2015). Isolations of LBV have mainly been associated with bat species in the Pteropodidae family, namely the African straw‐coloured fruit bat, Wahlberg's epauletted fruit bat (Epomophorus wahlbergi) and Egyptian rousette bats with single isolates reported from Peters's dwarf epauletted fruit bat (Micropterus pusillus) and the insectivorous Gambian slit‐faced bat (Nycteris gambiensis) (Swanepoel et al., 1993). Spillover infections in domestic animals (cats and dogs) were first recognized several decades ago, though <10 cases have been reported to date (Markotter & Coertse, 2018). A single spillover infection was also reported in the water mongoose (Atilax paludinosus) (Markotter et al., 2006). No human infections have been documented.
In 1970, a rabies‐related lyssavirus was described from a fatal human rabies case in South Africa. The virus was named Duvenhage virus (DUVV) after this patient (Meredith, Rossouw & Van Praag Koch, 1971). The bat involved in this specific case was never positively identified, however, due to the proximity of the human case to a maternal colony of Natal long‐fingered bats (M. natalensis previously M. schreibersii) and abundance of the bat in the area, this species was implicated based on circumstantial evidence (Van Der Merwe, 1982). Duvenhage virus was only detected again more than a decade later in 1981, from an unidentified bat that was caught by a cat in South Africa (Van Der Merwe, 1982). This was followed by virus isolations from the Egyptian slit‐faced bat (Nycteris thebaica) in 1986 from Zimbabwe (Foggin, 1988) and in 2012 from the same species in South Africa (Markotter & Coertse, 2018). An additional two human cases have been reported. In 2006, a man was scratched in the face by an unidentified bat and developed rabies (Paweska et al., 2006). The last human case, and first DUVV case outside of southern Africa, was reported in 2007 (Fig. 4). A Dutch woman developed rabies after receiving two small superficial wounds on the face from an unidentified bat while on holiday in Kenya during 2007 (Van Thiel et al., 2009). With limited detections of DUVV, and laboratory confirmation of infection reported in only the Egyptian slit‐faced bat, it is not currently possible to make definite conclusions about the potential reservoir bat species.
Only a single isolate of SHIBV has been described to date from the striped leaf‐nosed bat (Macronycteris vittatus previously Hipposideros vittatus) after a partly decomposed bat was found in a cave in the coastal region of Kenya in 2009 (Kuzmin et al., 2010) (Fig. 4). Following this detection, a serosurvey in Kenya indicated that the striped leaf‐nosed bat is the likely reservoir host for SHIBV (Kuzmin et al., 2011). Mokola virus and IKOV have not yet been linked to bats, and the potential reservoir species is unknown. Mokola virus was first isolated from shrews (Crocidura flavescens manni) in Nigeria in 1968 (Shope et al., 1970), and since then, the virus has been isolated from a variety of different mammalian species including cats, dogs and a rodent from various African countries including Cameroon, Central African Republic, Ethiopia, South Africa and Zimbabwe (Kgaladi et al., 2013; Coertse et al., 2016) and implicated in two human fatalities in Nigeria. Serological evidence of MOKV exposure in bats has been reported, but it should be noted that cross‐reaction between lyssavirus species occurs (Wright et al., 2010; Markotter & Coertse, 2018), and no MOKV nucleic acids or isolations have been reported from bats. Ikoma lyssavirus was reported for the first and only time from a spillover infection in an African civet (Civettictis civetta), from an unknown reservoir host, after it attacked a child in Tanzania in 2009 (Marston et al., 2012).
Most bats are resilient to lyssavirus infection without the development of fatal disease, leading to the hypothesis that during such infections an appropriate immune response will develop resulting in viral clearance with no viral transmission (Kuzmin & Rupprecht, 2015). As such, significant antibody prevalence in bat populations is attributed to abortive peripheral infection, also referred to as exposure (Kuzmin & Rupprecht, 2015) corresponding with limited nucleic acid detection (<1%) (Markotter & Coertse, 2018). Limited human infections due to rabies‐related lyssaviruses (eleven) have been reported, but experimental studies indicated that these viruses are as pathogenic as RABV. Existing rabies vaccines, all based on RABV strains, may not provide protection against more divergent lyssavirus species such as LBV and SHIBV (Banyard & Fooks, 2017).
Concluding remarks
A high diversity of viruses is reported from bats, and it is challenging to predict zoonotic potential. With a few exceptions, most connections between bat‐borne viruses and diseases have been established based on the detection of the same or similar viruses in bats, with limited evidence supporting their direct roles in the epidemiology of emerging diseases. However, there are also convincing scientific evidence implicating bats in deadly human diseases that cannot be ignored. Although a clear link between bats and the West African Ebola outbreak could not be established, we should not forget the impact of this epidemic that started in 2013 and ended in 2016 with over 11 000 people losing their lives. Not only did the outbreak have an economic impact on the countries where the outbreak occurred, but also an estimated global cost of more than US$32 billion. Similarly, a previous global pandemic caused by SARS coronavirus in 2002 resulted in over 800 human deaths with an economic impact of US$50 billion. The World Bank estimated economic losses from fatal zoonoses between 1997 and 2009 at US$80 billion and appraises that US$6.7 billion per year could be saved globally by preventing emerging disease outbreaks (Mazet et al., 2015).
Historically, rabies was the only disease risk to be considered with route of transmission through contact of infected saliva on broken skin or mucosal membranes, and pre‐ and post‐exposure rabies prophylaxis is available. However, the diversity of the rabies‐related lyssaviruses has expanded and it became clear that the current vaccine may not protect against this viral diversity. As more virus families are identified in bats, additional transmission routes including contact with bat body fluids and excreta (faeces and urine) became apparent and no treatment or prophylaxis for these viruses exists. People who are in contact with bats frequently, including fieldworkers, are at a greater risk of being infected than the general public. It is unknown what pathogens will be present in bats and although the probability of being infected is extremely low, all animals and samples must therefore be considered infectious. In addition should we also recognize that humans can transmit diseases to bats and between environments for example white‐nose syndrome that emerged in North America and devastated the bat populations. Biosafety must be of utmost importance in all fieldwork investigations (Moratelli & Calisher, 2015) and must include pre‐exposure prophylaxis for rabies according to recommendations (Markotter & Coertse, 2018). Fieldworkers must be made aware of the risk involve and a basic medical surveillance programme must be in place, especially to monitor exposures. Bites, scratches and contact with excretions and body fluids must be avoided and are therefore recommended to wear appropriate handling gloves over a double layer of disposable gloves, disposable clothing (surgical gowns, Tyvek coveralls) and rubber boots with shoe coverings. Aerosols are also a potential transmission route, and therefore, a respirator with high‐efficiency particulate filters is the only efficient way to protect against infective particles. Mucous membranes including the eyes must also be covered. Fieldworkers must be properly trained in wearing of personal protective equipment including how to disinfect and remove it safely after activities. All reusable equipment and supplies must also be disinfected with an appropriate disinfectant (e.g. 10% chlorine), and biowaste must be sterilized using appropriate methods before disposal.
As discussed in this review, the detection of a virus in bats does not constitute spillover and several ecological and biological factors can play a role in disease emergence. Seasonality has been associated with paramyxoviruses, where virus excretion has been observed in the dry winter months in lower latitudes (McFarlane, Becker & Field, 2011; Field et al., 2015; McMichael et al., 2017; Paez et al., 2017). Bat densities, nutritional stress and events during the reproductive season of bats (waning maternal antibodies, late pregnancy and breeding) have been found to correspond with peaks in virus shedding (Plowright et al., 2008; Amman et al., 2012; Dietrich et al., 2015; Paez et al., 2017; Paweska et al., 2018; Mortlock et al., 2019). The role that meta‐population dynamics play in pathogen prevalence and viral sharing is also unknown, with very few studies investigating movement patterns of bats (Amman et al., 2012; Fahr et al., 2015; Abedi‐Lartey et al., 2016; Olson et al., 2019). Viral dynamics may additionally vary across host bat species, viral genera or even individual virus species. Environmental and anthropogenic changes act as a major contributor to disease emergence and include activities such as changes in land use, human population growth and increased mobility across landscapes, changes in human socioeconomic behaviour or social structure, trade increase, forest fires, extreme weather events, wars and breakdown in public health infrastructure, to name a few. These activities also result in increased contact with wildlife such as bats, ultimately leading to a higher risk of spillover. Furthermore, correct morphological bat species identification correlated with publically available genetic sequence information that is accurate is becoming essential for species identification. Misinformed host identification may implicate incorrect species but is also important to determine accurate distribution maps and potential geographically risk of pathogens.
Further understanding of the many factors that may play a role in spillover of pathogens from bats to humans will require structured, systematic, longitudinal surveillance of bat populations (Wacharapluesadee et al., 2018) that includes multi‐ and transdisciplinary approaches in addition to virological investigations. It is also essential to involve a multidisciplinary team to develop appropriate management plans to minimize risks of human infection without causing significant harm to the bat populations. We also cannot ignore the critical ecological services that bats provide, that is their participation as fruit pollinators and controlling insect pest populations [reviewed in (Kunz et al., 2011; Kasso & Balakrishnan, 2013; López‐Baucells, Rocha & Fernández‐Llamazares, 2018)]. As such, removing bats from a specific ecosystem can have devastating consequences (Amman et al., 2014). Finally, can we confidently link bats with emerging viruses? In the majority of cases, the answer is no with essential evidence still missing.
Acknowledgements
This work is based on research supported by the South African Research Chair initiative (held by Professor Wanda Markotter) of the Department of Science and Innovation and administered by the National Research Foundation of South Africa (Grant No. 98339).
Editor: Nigel Bennett
References
- Abedi‐Lartey, M. , Dechmann, D.K.N. , Wikelski, M. , Scharf, A.K. & Fahr, J. (2016). Long‐distance seed dispersal by straw‐coloured fruit bats varies by season and landscape. Glob. Eco. Conserv. 7, 12–24. [Google Scholar]
- ACR . (2018). African chiroptera report 2018. Pretoria: AfricanBats NPC. [Google Scholar]
- Afonso, C.L. , Amarasinghe, G.K. , Bányai, K. , Bào, Y. , Basler, C.F. , Bavari, S. , Bejerman, N. , Blasdell, K.R. , Briand, F.‐X. , Briese, T. , Bukreyev, A. , Calisher, C.H. , Chandran, K. , Chéng, J. , Clawson, A.N. , Collins, P.L. , Dietzgen, R.G. , Dolnik, O. , Domier, L.L. , Dürrwald, R. , Dye, J.M. , Easton, A.J. , Ebihara, H. , Farkas, S.L. , Freitas‐Astúa, J. , Formenty, P. , Fouchier, R.A.M. , Fù, Y. , Ghedin, E. , Goodin, M.M. , Hewson, R. , Horie, M. , Hyndman, T.H. , Jiāng, D. , Kitajima, E.W. , Kobinger, G.P. , Kondo, H. , Kurath, G. , Lamb, R.A. , Lenardon, S. , Leroy, E.M. , Li, C.‐X. , Lin, X.‐D. , Liú, L. , Longdon, B. , Marton, S. , Maisner, A. , Mühlberger, E. , Netesov, S.V. , Nowotny, N. , Patterson, J.L. , Payne, S.L. , Paweska, J.T. , Randall, R.E. , Rima, B.K. , Rota, P. , Rubbenstroth, D. , Schwemmle, M. , Shi, M. , Smither, S.J. , Stenglein, M.D. , Stone, D.M. , Takada, A. , Terregino, C. , Tesh, R.B. , Tian, J.‐H. , Tomonaga, K. , Tordo, N. , Towner, J.S. , Vasilakis, N. , Verbeek, M. , Volchkov, V.E. , Wahl‐Jensen, V. , Walsh, J.A. , Walker, P.J. , Wang, D. , Wang, L.‐F. , Wetzel, T. , Whitfield, A.E. , Xiè, J. , Yuen, K.‐Y. , Zhang, Y.‐Z. & Kuhn, J.H. (2016). Taxonomy of the order Mononegavirales: update 2016. Arch. Virol. 161, 2351–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albariño, C.G. , Foltzer, M. , Towner, J.S. , Rowe, L.A. , Campbell, S. , Jaramillo, C.M. , Bird, B.H. , Reeder, D.M. , Vodzak, M.E. , Rota, P. , Metcalfe, M.G. , Spiropoulou, C.F. , Knust, B. , Vincent, J.P. , Frace, M.A. , Nichol, S.T. , Rollin, P.E. & Ströher, U. (2014). Novel paramyxovirus associated with severe acute febrile disease, South Sudan and Uganda, 2012. Emerg. Infect. Dis. 20, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, T. , Murray, K.A. , Zambrana‐torrelio, C. , Morse, S.S. , Rondinini, C. , Marco, M.Di , Breit, N. , Olival, K.J. & Daszak, P. (2017). Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe, G.K. , Aréchiga Ceballos, N.G. , Banyard, A.C. , Basler, C.F. , Bavari, S. , Bennett, A.J. , Blasdell, K.R. , Briese, T. , Bukreyev, A. , Caì, Y. , Calisher, C.H. , Campos Lawson, C. , Chandran, K. , Chapman, C.A. , Chiu, C.Y. , Choi, K.S. , Collins, P.L. , Dietzgen, R.G. , Dolja, V.V. , Dolnik, O. , Domier, L.L. , Dürrwald, R. , Dye, J.M. , Easton, A.J. , Ebihara, H. , Echevarría, J.E. , Fooks, A.R. , Formenty, P.B.H. , Fouchier, R.A.M. , Freuling, C.M. , Ghedin, E. , Goldberg, T.L. , Hewson, R. , Horie, M. , Hyndman, T.H. , Jiāng, D. , Kityo, R. , Kobinger, G.P. , Kondō, H. , Koonin, E.V. , Krupovic, M. , Kurath, G. , Lamb, R.A. , Lee, B. , Leroy, E.M. , Maes, P. , Maisner, A. , Marston, D.A. , Mor, S.K. , Müller, T. , Mühlberger, E. , Ramírez, V.M.N. , Netesov, S.V. , Ng, T.F.F. , Nowotny, N. , Palacios, G. , Patterson, J.L. , Pawęska, J.T. , Payne, S.L. , Prieto, K. , Rima, B.K. , Rota, P. , Rubbenstroth, D. , Schwemmle, M. , Siddell, S. , Smither, S.J. , Song, Q. , Song, T. , Stenglein, M.D. , Stone, D.M. , Takada, A. , Tesh, R.B. , Thomazelli, L.M. , Tomonaga, K. , Tordo, N. , Towner, J.S. , Vasilakis, N. , Vázquez‐Morón, S. , Verdugo, C. , Volchkov, V.E. , Wahl, V. , Walker, P.J. , Wang, D. , Wang, L.F. , Wellehan, J.F.X. , Wiley, M.R. , Whitfield, A.E. , Wolf, Y.I. , Yè, G. , Zhāng, Y.Z. & Kuhn, J.H. (2017). Taxonomy of the order Mononegavirales: update 2017. Arch. Virol. 162, 2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe, G.K. , Ayllón, M.A. , Bào, Y. , Basler, C.F. , Bavari, S. , Blasdell, K.R. , Briese, T. , Brown, P.A. , Bukreyev, A. , Balkema‐Buschmann, A. , Buchholz, U.J. , Chabi‐Jesus, C. , Chandran, K. , Chiapponi, C. , Crozier, I. , de Swart, R.L. , Dietzgen, R.G. , Dolnik, O. , Drexler, J.F. , Dürrwald, R. , Dundon, W.G. , Duprex, W.P. , Dye, J.M. , Easton, A.J. , Fooks, A.R. , Formenty, P.B.H.H. , Fouchier, R.A.M.M. , Freitas‐Astúa, J. , Griffiths, A. , Hewson, R. , Horie, M. , Hyndman, T.H. , Jiāng, D. , Kitajima, E.W. , Kobinger, G.P. , Kondō, H. , Kurath, G. , Kuzmin, I.V. , Lamb, R.A. , Lavazza, A. , Lee, B. , Lelli, D. , Leroy, E.M. , Lǐ, J. , Maes, P. , Marzano, S.‐Y.L.Y.L. , Moreno, A. , Mühlberger, E. , Netesov, S.V. , Nowotny, N. , Nylund, A. , Økland, A.L. , Palacios, G. , Pályi, B. , Pawęska, J.T. , Payne, S.L. , Prosperi, A. , Ramos‐González, P.L. , Rima, B.K. , Rota, P. , Rubbenstroth, D. , Shī, M. , Simmonds, P. , Smither, S.J. , Sozzi, E. , Spann, K. , Stenglein, M.D. , Stone, D.M. , Takada, A. , Tesh, R.B. , Tomonaga, K. , Tordo, N. , Towner, J.S. , van den Hoogen, B. , Vasilakis, N. , Wahl, V. , Walker, P.J. , Wang, L.‐F.F. , Whitfield, A.E. , Williams, J.V. , Zerbini, F.M. , Zhāng, T. , Zhang, Y.‐Z.Z. & Kuhn, J.H. (2019). Taxonomy of the order Mononegavirales: update 2019. Arch. Virol. 164, 1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman, B.R. , Carroll, S.A. , Reed, Z.D. , Sealy, T.K. , Balinandi, S. , Swanepoel, R. , Kemp, A. , Erickson, B.R. , Comer, J.A. , Campbell, S. , Cannon, D.L. , Khristova, M.L. , Atimnedi, P. , Paddock, C.D. , Kent Crockett, R.J. , Flietstra, T.D. , Warfield, K.L. , Unfer, R. , Katongole‐Mbidde, E. , Downing, R. , Tappero, J.W. , Zaki, S.R. , Rollin, P.E. , Ksiazek, T.G. , Nichol, S.T. & Towner, J.S. (2012). Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8, e1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman, B.R. , Nyakarahuka, L. , McElroy, A.K. , Dodd, K.A. , Sealy, T.K. , Schun, A.J. , Shoemaker, T.R. , Balinandi, S. , Atimnedi, P. , Kaboyo, W. , Nichol, S.T. & Towner, J.S. (2014). Marburgvirus resurgence in Kitaka mine bat population after extermination attempts. Uganda. Emerg. Infect. Dis. 20, 1761–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman, B.R. , Albariño, C.G. , Bird, B.H. , Nyakarahuka, L. , Sealy, T.K. , Balinandi, S. , Schuh, A.J. , Campbell, S.M. , Ströher, U. , Megan, E.B. , Vodzack, M.E. , Reeder, D.M. , Kaboyo, W. , Nichol, S.T. & Towner, J.S. (2015a). A recently discovered pathogenic paramyxovirus, Sosuga Virus, is present in Rousettus aegyptiacus fruit bats at multiple locations in Uganda. J. Wildl. Dis. 51, 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman, B.R. , Jones, M.E.B. , Sealy, T.K. , Uebelhoer, L.S. , Schuh, A.J. , Bird, B.H. , Coleman‐McCray, J.D. , Martin, B.E. , Nichol, S.T. & Towner, J.S. (2015b). Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus). J. Wildl. Dis. 51, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, B.S.P. , Lim, T.C.C. & Wang, L. (2018). Nipah virus infection. J. Clin. Microbiol. 56, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anindita, P.D. , Sasaki, M. , Setiyono, A. , Handharyani, E. , Orba, Y. , Kobayashi, S. , Rahmadani, I. , Taha, S. , Adiani, S. , Subangkit, M. , Nakamura, I. , Sawa, H. & Kimura, T. (2015). Detection of coronavirus genomes in Moluccan naked‐backed fruit bats in Indonesia. Arch. Virol. 160, 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annan, A. , Baldwin, H.J. , Corman, V.M. , Klose, S.M. , Owusu, M. , Nkrumah, E.E. , Badu, E.K. , Anti, P. , Agbenyega, O. , Meyer, B. , Oppong, S. , Sarkodie, Y.A. , Kalko, E.K.V. , Lina, P.H.C. , Godlevska, E.V. , Reusken, C. , Seebens, A. , Gloza‐rausch, F. , Vallo, P. , Tschapka, M. , Drosten, C. & Drexler, J.F. (2013). Human Betacoronavirus 2c EMC/2012‐related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 19, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, S.J. , Gilardi, K. , Menachery, V.D. , Goldstein, T. , Ssebide, B. , Mbabazi, R. , Navarrete‐Macias, I. , Liang, E. , Wells, H. , Hicks, A. , Petrosov, A. , Byarugaba, D.K. , Debbink, K. , Dinnon, K.H. , Scobey, T. , Randell, S.H. , Yount, B.L. , Cranfield, M. , Johnson, C.K. , Baric, R.S. , Lipkin, W.I. & Mazet, J.A.K. (2017a). Further Evidence for Bats as the Evolutionary Source of Middle East Respiratory Syndrome Coronavirus. MBio 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, S.J. , Johnson, C.K. , Greig, D.J. , Kramer, S. , Che, X. , Wells, H. , Hicks, A.L. , Joly, D.O. , Wolfe, N.D. , Daszak, P. , Karesh, W. , Lipkin, W.I. , Morse, S.S. , Mazet, J.A.K. & Goldstein, T. (2017b). Global patterns in coronavirus diversity. Virus Evol. 3, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert, M.F.A. 1999. Rabies in individual countries. France. Rabies Bull. Eur. 23, 6. [Google Scholar]
- Baker, K.S. , Todd, S. , Marsh, G. , Fernandez‐Loras, A. , Suu‐Ire, R. , Wood, J.L.N. , Wang, L.F. , Murcia, P.R. & Cunningham, A.A. (2012). Co‐circulation of diverse paramyxoviruses in an urban African fruit bat population. J. Gen. Virol. 93, 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, K.S. , Todd, S. , Marsh, G.A. , Crameri, G. , Barr, J.A. , Kamins, A.O. , Peel, A.J. , Yu, M. , Hayman, D.T.S. , Nadjm, B. , Mtove, G. , Amos, B. , Reyburn, H. , Nyarko, E. , Suu‐Ire, R. , Murcia, P.R. , Cunningham, A.A. , Wood, J.L.N. & Wang, L.‐F. (2013). Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J. Virol 87, 1348–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyard, A.C. & Fooks, A.R. (2017). The impact of novel lyssavirus discovery. Microbiol. Aust. 17–21. [Google Scholar]
- Bernard, R.T.F. & Cumming, G.S. (1997). African bats: Evolution of reproductive patterns and delays. Q. Rev. Biol. 72, 253–274. [DOI] [PubMed] [Google Scholar]
- Berry, M. , Gamieldien, J. & Fielding, B. (2015). Identification of New Respiratory Viruses in the New Millennium. Viruses 7, 996–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulger, L.R. & Porterfield, J.S. (1958). Isolation of a virus from Nigerian fruit bats. Trans. R. Soc. Trop. Med. Hyg. 52, 421–424. [DOI] [PubMed] [Google Scholar]
- Bourgarel, M. , Pfukenyi, D.M. , Boué, V. , Talignani, L. , Chiweshe, N. , Diop, F. , Caron, A. , Matope, G. , Missé, D. & Liégeois, F. (2018). Circulation of Alphacoronavirus, Betacoronavirus and Paramyxovirus in Hipposideros bat species in Zimbabwe. Infect. Genet. Evol. 58, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauburger, K. , Hume, A.J. , Mühlberger, E. & Olejnik, J. (2012). Forty‐five years of Marburg virus research. Viruse 4, 1878–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher, C.H. , Childs, J.E. , Field, H.E. , Holmes, K.V. & Schountz, T. (2006). Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chege, H.N. , Schepers, C. & Wolfaardt, G.J.J. (2015). Documenting the bat species assemblages of the Meletse Bat Research and Conservation Training Centre in Limpopo Province, Thabazimbi. South Africa. African Bat Conserv. News 38, 5–8. [Google Scholar]
- Chen, L. , Liu, B. , Yang, J. & Jin, Q. (2014). DBatVir: the database of bat‐associated viruses. Database (Oxford). 2014, bau021–bau021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D.K.W. , Hui, K.P.Y. , Perera, R.A.P.M. , Miguel, E. , Niemeyer, D. , Zhao, J. , Channappanavar, R. , Dudas, G. , Oladipo, J.O. , Traoré, A. , Fassi‐Fihri, O. , Ali, A. , Demissié, G.F. , Muth, D. , Chan, M.C.W. , Nicholls, J.M. , Meyerholz, D.K. , Kuranga, S.A. , Mamo, G. , Zhou, Z. , So, R.T.Y. , Hemida, M.G. , Webby, R.J. , Roger, F. , Rambaut, A. , Poon, L.L.M. , Perlman, S. , Drosten, C. , Chevalier, V. & Peiris, M. (2018). MERS coronaviruses from camels in Africa exhibit region‐dependent genetic diversity. Proc. Natl. Acad. Sci. 115, 3144–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, K.B. , Goh, K.J. , Wong, K.T. , Kamarulzaman, A. , Tan, P.S.K. , Ksiazek, T.G. , Zaki, S.R. , Paul, G. , Lam, S.K. & Tan, C.T. (1999). Fatal encephalitis due to Nipah virus among pig‐farmers in Malaysia. Lancet 354, 1257–1259. [DOI] [PubMed] [Google Scholar]
- Chua, K.B. , Koh, C.L. , Hooi, P.S. , Wee, K.F. , Khong, J.H. , Chua, B.H. , Chan, Y.P. , Lim, M.E. & Lam, S.K. (2002). Isolation of Nipah virus from Malaysian Island flying‐foxes. Microbes Infect. 4, 145–151. [DOI] [PubMed] [Google Scholar]
- Coertse, J. , Markotter, W. , le Roux, K. , Stewart, D. , Sabeta, C.T. & Nel, L.H. (2016). New isolations of the rabies‐related Mokola virus from South Africa. BMC Vet. Res. 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrardy, C. , Tao, Y. , Kuzmin, I.V. , Niezgoda, M. , Agwanda, B. , Breiman, R.F. , Anderson, L.J. , Rupprecht, C.E. & Tong, S. (2014). Molecular detection of adenoviruses, rhabdoviruses, and paramyxoviruses in bats from Kenya. Am. J. Trop. Med. Hyg. 91, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman, V.M. , Baldwin, H.J. , Tateno, A.F. , Zerbinati, R.M. , Annan, A. , Owusu, M. , Nkrumah, E.E. , Maganga, G.D. , Oppong, S. , Adu‐Sarkodie, Y. , Vallo, P. , da Silva Filho, L.V.R.F. , Leroy, E.M. , Thiel, V. , van der Hoek, L. , Poon, L.L.M. , Tschapka, M. , Drosten, C. & Drexler, J.F. (2015). Evidence for an Ancestral Association of Human Coronavirus 229E with Bats. J. Virol. 89, 11858–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J. , Li, F. & Shi, Z.‐L. (2019). Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, M. , Wilkinson, D.A. , Benlali, A. , Lagadec, E. , Ramasindrazana, B. , Dellagi, K. & Tortosa, P. (2015). Leptospira and paramyxovirus infection dynamics in a bat maternity enlightens pathogen maintenance in wildlife. Environ. Microbiol. 17, 4280–4289. [DOI] [PubMed] [Google Scholar]
- Dighe, A. , Jombart, T. , Van Kerkhove, M.D. & Ferguson, N. (2019). A systematic review of MERS‐CoV seroprevalence and RNA prevalence in dromedary camels: Implications for animal vaccination. Epidemics 100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, G.E. (1875). XLVII.—Conspectus of the suborders, families, and genera of Chiroptera arranged according to their natural affinities. Ann. Mag. Nat. Hist. 16, 345–57. [Google Scholar]
- Drexler, J.F. , Corman, V.M. , Gloza‐Rausch, F. , Seebens, A. , Annan, A. , Ipsen, A. , Kruppa, T. , Müller, M.A. , Kalko, E.K.V. , Adu‐Sarkodie, Y. , Oppong, S. & Drosten, C. (2009). Henipavirus RNA in African bats. PLoS One 4, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler, J.F. , Corman, V.M. , Müller, M.A. , Maganga, G.D. , Vallo, P. , Binger, T. , Gloza‐Rausch, F. , Rasche, A. , Yordanov, S. , Seebens, A. , Oppong, S. , Sarkodie, Y.A. , Pongombo, C. , Lukashev, A.N. , Schmidt‐Chanasit, J. , Buettner, R. , Kalko, E.K.V. , Kruppa, T. , Franke, C.R. , Kallies, R. , Yandoko, E.R.N. , Herrler, G. , Reusken, C. , Hassanin, A. , Krüger, D.H. , Matthee, S. , Ulrich, R.G. , Leroy, E.M. & Drosten, C. (2012). Bats host major mammalian paramyxoviruses. PLoS One 4, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzikwi, A.A. , Kuzmin, I.I. , Umoh, J.U. , Kwaga, J.K.P. , Ahmad, A.A. and Rupprecht, C.E. 2010. Evidence of Lagos bat virus circulation among Nigerian fruit bats. J. Wildl. Dis. 46, 267–271. [DOI] [PubMed] [Google Scholar]
- Fahr, J. , Abedi‐Lartey, M. , Esch, T. , Machwitz, M. , Suu‐Ire, R. , Wikelski, M. & Dechmann, D.K.N. (2015). Pronounced seasonal changes in the movement ecology of a highly gregarious central‐place Forager, the African Straw‐Coloured Fruit Bat (Eidolon helvum). PLoS One 10, e0138985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton, M.B. & Simmons, N.B. (2015). Bats: a world of science and mystery. Chicago: University of Chicago Press. [Google Scholar]
- Field, H. , Jordan, D. , Edson, D. , Morris, S. , Melville, D. , Parry‐Jones, K. , Broos, A. , Divljan, A. , McMichael, L. , Davis, R. , Kung, N. , Kirkland, P. & Smith, C. (2015). Spatiotemporal aspects of Hendra virus infection in pteropid bats (flying‐foxes) in Eastern Australia. PLoS One 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foggin, C.M. (1988). Rabies and rabies‐related viruses in zimbabwe historical, virological and ecological aspects. PhD thesis.
- Foley, N.M. , Goodman, S.M. , Whelan, C.V. , Puechmaille, S.J. & Teeling, E. 2017. Towards navigating the minotaur's labyrinth: Cryptic diversity and taxonomic revision within the speciose genus Hipposideros (Hipposideridae). Acta Chiropt. 19, 1–18. [Google Scholar]
- Forbes, K.M. , Webala, P.W. , Jääskeläinen, A.J. , Abdurahman, S. , Ogola, J. , Masika, M.M. , Kivistö, I. , Alburkat, H. , Plyusnin, I. , Levanov, L. , Korhonen, E.M. , Huhtamo, E. , Mwaengo, D. , Smura, T. , Mirazimi, A. , Anzala, O. , Vapalahti, O. & Sironen, T. (2019). Bombali virus in mops condylurus bat, kenya. Emerg. Infect. Dis. 25, 955–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuling, C.M. , Binger, T. , Beer, M. , Adu‐Sarkodie, Y. , Schatz, J. , Fischer, M. , Hanke, D. , Hoffmann, B. , Höper, D. , Mettenleiter, T.C. , Oppong, S.K. , Drosten, C. & Müller, T. (2015). Lagos bat virus transmission in an Eidolon helvum bat colony, Ghana. Virus Res. 210, 42–45. [DOI] [PubMed] [Google Scholar]
- Ge, X. , Li, J. , Yang, X. , Chmura, A.A. , Zhu, G. , Epstein, J.H. , Mazet, J.K. , Hu, B. , Zhang, W. , Peng, C. , Zhang, Y. , Luo, C. , Tan, B. , Wang, N. , Zhu, Y. , Crameri, G. , Zhang, S. , Wang, L. , Daszak, P. & Shi, Z. (2013). Isolation and characterization of a bat SARS‐like coronavirus that uses the ACE2 receptor. Nature 503, 535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser, F. & Stawski, C. (2011). Hibernation and Torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integr. Comp. Biol. 51, 337–348. [DOI] [PubMed] [Google Scholar]
- Geldenhuys, M. , Mortlock, M. , Weyer, J. , Bezuidt, O. , Seamark, E.C.J. , Kearney, T. , Gleasner, C. , Erkkila, T.H. , Cui, H. & Markotter, W. (2018). A metagenomic viral discovery approach identifies potential zoonotic and novel mammalian viruses in Neoromicia bats within South Africa. PLoS ONE 13, e0194527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerow, C.M. , Rapin, N. , Voordouw, M.J. , Elliot, M. , Misra, V. & Subudhi, S. (2019). Arousal from hibernation and reactivation of Eptesicus fuscus gammaherpesvirus ( Ef HV ) in big brown bats. Transbound. Emerg. Dis. 66, 1054–1062. [DOI] [PubMed] [Google Scholar]
- Glennon, E.E. , Jephcott, F.L. , Restif, O. & Wood, J.L.N. (2019). Estimating undetected Ebola spillovers. PLoS Negl. Trop. Dis. 13, e0007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, T. , Anthony, S.J. , Gbakima, A. , Bird, B.H. , Bangura, J. , Tremeau‐Bravard, A. , Belaganahalli, M.N. , Wells, H.L. , Dhanota, J.K. , Liang, E. , Grodus, M. , Jangra, R.K. , DeJesus, V.A. , Lasso, G. , Smith, B.R. , Jambai, A. , Kamara, B.O. , Kamara, S. , Bangura, W. , Monagin, C. , Shapira, S. , Johnson, C.K. , Saylors, K. , Rubin, E.M. , Chandran, K. , Lipkin, W.I. & Mazet, J.A.K. (2018). The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol. 3, 1486–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, R.J. , Baker, S.C. , Baric, R.S. , Brown, C.S. , Drosten, C. , Enjuanes, L. , Fouchier, R.A.M. , Galiano, M. , Gorbalenya, A.E. , Memish, Z.A. , Perlman, S. , Poon, L.L.M. , Snijder, E.J. , Stephens, G.M. , Woo, P.C.Y. , Zaki, A.M. , Zambon, M. & Ziebuhr, J. (2013). Middle East respiratory syndrome coronavirus (MERS‐CoV): Announcement of the Coronavirus Study Group. J. Virol. 87, 7790–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Y. , Zheng, B.J. , He, Y.Q. , Liu, X.L. , Zhuang, Z.X. , Cheung, C.L. , Luo, S.W. , Li, P.H. , Zhang, L.J. , Guan, Y.J. , Butt, K.M. , Wong, K.L. , Chan, K.W. , Lim, W. , Shortridge, K.F. , Yuen, K.Y. , Peiris, J.S.M. & Poon, L.L.M. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278. [DOI] [PubMed] [Google Scholar]
- Halpin, K. , Young, P.L. , Field, H.E. & Mackenzie, J.S. (2000). Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J. Gen. Virol. 81, 1927–1932. [DOI] [PubMed] [Google Scholar]
- Han, B.A. , Kramer, A.M. & Drake, J.M. (2016). Global Patterns of Zoonotic Disease in Mammals. Trends Parasitol. 32(7), 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M.A. & Piaggio, A.J. (2018). Assessing the potential impacts of a changing climate on the distribution of a rabies virus vector. PLoS One 13, e0192887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D.T. , Emmerich, P. , Yu, M. , Wang, L.F. , Suu‐Ire, R. , Fooks, A.R. & Wood, J.L. 2010. Long‐term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. PLoS ONE, 5(8), e11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D.T. , Fooks, A.R. , Horton, D. , Suu‐Ire, R. , Breed, A.C. , Cunningham, A.A. & Wood, J.L. 2008b. Antibodies against Lagos bat virus in megachiroptera from West Africa. Emerg. Infect. Dis., 14, 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D.T.S. , Fooks, A.R. , Rowcliffe, J.M. , McCrea, R. , Restif, O. , Baker, K.S. , Horton, D.L. , Suu‐ire, R. , Cunningham, A.A. & Wood, J.L.N. 2012b. Endemic Lagos bat virus infection in Eidolon helvum. Epidemiol. Infect. 140, 2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D.T. , Suu‐Ire, R. , Breed, A.C. , McEachern, J.A. , Wang, L. , Wood, J.L. & Cunningham, A.A. 2008a. Evidence of henipavirus infection in West African fruit bats. PLoS ONE, 3, 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D.T.S. , Wang, L.F. , Barr, J. , Baker, K.S. , Suu‐Ire, R. , Broder, C.C. , Cunningham, A.A. & Wood, J.L.N. (2011). Antibodies to henipavirus or henipa‐like viruses in domestic pigs in Ghana, West Africa. PLoS One 6, 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D.T. , Yu, M. , Crameri, G. , Wang, L.F. , Suu‐Ire, R. , Wood, J.L. & Cunningham, A.A. 2012a.Ebola virus antibodies in fruit bats, Ghana, West Africa. Emerg. Infect. Dis., 18, 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, B. , Zeng, L.‐P. , Yang, X.‐L. , Ge, X.‐Y. , Zhang, W. , Li, B. , Xie, J.‐Z. , Shen, X.‐R. , Zhang, Y.‐Z. , Wang, N. , Luo, D.‐S. , Zheng, X.‐S. , Wang, M.‐N. , Daszak, P. , Wang, L.‐F. , Cui, J. & Shi, Z.‐L. (2017). Discovery of a rich gene pool of bat SARS‐related coronaviruses provides new insights into the origin of SARS coronavirus. PLOS Pathog. 13, e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S.‐C. , Hsu, C.‐L. , Lee, M.‐S. , Tu, Y.‐C. , Chang, J.‐C. , Wu, C.‐H. , Lee, S.‐H. , Ting, L.‐J. , Tsai, K.‐R. , Cheng, M.‐C. , Tsu, W.‐J. & Hsu, W.‐C. (2018). Lyssavirus in Japanese Pipistrelle. Taiwan. Emerg. Infect. Dis. 24, 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon, J. & Kirsch, J. (2006). A moveable face: deconstructing the Microchiroptera and a new classification of extant bats. Acta Chiropterologica 8, 1–10. [Google Scholar]
- ICTV . (2019). Virus taxonomy: 2018b Release. https://talk.ictvonline.org/taxonomy/ [Google Scholar]
- Islam, M.S. , Hossain, M.J. , Hasan, M. , Rahman, M. , Campbell, S. , Cannon, D.L. , Ströher, U. , Daszak, P. , Luby, S.P. & Gurley, E.S. (2016). Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 22, 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithete, N.L. , Stoffberg, S. , Corman, V.M. , Cottontail, V.M. , Richards, L.R. , Schoeman, M.C. , Drosten, C. , Drexler, J.F. & Preiser, W. (2013). Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg. Infect. Dis. 19, 1697–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, N.H.G. & Du Plessis, E. (1976). Observations on the ecology and biology of the Cape fruit bat Rousettus aegyptiacus leachi in the Eastern Transvaal. S. Afr. J. Sci. 72, 270–273. [Google Scholar]
- Jones, K.E. , Patel, N.G. , Levy, M.A. , Storegard, A. , Balk, D. , Gittleman, J.L. & Daszak, P. (2008). Global trends in emerging infectious diseases. Nature 451, 990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalemba, L.N. , Niezgoda, M. , Gilbert, A.T. , Doty, J.B. , Wallace, R.M. , Malekani, J.M. & Carroll, D.S. 2017. Exposure to Lyssaviruses in Bats of the Democratic Republic of the Congo. J. Wildl. Dis. 53, 408–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasso, M. & Balakrishnan, M. (2013). Ecological and economic importance of bats (Order Chiroptera). ISRN Biodivers. 2013, 1–9. [Google Scholar]
- Kemenesi, G. , Kurucz, K. , Dallos, B. , Zana, B. , Földes, F. , Boldogh, S. , Görföl, T. , Carroll, M.W. & Jakab, F. (2018). Re‐emergence of Lloviu virus in Miniopterus schreibersii bats, Hungary, 2016. Emerg. Microbes Infect. 7, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kgaladi, J. , Wright, N. , Coertse, J. , Markotter, W. , Marston, D. , Fooks, A.R. , Freuling, C.M. , Müller, T.F. , Sabeta, C.T. & Nel, L.H. (2013). Diversity and epidemiology of Mokola Virus. PLoS Negl. Trop. Dis. 7, e2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia, G.S. , Kuzmin, I.I. , Umoh, J.U. , Kwaga, J.K. , Kazeem, H.M. , Osinubi, M.O. & Rupprecht, C.E. 2014.Detection of some lyssaviruses from fruigivorous and insectivorous bats in Nigeria. Online J. Public Health Inform. 6, 2579. [Google Scholar]
- Koopman, K.F. (1994). Chiroptera: systematics. In Niethammer, J. , Schliemann, H. & Stark, D. (Eds). Handbook of zoology Vol VIII Mammalia, Part 6: 1–217. New York: Walter de Gruyter. [Google Scholar]
- Kuhn, J.H. , Amarasinghe, G.K. , Basler, C.F. , Bavari, S. , Bukreyev, A. , Chandran, K. , Crozier, I. , Dolnik, O. , Dye, J.M. , Formenty, P.B.H. , Griffiths, A. , Hewson, R. , Kobinger, G.P. , Leroy, E.M. , Mühlberger, E. , Netesov (Нетёсов Сергей Викторович), S. V. , Palacios, G. , Pályi, B. , Pawęska, J.T. , Smither, S.J. , Takada (高田礼人), A. , Towner, J.S. & Wahl, V. (2019). ICTV virus taxonomy profile: Filoviridae. J. Gen. Virol. 100, 911–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, T.H. , Braun de Torrez, E. , Bauer, D. , Lobova, T. & Fleming, T.H. (2011). Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 1223, 1–38. [DOI] [PubMed] [Google Scholar]
- Kuzmin, I.V. & Rupprecht, C.E. (2015). Bat Lyssaviruses. In Bats and viruses: 47–97. Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Kuzmin, I.V. , Niezgoda, M. , Franka, R. , Agwanda, B. , Markotter, W. , Beagley, J.C. , Urazova, O.Y. , Breiman, R.F. & Rupprecht, C.E. (2008). Lagos bat virus in Kenya. J. Clin. Microbiol. 46, 1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin, I.V. , Mayer, A.E. , Niezgoda, M. , Markotter, W. , Agwanda, B. , Breiman, R.F. & Rupprecht, C.E. (2010). Shimoni bat virus, a new representative of the Lyssavirus genus. Virus Res. 149, 197–210. [DOI] [PubMed] [Google Scholar]
- Kuzmin, I.V. , Turmelle, A.S. , Agwanda, B. , Markotter, W. , Niezgoda, M. , Breiman, R.F. & Rupprecht, C.E. (2011). Commerson’s Leaf‐Nosed Bat (Hipposideros commersoni) is the likely reservoir of Shimoni Bat virus. Vector‐Borne Zoonotic Dis. 11, 1465–1470. [DOI] [PubMed] [Google Scholar]
- Lau, S.K.P. , Woo, P.C.Y. , Li, K.S.M. , Huang, Y. , Tsoi, H.‐W. , Wong, B.H.L. , Wong, S.S.Y. , Leung, S.‐Y. , Chan, K.‐H. & Yuen, K.‐Y. (2005). Severe acute respiratory syndrome coronavirus‐like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. 102, 14040–14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi, S. , Holmes, E.C. , Gastaldelli, M. , Tassoni, L. , Priori, P. , Scaravelli, D. , Zamperin, G. & De Benedictis, P. (2018). Interplay between co‐divergence and cross‐species transmission in the evolutionary history of bat coronaviruses. Infect. Genet. Evol. 58, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, E.M. , Kumulungui, B. , Pourrut, X. , Rouquet, P. , Hassanin, A. , Yaba, P. , Délicat, A. , Paweska, J.T. , Gonzalez, J.‐P. & Swanepoel, R. (2005). Fruit bats as reservoirs of Ebolavirus. Nature 438, 575–576. [DOI] [PubMed] [Google Scholar]
- Li, W. , Shi, Z. , Yu, M. , Ren, W. , Smith, C. , Hu, Z. , Zhang, H. , Zhang, J. , Eaton, B.T. , Zhang, S. & Wang, L. (2005). Bats are natural reservoirs of SARS‐Like coronaviruses. Science 310, 676–679. [DOI] [PubMed] [Google Scholar]
- López‐Baucells, A. , Rocha, R. & Fernández‐Llamazares, Á. (2018). When bats go viral: negative framings in virological research imperil bat conservation. Mamm. Rev. 48, 62–66. [Google Scholar]
- Luis, A.D. , Hayman, D.T.S. , O’Shea, T.J. , Cryan, P.M. , Gilbert, A.T. , Pulliam, J.R.C. , Mills, J.N. , Timonin, M.E. , Willis, C.K.R. , Cunningham, A.A. , Fooks, A.R. , Rupprecht, C.E. , Wood, J.L.N. & Webb, C.T. (2013). A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B Biol. Sci. 280, 20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis, A.D. , O’Shea, T.J. , Hayman, D.T.S. , Wood, J.L.N. , Cunningham, A.A. , Gilbert, A.T. , Mills, J.N. & Webb, C.T. (2015). Network analysis of host‐virus communities in bats and rodents reveals determinants of cross‐species transmission. Ecol. Lett. 18, 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganga, G.D. , Bourgarel, M. , Vallo, P. , Dallo, T.D. , Ngoagouni, C. , Drexler, J.F. , Drosten, C. , Nakouné, E.R. , Leroy, E.M. & Morand, S. (2014). Bat distribution size or shape as determinant of viral richness in African Bats. PLoS One 9, e100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marí Saéz, A. , Weiss, S. , Nowak, K. , Lapeyre, V. , Zimmermann, F. , Düx, A. , Kühl, H.S. , Kaba, M. , Regnaut, S. , Merkel, K. , Sachse, A. , Thiesen, U. , Villányi, L. , Boesch, C. , Dabrowski, P.W. , Radonić, A. , Nitsche, A. , Leendertz, S.A.J. , Petterson, S. , Becker, S. , Krähling, V. , Couacy‐Hymann, E. , Akoua‐Koffi, C. , Weber, N. , Schaade, L. , Fahr, J. , Borchert, M. , Gogarten, J.F. , Calvignac‐Spencer, S. & Leendertz, F.H. (2015). Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 7, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markotter, W. & Coertse, J. (2018). Bat lyssaviruses. Rev. Sci. Tech. l’OIE 37, 385–400. [DOI] [PubMed] [Google Scholar]
- Markotter, W. , Kuzmin, I. , Rupprecht, C.E. , Randles, J. , Sabeta, C.T. , Wandeler, A.I. & Nel, L.H. (2006). Isolation of Lagos bat virus from water mongoose. Emerg. Infect. Dis. 12, 1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markotter, W. , Kuzmin, I. , Rupprecht, C.E. & Nel, L.H. (2008). Phylogeny of Lagos bat virus: challenges for lyssavirus taxonomy. Virus Res. 135, 10–21. [DOI] [PubMed] [Google Scholar]
- Markotter, W. , Monadjem, A. & Nel, L.H. 2013.Antibodies against duvenhage virus in insectivorous bats in Swaziland. J. Wildl. Dis. 49, 1000–1003. [DOI] [PubMed] [Google Scholar]
- Markotter, W. , Geldenhuys, M. , Jansen van Vuren, P. , Kemp, A. , Mortlock, M. , Mudakikwa, A. , Nel, L. , Nziza, J. , Paweska, J. & Weyer, J. (2019). Paramyxo‐ and Coronaviruses in Rwandan Bats. Trop. Med. Infect. Dis. 4, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, D.A. , Horton, D.L. , Ngeleja, C. , Hampson, K. , Mcelhinney, L.M. , Banyard, A.C. , Haydon, D. , Cleaveland, S. , Rupprecht, C.E. , Bigambo, M. , Fooks, A.R. & Lembo, T. (2012). Ikoma lyssavirus, highly divergent Novel lyssavirus in an African civet. Emerg. Infect. Dis. 18, 664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazet, J.A.K. , Wei, Q. , Zhao, G. , Cummings, D.A.T. , Desmond, J.S. , Rosenthal, J. , King, C.H. , Cao, W. , Chmura, A.A. , Hagan, E.A. , Zhang, S. , Xiao, X. , Xu, J. , Shi, Z. , Feng, F. , Liu, X. , Pan, W. , Zhu, G. , Zuo, L. & Daszak, P. (2015). Joint China‐US call for employing a transdisciplinary approach to emerging infectious diseases. EcoHealth 12, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane, R. , Becker, N. & Field, H. (2011). Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLoS One 6, 1994–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael, L. , Edson, D. , Smith, C. , Mayer, D. , Smith, I. , Kopp, S. , Meers, J. & Field, H. (2017). Physiological stress and Hendra virus in flying‐foxes (Pteropus spp.), Australia. PLoS One 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish, Z.A. , Mishra, N. , Olival, K.J. , Fagbo, S.F. , Kapoor, V. , Epstein, J.H. , AlHakeem, R. , Durosinloun, A. , Al Asmari, M. , Islam, A. , Kapoor, A. , Briese, T. , Daszak, P. , Al Rabeeah, A.A. & Lipkin, W.I., (2013a). Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 19, 1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish, Z.A. , Mishra, N. , Olival, K.J. , Fagbo, S.F. , Kapoor, V. , Epstein, J.H. , Alhakeem, R. , Asmari, M.Al , Islam, A. , Kapoor, A. , Briese, T. , Daszak, P. , Rabeeah, A.A.A. & Lipkin, W.I. (2013b). Middle east respiratory syndrome Coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 19, 1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish, Z.A. , Cotten, M. , Meyer, B. , Watson, S.J. , Alsahafi, A.J. , Al Rabeeah, A.A. , Corman, V.M. , Sieberg, A. , Makhdoom, H.Q. , Assiri, A. , Al Masri, M. , Aldabbagh, S. , Bosch, B. , Beer, M. , Müller, M.A. , Kellam, P. & Drosten, C. (2014). Human infection with MERS Coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. 20, 1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall, I.H. , Borthwick, S. , Neves, E.S. , Low, D. , Linster, M. , Liang, B. , Skiles, M. , Jayakumar, J. , Han, H. , Gunalan, V. , Lee, B.P.Y.‐H. , Okahara, K. , Wang, L.‐F. , Maurer‐Stroh, S. , Su, Y.C.F. & Smith, G.J.D. (2017). Identification of a lineage D Betacoronavirus in Cave Nectar Bats (Eonycteris spelaea) in Singapore and an overview of lineage D reservoir ecology in SE Asian Bats. Transbound. Emerg. Dis. 64, 1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith, C.D. , Rossouw, A.P. & Van Praag Koch, H. (1971). An unusual case of human rabies thought to be of chiropteran origin. South African Med. J. 45, 767–769. [PubMed] [Google Scholar]
- Miller, M.R. , McMinn, R.J. , Misra, V. , Schountz, T. , Müller, M.A. , Kurth, A. & Munster, V.J. (2016). Broad and temperature independent replication potential of Filoviruses on cells derived from old and new world bat species. J. Infect. Dis. 214, S297–S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratelli, R. & Calisher, C.H. (2015). Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Mem. Inst. Oswaldo Cruz 110, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortlock, M. (2019). Detection and genetic characterization of potential zoonotic paramyxoviruses in an Egyptian fruit bat (Rousettus aegyptiacus) population within South Africa. University of Pretoria.
- Mortlock, M. , Kuzmin, I.V. , Weyer, J. , Gilbert, A.T. , Agwanda, B. , Rupprecht, C.E. , Nel, L.H. , Kearney, T. , Malekani, J.M. & Markotter, W. (2015). Novel paramyxoviruses in bats from Sub‐Saharan Africa, 2007–2012. Emerg. Infect. Dis. 21, 1840–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortlock, M. , Dietrich, M. , Weyer, J. , Paweska, J.T. & Markotter, W. (2019). Co‐circulation and excretion dynamics of diverse rubula‐and related viruses in Egyptian rousette bats from South Africa. Viruses 11, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muleya, W. , Sasaki, M. , Orba, Y. , Ishii, A. , Thomas, Y. , Nakagawa, E. , Ogawa, H. , Hang’ombe, B. , Namangala, B. , Mweene, A. , Takada, A. , Kimura, T. & Sawa, H. (2014). Molecular epidemiology of Paramyxoviruses in frugivorous Eidolon helvum bats in Zambia. J. Vet. Med. Sci. 76, 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, M.A. , Paweska, J.T. , Leman, P.A. , Drosten, C. , Grywna, K. , Kemp, A. , Braack, L. , Sonnenberg, K. , Niedrig, M. & Swanepoel, R. 2007. Coronavirus antibodies in African bat species. Emerg. Infect. Dis. 13, 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, K. , Selleck, P. , Hooper, P. , Hyatt, A. , Gould, A. , Gleeson, L. , Westbury, H. , Hiley, L. , Selvey, L. , Rodwell, B. , Ketterer, P. , Murray, K. , Selleck, P. , Hooper, P. , Hyatt, A. , Gould, A. , Gleeson, L. , Westbury, H. , Hiley, L. , Selvey, L. , Rodwell, B. & Ketterer, P. (1995). A morbillivirus that caused fatal disease in horses and humans. Science. 268, 94–97. [DOI] [PubMed] [Google Scholar]
- Nowak, R.M. , Walker, E.P. , Kunz, T.H. & Pierson, E.D. (1994). Walker’ Bats of the World. Baltimore: JHU Press. [Google Scholar]
- O’Shea, T.J. , Cryan, P.M. , Cunningham, A.A. , Fooks, A.R. , Hayman, D.T.S.S. , Luis, A.D. , Peel, A.J. , Plowright, R.K. & Wood, J.L.N.N. (2014). Bat flight and zoonotic viruses. Emerg. Infect. Dis. 20, 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival, K.J. & Hayman, D.T.S. (2014). Filoviruses in bats: current knowledge and future directions. Viruses 6, 1759–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival, K.J. , Hosseini, P.R. , Zambrana‐Torrelio, C. , Ross, N. , Bogich, T.L. & Daszak, P. (2017). Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, S.H. , Bounga, G. , Ondzie, A. , Bushmaker, T. , Seifert, S.N. , Kuisma, E. , Taylor, D.W. , Munster, V.J. & Walzer, C. (2019). Lek‐associated movement of a putative Ebolavirus reservoir, the hammer‐headed fruit bat (Hypsignathus monstrosus), in northern Republic of Congo.". PLoS ONE 14, e0223139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez, D.J. , Giles, J. , McCallum, H. , Field, H. , Jordan, D. , Peel, A.J. & Plowright, R.K. (2017). Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Epidemiol. Infect. 145, 3143–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich, S.S. , Lovett, S.P. , Koroleva, G. , Guito, J.C. , Arnold, C.E. , Nagle, E.R. , Kulcsar, K. , Lee, A. , Thibaud‐Nissen, F. , Hume, A.J. , Mühlberger, E. , Uebelhoer, L.S. , Towner, J.S. , Rabadan, R. , Sanchez‐Lockhart, M. , Kepler, T.B. & Palacios, G. (2018). The Egyptian rousette genome reveals unexpected features of bat antiviral immunity. Cell 173, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweska, J.T. , Blumberg, L.H. , Liebenberg, C. , Hewlett, R.H. , Grobbelaar, A.A. , Leman, P.A. , Croft, J.E. , Nel, L.H. , Nutt, L. & Swanepoel, R. (2006). Fatal human infection with rabies‐related Duvenhage virus, South Africa. Emerg. Infect. Dis. 12, 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweska, J.T. , Jansen van Vuren, P. , Kemp, A. , Storm, N. , Grobbelaar, A.A. , Wiley, M.R. , Palacios, G. & Markotter, W. (2018). Marburg virus infection in Egyptian rousette bats, South Africa, 2013–2014. Emerg. Infect. Dis. 24, 1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel, A.J. , Sargan, D.R. , Baker, K.S. , Hayman, D.T.S. , Barr, J.A. , Crameri, G. , Suu‐ire, R. , Broder, C.C. , Lembo, T. , Wang, L. , Fooks, A.R. , Rossiter, S.J. , Wood, J.L.N. & Cunningham, A.A. 2013. Continent‐wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 4, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet, O. , Schneider, B.S. , Beaty, S.M. , LeBreton, M. , Yun, T.E. , Park, A. , Zachariah, T.T. , Bowden, T.A. , Hitchens, P. , Ramirez, C.M. , Daszak, P. , Mazet, J. , Freiberg, A.N. , Wolfe, N.D. & Lee, B. (2014). Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle, S. , Oppong, S. , Drexler, J.F. , Gloza‐Rausch, F. , Ipsen, A. , Seebens, A. , Müller, M.A. , Annan, A. , Vallo, P. , Adu‐Sarkodie, Y. , Kruppa, T.F. & Drosten, C. (2009). Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats. Ghana. Emerg. Infect. Dis. 15, 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright, R.K. , Field, H.E. , Smith, C. , Divljan, A. , Palmer, C. , Tabor, G. , Daszak, P. & Foley, J.E. (2008). Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. R. Soc. B Biol. Sci. 275, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright, R.K. , Eby, P. , Hudson, P.J. , Smith, I.L. , Westcott, D. , Bryden, W.L. , Middleton, D. , Reid, P.A. , McFarlane, R.A. , Martin, G. , Tabor, G.M. , Skerratt, L.F. , Anderson, D.L. , Crameri, G. , Quammen, D. , Jordan, D. , Freeman, P. , Wang, L.‐F. , Epstein, J.H. , Marsh, G.A. , Kung, N.Y. & McCallum, H. (2015). Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B Biol. Sci. 282, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlutsky, A.J. , Khritankov, A.M. , Ovodov, N.D. & Austad, S.N. (2005). A new field record for bat longevity. J. Gerontol. A Biol. Sci. Med. Sci. 60A, 1366–1368. [DOI] [PubMed] [Google Scholar]
- Pourrut, X. , Souris, M. , Towner, J.S. , Rollin, P.E. , Nichol, S.T. , Gonzalez, J.P. & Leroy, E. 2009. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect. Dis. 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, P.‐L. , Firth, C. , Street, C. , Henriquez, J.A. , Petrosov, A. , Tashmukhamedova, A. , Hutchison, S.K. , Egholm, M. , Osinubi, M.O.V. , Niezgoda, M. , Ogunkoya, A.B. , Briese, T. , Rupprecht, C.E. & Lipkin, W.I. (2010). Identification of a severe acute respiratory syndrome coronavirus‐like virus in a leaf‐nosed bat in Nigeria. MBio 1, e00208–10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken, C.B.E.M. , Messadi, L. , Feyisa, A. , Ularamu, H. , Godeke, G.‐J. , Danmarwa, A. , Dawo, F. , Jemli, M. , Melaku, S. , Shamaki, D. , Woma, Y. , Wungak, Y. , Gebremedhin, E.Z. , Zutt, I. , Bosch, B.‐J. , Haagmans, B.L. & Koopmans, M.P.G. (2014). Geographic distribution of MERS Coronavirus among Dromedary Camels. Africa. Emerg. Infect. Dis. 20, 1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, H.V. & Cumming, G.S. (2006). Food availability and annual migration of the straw‐colored fruit bat (Eidolon helvum). J. Zool. 268, 35–44. [Google Scholar]
- Roddy, P. , Thomas, S.L. , Jeffs, B. , Nascimento Folo, P. , Pablo Palma, P. , Moco Henrique, B. , Villa, L. , Damiao Machado, F.P. , Bernal, O. , Jones, S.M. , Strong, J.E. , Feldmann, H. & Borchert, M. (2010). Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige. Angola. J. Infect. Dis. 201, 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanadan, R. , Arunkumar, G. , Laserson, K.F. , Heretik, K.H. , Singh, S. , Mourya, D.T. , Gangakhedkar, R.R. , Gupta, N. , Sharma, R. , Dhuria, M. , Jain, S.K. , Nichol, S. , Gupta, P. & Bhargava, B. (2018). Towards global health security: response to the May 2018 Nipah virus outbreak linked to Pteropus bats in Kerala, India. BMJ Glob. Heal. 3, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, A.B. , Leonard, S. , Masamsetti, V. , Pierce, A. , Podlutsky, A.J. , Podlutskaya, N. , Richardson, A. , Austad, S.N. & Chaudhuri, A.R. (2009). The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 23, 2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schountz, T. , Baker, M.L. , Butler, J. & Munster, V. (2017). Immunological control of viral infections in bats and the emergence of viruses highly pathogenic to humans. Front. Immunol. 8, 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata, M. , Chu, D.K.W. , Gomaa, M.R. , Abisaid, M. , Shesheny, R.El , Kandeil, A. , Bagato, O. , Chan, S.M.S. , Barbour, E.K. , Shaib, H.S. , Mckenzie, P.P. , Webby, R.J. , Ali, M.A. , Peiris, M. & Kayali, G. (2016). Surveillance for Coronaviruses in Bats, Lebanon and Egypt, 2013–2015. Emerg. Infect. Dis. 22, 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin, H.A. , Montgomery, W.I. & Lundy, M.G. (2013). The impact and implications of climate change for bats. Mamm. Rev. 43, 171–182. [Google Scholar]
- Shope, R.E. , Murphy, F.A. , Harrison, A.K. , Causey, O.R. , Kemp, G.E. , Simpson, D.I.H. & Moore, D.L. (1970). Two African viruses serologically and morphologically related to rabies virus. J. Virol. 6, 690–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, N.B. , Seymour, K.L. , Habersetzer, J. & Gunnell, G.F. (2008). Primitive early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451, 818–821. [DOI] [PubMed] [Google Scholar]
- Smith, I. , Broos, A. , de Jong, C. , Zeddeman, A. , Smith, C. , Smith, G. , Moore, F. , Barr, J.A. , Crameri, G. , Marsh, G. , Tachedjian, M. , Yu, M. , Kung, Y.H. , Wang, L.F. & Field, H. (2011). Identifying Hendra virus diversity in pteropid bats. PLoS One 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler, J.R. , Ervin, E.D. , Towner, J.S. , Rollin, P.E. & Nichol, S.T. (2016). Perspectives on West Africa Ebolavirus disease outbreak, 2013–2016. Emerg. Infect. Dis. 22, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, M.S. , Teeling, E. & Stanhope, M.J. (2001). External nasal cartilages in Bats: evidence for Microchiropteran Monophyly? J. Mamm. Evol. 8, 231–236. [Google Scholar]
- Sureau, P. , Germain, M. , Hervé, J. , Geoffroy, B. , Cornet, J. , Heme, G. & Robin, Y. 1977. Isolement du virus Lagos bat en Empire Centrafricain. Bull. Exotic Pathol. Soc. 70, 467–470. [PubMed] [Google Scholar]
- Swanepoel, R. , Barnard, B.J. , Meredith, C.D. , Bishop, G.C. , Brückner, G.K. , Foggin, C.M. & Hübschle, O.J. (1993). Rabies in southern Africa. Onderstepoort J. Vet. Res. 60, 325–346. [PubMed] [Google Scholar]
- Swanepoel, R. , Leman, P.A. , Burt, F.J. , Zachariades, N.A. , Braack, L.E.O. , Ksiazek, T.G. , Rollin, P.E. , Zaki, S.R. & Peters, C.J. (1996). Experimental Inoculation of plants and animals with Ebolavirus. Emerg. Infect. Dis. 2, 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanepoel, R. , Smit, S.B. , Rollin, P.E. , Formenty, P. , Leman, P.A. , Kemp, A. , Burt, F.J. , Grobbelaar, A.A. , Croft, J. , Bausch, D.G. , Zeller, H. , Leirs, H. , Braack, L.E.O. , Libande, M.L. & Zaki, S. (2007). Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 13, 1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y. , Shi, M. , Chommanard, C. , Queen, K. , Zhang, J. , Markotter, W. , Kuzmin, I.V. , Holmes, E.C. & Tong, S. (2017). Surveillance of Bat Coronaviruses in Kenya Identifies Relatives of Human Coronaviruses NL63 and 229E and Their Recombination, History. J. Virol. 91, JVI–01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, S. , Conrardy, C. , Ruone, S. , Kuzmin, I.V. , Guo, X. , Tao, Y. , Niezgoda, M. , Haynes, L. , Agwanda, B. , Breiman, R.F. , Anderson, L.J. & Rupprecht, C.E. (2009). Detection of novel SARS‐like and other coronaviruses in bats from Kenya. Emerg. Infect. Dis. 15, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner, J.S. , Amman, B.R. , Sealy, T.K. , Reeder Carroll, S.A. , Comer, J.A. , Kemp, A. , Swanepoel, R. , Paddock, C.D. , Balinandi, S. , Khristova, M.L. , Formenty, P.B.H. , Albarino, C.G. , Miller, D.M. , Reed, Z.D. , Kayiwa, J.T. , Mills, J.N. , Cannon, D.L. , Greer, P.W. , Byaruhanga, E. , Farnon, E.C. , Atimnedi, P. , Okware, S. , Katongole‐Mbidde, E. , Downing, R. , Tappero, J.W. , Zaki, S.R. , Ksiazek, T.G. , Nichol, S.T. & Rollin, P.E. (2009). Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bussche, R.A. & Hoofer, S.R. (2004). Phylogenetic relationships among recent Chripteran families and the importance of choosing appropriate out‐group taxa. J. Mammal. 85, 321–330. [Google Scholar]
- Van Der Merwe, M. (1982). Bats as vectors of rabies. S. Afr. J. Sci. 78, 421–422. [Google Scholar]
- Van Thiel, P.‐P.A.M. , de Bie, R.M.A. , Eftimov, F. , Tepaske, R. , Zaaijer, H.L. , van Doornum, G.J.J. , Schutten, M. , Osterhaus, A.D.M.E. , Majoie, C.B.L.M. , Aronica, E. , Fehlner‐Gardiner, C. , Wandeler, A.I. & Kager, P.A. (2009). Fatal human rabies due to Duvenhage virus from a bat in Kenya: failure of treatment with coma‐induction, Ketamine, and antiviral drugs. PLoS Negl. Trop. Dis. 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue, E.R. , Marsh, G.A. & Wang, L. (2009). Paramyxoviruses infecting humans: the old, the new and the unknown. Future Microbiol. 4, 537–554. [DOI] [PubMed] [Google Scholar]
- Wacharapluesadee, S. , Duengkae, P. , Chaiyes, A. , Kaewpom, T. , Rodpan, A. , Yingsakmongkon, S. , Petcharat, S. , Phengsakul, P. , Maneeorn, P. & Hemachudha, T. (2018). Longitudinal study of age‐specific pattern of coronavirus infection in Lyle’s flying fox (Pteropus lylei) in Thailand. Virol. J. 15, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.F. , Walker, P.J. & Poon, L.L.M. (2011). Mass extinctions, biodiversity and mitochondrial function: Are bats “special” as reservoirs for emerging viruses? Curr. Opin. Virol. 1, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waruhiu, C. , Ommeh, S. , Obanda, V. , Agwanda, B. , Gakuya, F. , Ge, X.Y. , Yang, X.Lou , Wu, L.J. , Zohaib, A. , Hu, B. & Shi, Z.L. (2017). Molecular detection of viruses in Kenyan bats and discovery of novel astroviruses, caliciviruses and rotaviruses. Virol. Sin. 32, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, S. , Nowak, K. , Fahr, J. , Wibbelt, G. , Mombouli, J.‐V. , Parra, H.‐J. , Wolfe, N.D. , Schneider, B.S. & Leendertz, F.H. (2012). Henipavirus‐related sequences in fruit bat bushmeat, Republic of Congo. Emerg. Infect. Dis. 18, 1536–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby, A.R. , Phelps, K.L. , Olival, K.J. & Consortium P . (2017). A comparative analysis of viral richness and viral sharing in cave‐roosting bats. Diversity 9, 1–16. [Google Scholar]
- Wilson, D.E. (1997). Bats in question: the Smithsonian answer book. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Wong, A.C. , Li, X. , Lau, S.K. & Woo, P.C. 2019. Global epidemiology of bat coronaviruses. Viruses 11, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P.C.Y. , Lau, S.K.P. , Li, K.S.M. , Poon, R.W.S. , Wong, B.H.L. , Tsoi, H. , Yip, B.C.K. , Huang, Y. , Chan, K. & Yuen, K. (2006). Molecular diversity of coronaviruses in bats. Virology 351, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P.C.Y. , Lau, S.K.P. , Lam, C.S.F. , Lau, C.C.Y. , Tsang, A.K.L. , Lau, J.H.N. , Bai, R. , Teng, J.L.L. , Tsang, C.C.C. , Wang, M. , Zheng, B.‐J. , Chan, K.‐H. & Yuen, K.‐Y. (2012). Discovery of seven novel Mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavi. J. Virol. 86, 3995–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E. , Hayman, D.T.S. , Vaughan, A. , Temperton, N.J. , Wood, J.L.N. , Cunningham, A.A. , Suu‐Ire, R. , Weiss, R.A. & Fooks, A.R. (2010). Virus neutralising activity of African fruit bat (Eidolon helvum) sera against emerging lyssaviruses. Virology 408, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X.‐L. , Hu, B. , Wang, B. , Wang, M.‐N. , Zhang, Q. , Zhang, W. , Wu, L.‐J. , Ge, X.‐Y. , Zhang, Y.‐Z. , Daszak, P. , Wang, L.‐F. & Shi, Z.‐L. (2016). Isolation and characterization of a novel bat Coronavirus closely related to the direct progenitor of severe acute respiratory syndrome Coronavirus. J. Virol. 90, 3253–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Tan, C.W. , Anderson, D.E. , Jiang, R. , Li, B. , Zhang, W. , Zhu, Y. , Lim, X.F. , Zhou, P. , Liu, X.‐L. , Guan, W. , Zhang, L. , Li, S.‐Y. , Zhang, Y.‐Z. , Wang, L.‐F. & Shi, Z.‐L. (2019). Characterization of a filovirus (Měnglà virus) from Rousettus bats in China. Nat. Microbiol. 4, 390–395. [DOI] [PubMed] [Google Scholar]
- Zaki, A.M. , van Boheemen, S. , Bestebroer, T.M. , Osterhaus, A.D.M.E. & Fouchier, R.A.M. (2012). Isolation of a novel coronavirus from a man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820. [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Cowled, C. , Shi, Z. , Huang, Z. , Bishop‐Lilly, K.A. , Fang, X. , Wynne, J.W. , Xiong, Z. , Baker, M.L. , Zhao, W. , Tachedjian, M. , Zhu, Y. , Zhou, P. , Jiang, X. , Ng, J. , Yang, L. , Wu, L. , Xiao, J. , Feng, Y. , Chen, Y. , Sun, X. , Zhang, Y. , Marsh, G.A. , Crameri, G. , Broder, C.C. , Frey, K.G. , Wang, L.‐F. & Wang, J. (2013). Comparative analysis of bat genomes. Science. 339, 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Tachedjian, M. , Wynne, J.W. , Boyd, V. , Cui, J. , Smith, I. , Cowled, C. , Ng, J.H.J. , Mok, L. , Michalski, W.P. , Mendenhall, I.H. , Tachedjian, G. , Wang, L.F. & Baker, M.L. (2016). Contraction of the type i IFN locus and unusual constitutive expression of IFN‐α in bats. Proc. Natl. Acad. Sci. U. S. A. 113, 2696–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]


