Abstract
The current outbreak of Coronavirus Disease 2019 (COVID-19) has raised great concern worldwide, but its impact on transplant recipients is unknown. We report here the clinical features and therapeutic course of the first reported renal transplant recipient with confirmed COVID-19 pneumonia. This is a 52-year-old man who received kidney transplantation 12 years ago. His overall clinical characteristics (symptoms, laboratory examinations, and chest CT) were similar to those of non-transplanted COVID-19 patients. Following a treatment regimen consisting of reduced immunosuppressant use and low dose methylprednisolone-based therapy, the COVID-19 pneumonia in this long-term immunosuppressive patient was successfully recovered. This effectively treated case has reference value for the future treatment of other transplant patients with COVID-19 pneumonia.
KEYWORDS: coronavirus, COVID-19, immunosuppression, pneumonia, renal transplantation
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; IL, interleukin; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; MP, methylprednisolone; PCR, polymerase chain reaction; Pred, prednisone; Scr, serum creatinine; Tac, tacrolimus; TNF-ɑ, tumor necrosis factor alpha
1. INTRODUCTION
In late December 2019, the epidemic of a novel coronavirus disease (COVID-19) broke out in Wuhan, China, and has subsequently spread to the rest of China and 37 other countries around the world.1, 2, 3, 4 As of February 26, 2020, there were 47 824 confirmed cases and 2104 deaths in Wuhan. Since Wuhan is one of the cities with the largest volume of kidney transplants in China, COVID-19 infection in renal transplant recipients deserves attention. As a population living with immunosuppression, the clinical manifestations, treatment and prognosis of COVID-19 pneumonia for renal transplant recipients may differ from those of the general population.5 However, no relevant reports have as yet been published. Herein, we report a case of COVID-19 pneumonia following kidney transplantation, which was successfully recovered after treatment with immunosuppressant reduction coupled with low dose methylprednisolone-based therapy. Our intervention protocol thus may serve as a template for the treatment of other such patients.
2. CASE REPORT
The patient is a 52-year-old man who underwent living-related kidney transplantation for his chronic glomerulonephritis 12 years ago following 4 years of hemodialysis. The donor was his father (63 years old at that time). The donor-recipient HLA-A, -B, -DR mismatch grade was 3. Since the transplant, this patient has received triple maintenance immunosuppressive therapy with oral tacrolimus (Tac), mycophenolate mofetil (MMF) and prednisone (Pred), and he has been regularly followed up at our hospital. At his most recent follow-up on December 4, 2019, his serum creatinine (Scr) level was 139 μmol/L, estimated glomerular filtration rate (eGFR) was 49.8 mL/min/1.73 m2, Tac trough level was 7 ng/mL, and there was no proteinuria.
On January 20, 2020, the patient came to Wuhan with his wife to visit a relative. Following this visit, he showed initial symptoms of fatigue, dyspnea, tightness and pain in the chest, nausea, loss of appetite, intermittent abdominal pain, and occasional dry coughs on January 28 (day one of illness). Two days later (January 30), he developed a fever (37.5°C) and headache ( Figure 1). However, he did not see a doctor and remained at his relative’s home until February 2 (day six of illness). On that same day (February 2) he visited a fever clinic in our hospital for treatment due to the intensification of his symptoms.
FIGURE 1.
Symptoms and treatments including immunosuppressive adjustment according to day of illness and day of hospitalization
At the fever clinic visit, the patient had a body temperature of 38.9°C. His laboratory ( Table 1) and radiological ( Figure 2A) results were as follows:
-
•
Lymphocyte count and lymphocyte percentage were significantly lower than baseline values;
-
•
Neutrophil and monocyte counts were markedly higher;
-
•
C-reactive protein (CRP) was significantly elevated (30.2 mg/L);
-
•
Chest computed tomography (CT) scan found multiple patchy ground-glass density shadows in the upper lobe of both lungs and the lower lobe of the left lung, as well as a small patchy ground-glass density shadow in the middle lobe of the right lung.
TABLE 1.
Clinical laboratory results
| Measure | Reference range | Baseline 2019/12/4 | Fever clinic 2020/2/2 | D2 2020/2/5 | D6 2020/2/9 | D9 2020/2/12 | D11 2020/2/14 |
|---|---|---|---|---|---|---|---|
| ALT (U/L) | ≤41 | 20 | 30 | 74 | 94 | 61 | |
| AST (U/L) | ≤40 | 22 | 29 | 41 | 33 | 23 | |
| Albumin (g/L) | 35-52 | 46.7 | 35.2 | 36.3 | 34.6 | 35.7 | |
| IgG (g/L) | 20-35 | 29.6 | 31.7 | 34.8 | 42.2 | 42.3 | |
| BUN (mmol/L) | 3.1-8.0 | 6.59 | 7.8 | 5.8 | 8.5 | 8 | |
| SCr (µmol/L) | 59-104 | 139 | 143 | 112 | 112 | 104 | |
| eGFR (mL/min/1.73m2) | >90 | 49.8 | 48.2 | 64.7 | 64.7 | 70.8 | |
| CRP (mg/L) | <3.0 | 30.2 | 54.0 | 29.8 | 3.0 | 1.4 | |
| WBC (×109/L) | 3.50-9.50 | 6.97 | 9.16 | 5.54 | 6.46 | 10.36 | 11.68 |
| NEUT (%) | 40.0-75 | 61.6 | 78.3 | 68.9 | 72.9 | 78.9 | 80.9 |
| NEUT (×109/L) | 1.8-6.3 | 4.29 | 7.17 | 3.82 | 4.71 | 8.17 | 9.45 |
| LYM (%) | 20.0-50.0 | 30.4 | 12.3 | 17.9 | 15.6 | 13.2 | 12.0 |
| LYM (×109/L) | 1.10-3.20 | 2.12 | 1.13 | 0.99 | 1.01 | 1.37 | 1.40 |
| MONO (%) | 3.0-10 | 6.9 | 9.5 | 13.0 | 11.3 | 7.8 | 6.9 |
| MONO (×109/L) | 0.1-0.6 | 0.48 | 0.84 | 0.72 | 0.73 | 0.81 | 0.81 |
| PLT (×109/L) | 125-350 | 190 | 126 | 125 | 199 | 354 | 367 |
| Urine protein | Negative | Negative | 1+ | Trace | |||
| Urine gravity | 1.101-1.025 | 1.022 | 1.022 | 1.016 | |||
| IL-1beta (pg/mL) | <5.0 | <5.0 | |||||
| IL-2 receptor (U/mL) | 223-710 | 863 | |||||
| IL-6 (pg/mL) | <7 | 19.53 | |||||
| IL-8 (pg/mL) | <62 | 9.6 | |||||
| IL-10 (pg/mL) | <9.1 | <5.0 | |||||
| TNF alpha | <8.1 | 13.1 |
Note: Values in bold were either above normal or below normal.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate, based on CKD-EPI creatinine 2009 equation method; IL, interleukin; LYM, lymphocyte; MONO, monocyte; NEUT, neutrophil; PLT, platelet; SCr, serum creatinine; TNF, tumor necrosis factor; WBC, white blood cell.
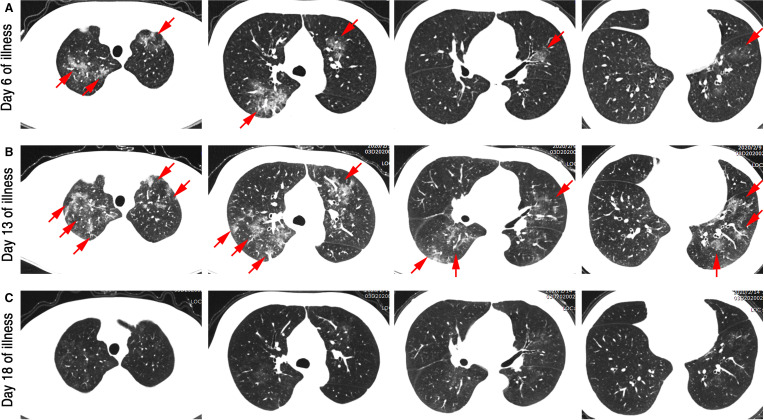
FIGURE 2.
High-resolution computed tomography images before and after treatment. A, Multiple ground glass density lesions were observed in the upper and lower lobe of the lung, some with consolidation, although without obvious subpleural distribution. B, The lesion range enlarged, and new lesions were observed. C, The lesion was almost completely absorbed, leaving only a few blurs [Color figure can be viewed at wileyonlinelibrary.com]
These abnormalities suggested the possibility of COVID-19 pneumonia. We therefore immediately advised the patient to discontinue all immunosuppressive agents and instead to take oral Umifenovir Tablets (0.2 g, tid) and Moxifloxacin Tablets (0.4 g, qd) (Figure 1). Meanwhile, we subjected a throat swab sample to reverse real-time polymerase chain reaction (PCR) assay in order to test for the presence of COVID-19.
Two days later (day eight of illness), the assay results indicated that the patient tested positive for COVID-19. The patient’s symptoms continued unabated and, in addition, he lost 10 kg of body weight within 1 week due to poor eating. Because of his ongoing symptoms, at 10 pm that night (day eight of illness), the patient was admitted to an isolation ward in our hospital. On the same day, his wife and the relative he had visited were also diagnosed with COVID-19, although their symptoms were noticeably milder than his.
Physical examination on admission to the isolation ward revealed a body temperature of 37.7°C, blood pressure of 136/100 mm Hg, a pulse of 68 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 96% while the patient breathed ambient air.
On the morning of the second day of admission (day nine of illness), the patient’s body temperature was 38°C, pulse was 90 beats per minute, and a blood gas analysis showed that the partial pressure of oxygen was 77.4 mm Hg, the partial pressure of carbon dioxide was 35.0 mm Hg, and pH level was 7.368. Oxygen was administered to the patient at 2 L/min via a nasal catheter. The results of laboratory examinations were as follows (Table 1):
-
•
No IgM antibody was detected against eight different respiratory pathogens which include respiratory syncytial virus, adenovirus, influenza virus A, influenza virus B, parainfluenza, legionella pneumoniae, mycoplasma, and chlamydia;
-
•
Peripheral blood lymphocyte count continued to decrease (0.99 × 109/L);
-
•
CRP continued to rise (54 mg/L);
-
•
Erythrocyte sedimentation rate (ESR) increased substantially (35 mm Hg);
-
•
Procalcitonin was normal;
-
•
Serum levels of some inflammatory cytokines were elevated, including interleukin 2 (IL-2) receptor, IL-6, and tumor necrosis factor alpha (TNF-ɑ);
-
•
There was a slight increase in urinary protein (1+), although renal graft function did not deteriorate significantly.
The patient was then given the following treatment (Figure 1):
-
•
Discontinue all immunosuppressive agents;
-
•
Methylprednisolone (MP 40 mg daily, intravenously);
-
•
Intravenous immunoglobulin (IVIG, 5 g on the first day and 10 g/d for the next 11 days);
-
•
Biapenem (0.3 g iv drip q12h);
-
•
Pantoprazole (60 mg iv qd);
-
•
Interferon α (5 million units daily, atomization inhalation).
On hospital day five (day 12 of illness), the patient’s body temperature returned to normal, nausea and chest tightness were relieved, and oral tacrolimus was resumed at half its original dosage following 5 days of complete discontinuation from February 2 to 7 (Figure 1). On hospital day six, the patient’s CRP started to decrease (29.8 mg/L). However, a second chest CT showed that the range of lesions in both lungs had enlarged and that new lesions had appeared (Figure 2B). Additionally, the patient had an increased level of serum alanine aminotransferase (ALT) (Table 1). Therefore, glycyrrhizic acid diamine (100 mg po, tid) was administered to protect liver function.
On hospital day nine (day 16 of illness), the patient’s weakness and dry cough had significantly improved. More importantly, analysis of a throat swab sample was negative for the presence of COVID-19. Additionally, CRP levels decreased to nearly normal (3 mg/L), and the lymphocyte count began to rise (1.37 × 109/L) (Table 1). On hospital day 11 (day 18 of illness), the patient felt well, and COVID-19 nucleic acid again tested negative. Meanwhile, a third chest CT showed that the lung lesions were almost completely resolved, with only a few indistinct shadows remaining (Figure 2C). Supplemental oxygen was discontinued, and the patient’s oxygen saturation values were maintained at above 96%. Moreover, we began administering oral tacrolimus and MMF to their full pre-illness dosage levels (Figure 1). Three days later (hospital day 13, illness day 21), the patient was discharged from hospital. To date, he has been in good health at home for a week.
3. DISCUSSION
The immune response of renal transplant recipients, particularly the T cell immune response, is significantly suppressed due to the long-term use of immunosuppressive agents. This means that some of the clinical manifestations of COVID-19 infection in this population may be distinctive and that treatment methods for COVID-19 pneumonia require careful consideration. Regarding the case discussed in this report, overall clinical characteristics (symptoms, laboratory examinations, and chest CT) were similar to those of other non-transplanted adult patients with COVID-19 pneumonia (ie, those patients without atypical or silent manifestations due to immunosuppression6). Regarding treatment, we thought that the peculiarity of transplant recipients may lie in the need to consider adjusting immunosuppressive agents while protecting graft function.
When treating pneumonia due to opportunistic virus infection following kidney transplantation, a reduction or even temporary discontinuation of immunosuppressants is a common strategy and allows recipients the opportunity to reacquire anti-infection immunity within a short period, which is conducive to eliminating the virus.7 , 8 In this patient’s case, we first discontinued all immunosuppressants to minimize the severity of symptom progression associated with his COVID-19 pneumonia. Next, considering the progress of the patient’s illness and his subsequent improvement, immunosuppressive agents were gradually reinstated. However, for the period prior to reinstating oral immunosuppressants, we considered it important to use appropriate doses of intravenous corticosteroids. The immunosuppressive effect of corticosteroids might protect the renal allograft from acute rejection and avoid imminent Addison crisis in the period of the discontinuation of oral steroid; moreover, the anti-inflammatory effect of corticosteroids may also reduce alveolar exudation and relieve systemic symptoms (such as fever or fatigue) caused by the storm of inflammatory factors.9 , 10 It is crucial to pay attention to the dosage and duration of steroid administration. Long duration and excessive doses of steroids may adversely affect recovery due to the inhibition of antiviral immunity, and may also result in other side effects related to steroids.11 In the present case, the patient was given 40mg of methylprednisolone daily for 12 days, which may have played an important role in the faster recovery from his pneumonia without the occurrence of severe side effects.
We reported the clinical features and therapeutic course of the first reported renal transplant recipient with COVID-19 pneumonia. Following a treatment regimen consisting of reduced immunosuppressant use and low dose methylprednisolone-based therapy, the COVID-19 pneumonia in this long-term immunosuppressive patient was successfully recovered, thus providing a reference case for treating such patients. However, we realize that a single successfully treated case cannot define effective therapy. The patient recovered well, but received a variety of novel therapies which may or may not have been helpful. It is difficult to extrapolate from this patient to recommendations for the transplant population. More experience needs to be accumulated to optimize the treatment protocol for immunocompromised transplant recipients with COVID-19.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. Study procedures were approved by the institutional review board (IRB) at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB approval number: TJ-C20200120). The clinical activities being reported are consistent with the principles of the declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. [published online ahead of print 2020]. 10.1001/jama.2020.2648. [DOI] [PubMed]
- 5.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. [published online ahead of print 2020]. 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed]
- 6.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 8.Kumar D, Michaels MG, Morris MI, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10(8):521–526. doi: 10.1016/S1473-3099(10)70133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansbury L, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2019;2:CD010406. doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.