We are living in times where a viral disease has brought normal life in much of the world to a halt. Named after its causative agent, coronavirus disease 2019 (COVID‐19) is caused by a novel coronavirus recently renamed as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 Coronavirus disease 2019 manifests as dry cough, frequent fevers and in severe cases pneumonia. Older patients and patients with underlying comorbidities are at a higher risk of death. 2 , 3 Until 11 March 2020, when the World Health Organization characterized the situation as a pandemic, there were 118 000 cases in 114 countries with 4291 deaths. It is the first pandemic caused by a coronavirus. 4 , 5
In such times, it would not be surprising if a lot of us were wondering, how something that is invisible even under a light microscope infects people and wreaks such a havoc. While we do not have all the answers yet, refreshing our basics and understanding the what, the why and the how of the virus would definitely help us better understand the situation.
1. WHAT IS A VIRUS?
A virus (Latin for poisonous liquid) is traditionally defined as a filterable agent that causes disease. 6 There are an estimated 1 × 1031 viruses on earth. 7 That number is astronomically large; if we put all of the viruses end to end, they would cover a distance of 100 million light years. 8 Luckily, most of them are bacteriophages, that is, viruses that infect bacteria. 7 The number of virus species that are able to infect humans was 219 in 2012, a number that has unfortunately increased by at least one. 9
All viruses are molecular parasites that cannot replicate outside a host cell. Once inside the cell, however, a virus hijacks the cellular machinery forcing it to produce more viruses. 10 Outside the host cell, a virus packs itself into individual infectious particles called virions. The chemical composition of a virion varies from virus to virus and is an important distinguishing character used in classification of viruses. 11 A virion typically contains a genome made up of one or several segments of single or double stranded DNA or RNA. A coat of structural viral proteins called the capsid surrounds this genome. In some viruses, the capsid itself is surrounded by a host cell‐membrane derived envelope. 10 , 11
Virions can be as small as 20 nm (adeno‐associated virus) in diameter and as large as 0.5 µm (mimiviruses). 12 , 13 The latter defy the traditional definition by being non‐filterable through bacteriological filters. 13 Virions also vary in shape. Filamentous viruses have their capsid proteins arranged in a helical array around the genome while “spherical” viruses have an icosahedral morphology. Moreover, the number of subunits forming the icosahedron and their arrangement are also used for classification. 11
2. SARS‐CoV‐2: STRUCTURE, ORIGIN AND TRANSMISSION
Coronaviruses (CoVs) are enveloped viruses with a positive‐sense single‐stranded RNA genome. The replicase gene occupies two‐thirds of the ~30 kb long genome and encodes the non‐structural proteins (NSPs). The remaining third encodes the structural proteins namely the spike (S), membrane (M), nucleocapsid (N) and envelope (E) proteins. The S protein is involved in the attachment and entry of the virus. 14
In the past 50 years, several veterinary and human CoVs that cause a wide array of diseases have emerged. CoVs were known to cause mild respiratory illness in humans until the SARS‐CoV outbreak in 2002‐2003. 14 Middle East respiratory syndrome CoV (MERS‐CoV) emerged in 2012 and has since spread to more than 27 countries. 15 The causative agent of the COVID‐19 pandemic, SARS‐CoV‐2, is also a zoonotic virus. Based on genome sequencing and evolutionary analysis, the virus is suspected to have originated in bats and transmitted to humans via an unknown intermediate. 16
Most emerging infectious diseases are zoonotic, that is, they are naturally transmitted between animals and humans. 17 Viruses, especially RNA viruses, are able to adapt to newer hosts and environments because of their higher mutation rates. 18 , 19 Zoonoses can occur in both urban and rural settings. While certain zoonoses like the bacterial anthrax show no secondary human‐to‐human transmission, many viral diseases like Ebola, and the currently ongoing COVID‐2 do. 20 , 21 If and how efficiently a zoonotic virus can transmit from human‐to‐human determines its ability to cause a major epidemic such as the one we are experiencing now. 22 The efficiency of transmission is measured as the virus' basic reproduction number, R 0, which denotes the average number of secondary infections caused by an infected individual in an immunologically naïve population. In general, a virus is able to cause an epidemic if R 0 > 1. An outbreak ends when R0 falls below one. 22 , 23
Severe acute respiratory syndrome coronavirus 2 transmits primarily via droplets, respiratory secretions and direct contact. Presence of the virus in faeces and blood suggests other potential modes of transmission. The incubation period for SARS‐CoV‐2 is between 1 and 14 days and asymptomatic individuals can transmit the virus during this period. 16 Coronavirus disease 2019 presents with mild flu‐like symptoms in most children and adults. However, patients with other comorbidities may develop acute respiratory distress syndrome, respiratory failure, multiple organ failure and even death. 2 , 3 , 16
3. CORONAVIRUS: LIFE CYCLE
One can think of viruses as molecular nanomachines that take over the host cell and force it to produce numerous copies of themselves. 24 Even though the replicative life cycle of viruses varies greatly depending on the species and the category of the virus, it consists of six basic stages, viz. attachment, entry, uncoating, replication, maturation and release. 25
Angiotensin converting enzyme 2 (ACE2) is the cellular receptor for the SARS‐CoV‐2. 26 , 27 The S protein of the virus interacts and binds to ACE2 in the first stage of virus replication called “attachment.” 16 , 27 The specificity of this binding or “attachment” determines which cell type a virus can infect, a phenomenon called cell tropism. 25 Therefore, all cells that express ACE2 including type II alveolar cells of the lung, upper and stratified epithelial cells of the oesophagus, absorptive enterocytes, myocardial cells, cholangiocytes, proximal tubule cells of the kidney and bladder urothelial cells are potentially susceptible to SARS‐CoV‐2 infection. 27
The second stage called “entry” leads to the insertion of the viral replication complex into the cytoplasm of the host cell. 25 In case of SARS‐CoV, the S protein is cleaved by the cellular transmembrane serine protease 2. 28 This exposes a fusion peptide, which then inserts itself into the cellular membrane initiating the fusion of the viral and cellular membranes. As a result, the viral genome enters the cytoplasm. 14 In the following stage, direct translation of the positive‐sense viral RNA genome leads to de novo synthesis of viral structural and NSPs. The NSPs, coded by the viral replicase gene, are responsible for the replication of the viral genome. This is followed by the "assembly" or “maturation” stage where newly synthesized viral structural proteins, viz. E, M and S are inserted into the endoplasmic reticulum‐trans Golgi intermediate compartment (ERGIC). Viral genomes coated with the N protein then enter the ERGIC via budding to form mature virions. Mature virions then travel to the cell surface inside vesicles and exit the cells by exocytosis. 14 A novel furin‐like cleavage site has recently been discovered in SARS‐CoV‐2 spike protein. This cleavage site, which is absent in SARS‐CoV, might be involved in viral egress and provide for the efficient spread of the virus in human population. 29
4. ANTIVIRALS AND VACCINE
Each of the six stages of replication is a potential target for antiviral drugs. The modes of action of approved antivirals include inhibition of virus entry or fusion (enfuvirtide), inhibition of uncoating (amantadine), inhibition of nucleic acid synthesis (acyclovir, ribavirin), maturation inhibitors (lopinavir, ritonavir) and inhibitors of virus release (oseltamivir). Nucleoside analogues and other inhibitors of nucleic acid synthesis are perhaps the largest group of antivirals available. 30 Currently, there are more than 90 antiviral drugs approved for use in humans. More than half of these are used to treat HIV infections, a third against herpes simplex virus, human cytomegalovirus, varicella zoster virus and influenza, and the remainder for miscellaneous viruses. 30 , 31 Drug repurposing is, therefore, a frequently applied strategy for developing treatment regimens in situations such as the current outbreak. 32 , 33
Currently, there is no effective antiviral treatment against COVID‐19. However, antivirals that have shown some effect on SARS and MERS are under trial. 16 The combination of two protease inhibitors used in anti‐retroviral therapy, viz. lopinavir/ritonavir, has shown some promise. 34 , 35 A combination of lopinavir/ritonavir and ribavirin was shown to improve disease outcome. Ribavirin is a nucleoside analogue used as broad‐spectrum antiviral. 35 Tests are also underway to assess the potential efficacy of remdesivir, a novel nucleotide analogue developed for treating Ebola virus. 33 , 35 Chloroquine, a widely used anti‐malaria drug, has also shown efficacy. 36 , 37 Angiotensin I receptor blockers such as losartan are thought to reduce lung injury in SARS cases and can potentially also be used in treatment of COVID‐19. 26
Between 6 and 15 different prophylactic vaccines are in the pipeline. 38 In silico predictions for several vaccine targets are also available. 39 , 40 , 41 The National Institutes of Health has begun a clinical trial of an mRNA vaccine, mRNA‐1273. 42 Besides the mRNA vaccine, several different kinds of vaccines including subunit vaccines, DNA vaccines, live‐attenuated vaccines, and virus like particles are at different stages of development. 38
5. OUTLOOK
In the absence of a prophylactic vaccine, we have two potential options to deal with the pandemic: impose restriction to break the spread of the virus or wait for herd immunity to develop. The boat has sailed on the former given that virus has already spread to more than 114 countries. 4 Amid fears that our healthcare system will not be able to keep up with the demand, we need to be patient with the latter option. The Italian system is already on the brink and some hospitals have issued guidelines on “catastrophic medicine”. 43 The only way forward, therefore, is to let the virus spread but slowly enough so that hospitals are not overwhelmed. “Flatten the curve” is the buzzword derived from the CDC's community mitigation guidelines for pandemic influenza and flatten the curve, we must. 44
CONFLICT OF INTEREST
We declare that we have no conflicts of interest to disclose.
6.
FIGURE 1.
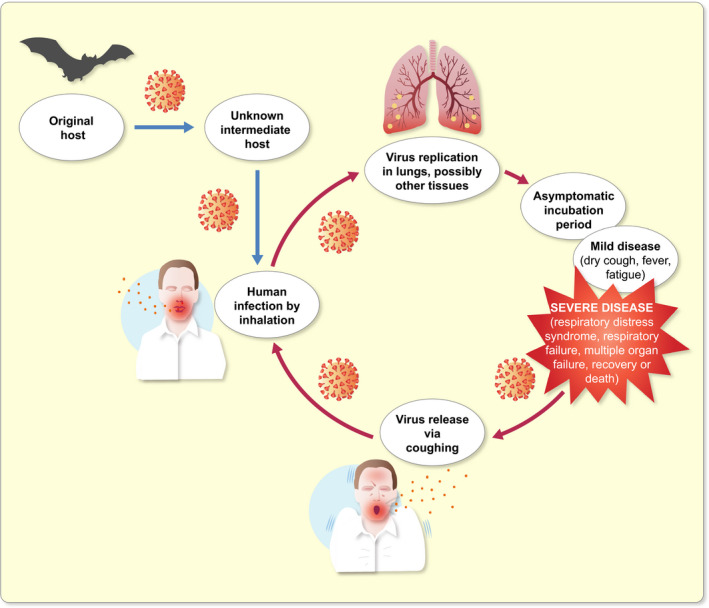
Transmission cycle of severe acute respiratory syndrome coronavirus 2
REFERENCES
- 1. World Health Organization . Naming the coronavirus disease (COVID‐19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/naming‐the‐coronavirus‐disease‐(covid‐2019)‐and‐the‐virus‐that‐causes‐it. Accessed March 17, 2020.
- 2. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China [published online ahead of print, 2020]. N Engl J Med. 1‐13. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ducharme J. World Health Organization declares COVID‐19 a ‘pandemic.’ Here's what that means. Time. 2020. https://time.com/5791661/who‐coronavirus‐pandemic‐declaration/. Accessed March 17, 2020. [Google Scholar]
- 5. World Health Organization . WHO Director‐General's opening remarks at the media briefing on COVID‐19. 2020;(March). https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19–‐11‐march‐2020. Accessed March 17, 2020.
- 6. Levine AJ, Enquist LW. History of virology. In: Knipe DM, Howley PM, eds. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:5‐6. [Google Scholar]
- 7. Breitbart M, Rohwer F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005;13(6):278‐284. [DOI] [PubMed] [Google Scholar]
- 8. Microbiology by numbers. Nat Rev Microbiol. 2011;9(9):628. [DOI] [PubMed] [Google Scholar]
- 9. Woolhouse M, Scott F, Hudson Z, Howey R, Chase‐Topping M. Human viruses: discovery and emergence. Philos Trans R Soc B Biol Sci. 2012;367(1604):2864‐2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Viruses: structure, function, and uses. In: Molecular Cell Biology. New York, NY: W. H. Freeman; 2001. https://www.ncbi.nlm.nih.gov/books/NBK21523/. Accessed March 17, 2020. [Google Scholar]
- 11. Gelderblom H. Structure and classification of viruses. In: Baron S, ed. Medical Microbiology. Galveston, TX: University of Texas Medical Branch; 1996. https://www.ncbi.nlm.nih.gov/books/NBK8174/. Accessed March 17, 2020. [PubMed] [Google Scholar]
- 12. Duan D. From the smallest virus to the biggest gene: marching towards gene therapy for duchenne muscular dystrophy. Discov Med. 2006;6(33):103‐108. [PMC free article] [PubMed] [Google Scholar]
- 13. Claverie J‐M, Abergel C. Family—Mimiviridae. In: King AM, Adams MJ, Carstens EB, Lefkowitz EJ, eds. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier Inc.; 2012:223‐228. [Google Scholar]
- 14. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chafekar A, Fielding BC. MERS‐CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2). 10.3390/v10020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—an update on the status. Mil Med Res. 2020;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stärk KDC, Morgan D. Emerging zoonoses: tackling the challenges. Epidemiol Infect. 2015;143(10):2015‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanjuán R, Domingo‐Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016;73(23):4433‐4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regoes RR, Hamblin S, Tanaka MM. Viral mutation rates: modelling the roles of within‐host viral dynamics and the trade‐off between replication fidelity and speed. Proc R Soc B. 2013;280(1750). 10.1098/rspb.2012.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cross AR, Baldwin VM, Roy S, Essex‐Lopresti AE, Prior JL, Harmer NJ. Zoonoses under our noses. Microbes Infect. 2019;21(1):10‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan J‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker JW, Han BA, Ott IM, Drake JM. Transmissibility of emerging viral zoonoses. PLoS ONE. 2018;13(11):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH. Complexity of the basic reproduction number (R0). Emerg Infect Dis. 2019;25(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hemminga MA, Vos WL, Nazarov PV, et al. Viruses: incredible nanomachines. New advances with filamentous phages. Eur Biophys J. 2010;39(4):541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harper DR. Virus replication. eLS. 2012;(May):1‐8. 10.1002/9780470015902.a0000438.pub2 [DOI] [Google Scholar]
- 26. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics [published online ahead of print February, 2020]. Drug Dev Res. 2‐5. 10.1002/ddr.21656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor article SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019‐nCoV contains a furin‐like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nováková L, Pavlík J, Chrenková L, Martinec O, Červený L. Current antiviral drugs and their analysis in biological materials—part I: antivirals against respiratory and herpes viruses. J Pharm Biomed Anal. 2018;147:400‐416. [DOI] [PubMed] [Google Scholar]
- 31. Chaudhuri S, Symons JA, Deval J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antiviral Res. 2018;155:76‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Hou Y, Shen J, Huang Y, Martin W. Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discov. 2020;6(14). 10.1038/s41421-020-0153-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for COVID‐19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim J, Jeon S, Shin H‐Y, et al. Letter to the editor: case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35(6):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discov Ther. 2020;14(1):58‐60. [DOI] [PubMed] [Google Scholar]
- 36. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72‐73. [DOI] [PubMed] [Google Scholar]
- 37. Colson P, Rolain J‐M, Lagier J‐C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents. 2020;105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pang J, Wang MX, Ang IYH, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): a systematic review. J Clin Med. 2020;9(3). 10.3390/jcm9030623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID‐19 coronavirus (SARS‐CoV‐2) based on SARS‐CoV immunological studies. Viruses. 2020;12(3). 10.1101/2020.02.03.933226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): immunoinformatics approach. J Med Virol. 2020. 10.1002/jmv.25736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol. 2020. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 42. National Insititutes of Health . NIH clinical trial of investigational vaccine for COVID‐19 begins. 2020. https://www.niaid.nih.gov/news‐events/nih‐clinical‐trial‐investigational‐vaccine‐covid‐19‐begins. Accessed March 18, 2020.
- 43. Horowitz J. Italy's health care system groans under coronavirus—a warning to the world. New York Times. 2020. https://www.nytimes.com/2020/03/12/world/europe/12italy‐coronavirus‐health‐care.html. Accessed March 19, 2020. [Google Scholar]
- 44. Qualls NL, Levitt AM, Kanade N, et al. Community mitigation guidelines to prevent pandemic influenza—United States, 2017. Morb Mortal Wkly Report Recomm Reports. 2017;66(1):1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]


