Abstract
The outbreak of the novel coronavirus in China (SARS‐CoV‐2) that began in December 2019 presents a significant and urgent threat to global health. This study was conducted to provide the international community with a deeper understanding of this new infectious disease. Epidemiological, clinical features, laboratory findings, radiological characteristics, treatment, and clinical outcomes of 135 patients in northeast Chongqing were collected and analyzed in this study. A total of 135 hospitalized patients with COVID‐19 were enrolled. The median age was 47 years (interquartile range, 36‐55), and there was no significant gender difference (53.3% men). The majority of patients had contact with people from the Wuhan area. Forty‐three (31.9%) patients had underlying disease, primarily hypertension (13 [9.6%]), diabetes (12 [8.9%]), cardiovascular disease (7 [5.2%]), and malignancy (4 [3.0%]). Common symptoms included fever (120 [88.9%]), cough (102 [76.5%]), and fatigue (44 [32.5%]). Chest computed tomography scans showed bilateral patchy shadows or ground glass opacity in the lungs of all the patients. All patients received antiviral therapy (135 [100%]) (Kaletra and interferon were both used), antibacterial therapy (59 [43.7%]), and corticosteroids (36 [26.7%]). In addition, many patients received traditional Chinese medicine (TCM) (124 [91.8%]). It is suggested that patients should receive Kaletra early and should be treated by a combination of Western and Chinese medicines. Compared to the mild cases, the severe ones had lower lymphocyte counts and higher plasma levels of Pt, APTT, d‐dimer, lactate dehydrogenase, PCT, ALB, C‐reactive protein, and aspartate aminotransferase. This study demonstrates the clinic features and therapies of 135 COVID‐19 patients. Kaletra and TCM played an important role in the treatment of the viral pneumonia. Further studies are required to explore the role of Kaletra and TCM in the treatment of COVID‐19.
Keywords: clinical features, cognition, COVID‐19, northeast Chongqing, treatment
Research Highlights
83.7% of the patients had contact history in Wuhan or had been to Wuhan or had contact with people from Wuhan.
Common symptoms included fever, cough, and fatigue. Other symptoms include myalgia, fatigue, dyspnea, anorexia, etc.
Common complications of the patients include acute respiratory distress syndrome, acute cardiac injury, acute kidney injury, secondary infection and shock. ICU patients were more likely to have these complications than non‐ICU patients.
Compared with non‐ICU patients, ICU patients had lower lymphocyte count, and higher plasma levels of the Pt, APTT, D‐dimer, LDH, PCT, ALB, CRP, AST.
All patients received antiviral therapy (kaletra or interferon), antibacterial therapy and corticosteroid and many received traditional chinese medicine. It was suggested that patients should use kaletra early.
1. INTRODUCTION
An outbreak of a series of pneumonia cases of unknown cause that began in Wuhan, China has continued since December 2019. This illness is reported to have affected more than 79 602 people so far (74 680 confirmed, 4922 suspected, 16 646 cured, and 2122 deaths). In addition to Hubei, Wanzhou, Chongqing ranks third in infection rates among all of the cities in the provinces of China. A literature review was performed, and it was found that there have been no studies regarding the characteristics of patients infected with COVID‐19 in Chongqing. Clinically, the disease is characterized by fever, dyspnea, dry cough, and fatigue. Upper‐respiratory tract symptoms are not prominent, but diarrhea was reported by some patients. Pulmonary imaging has shown multiple ground glass and infiltrative shadows in both lungs. Severe cases have been shown to develop into acute respiratory distress syndrome (ARDS) and septic shock. On 7 January 2019, scientists successfully isolated the pathogen that causes the pneumonia, a new type of β‐coronavirus, and it was named 2019‐nCoV. 1 Subsequently, WHO named it coronavirus disease (COVID‐19). An epidemiological survey showed that the first occurrence of COVID‐19 patients was closely related to a seafood market in south China. Due to the “Spring Festival Movement” (known as the “annual migration of the population in China”), COVID‐19 rapidly spread throughout the country, and the number of infected people gradually increased. The spread of COVID‐19 among people has been confirmed to occur through multiple channels, such as droplets, aerosols, feces, and mouth mucus membranes. 1
The aim of this study is to describe the epidemiological and clinical features, laboratory findings, radiological characteristics, treatment, and outcomes of COVID‐19 patients in northeast Chongqing. It is hoped that these findings will assist the global community to more clearly understand and treat this new infectious disease.
2. METHODS
2.1. Study design and participants
This case series was approved by the Institutional Ethics Board of Chongqing University Three Gorges Hospital (also named Three Gorges Central Hospital, No: 2020‐2). All of the patients were from northeast Chongqing admitted to the Chongqing University Three Gorges Hospital from 23 January to 8 February 2020 with subsequent confirmed cases of COVID‐19. A total of 135 patients were enrolled in this study. Oral consent was obtained from all of the patients. The Chongqing University Three Gorges Hospital, located in northeast Chongqing, is a teaching hospital consisting of nine medical colleges and universities, including the Third Military Medical University, the Southwest Medical University, and the North Sichuan Medical College. This facility is responsible for the treatment of COVID‐19 patients as assigned by the government. All of the patients with COVID‐19 enrolled in this study were diagnosed according to WHO interim guidance 2 and were jointly diagnosed by a multidisciplinary diagnosis and treatment team composed of infectious disease experts, respiratory medicine staff, critical care medical staff, and emergency medicine staff. The clinical outcomes (eg, discharge, mortality, and length of stay) were monitored from 8 February 2020 to the final date of follow‐up.
2.2. Case collection
Clinical data of 135 COVID‐19 patients were collected from 23 January 2020, when the first case in northeast Chongqing was found, to 8 February 2020. The research team collected the clinical data of patients using the electronic medical record system (HIS). General information included epidemiological histories, current medical histories, symptoms, physical signs, laboratory examination results, chest computed tomography (CT) manifestations, treatment measures, complications, admissions to intensive care unit (ICU), and other parameters. The patients were divided into mild (including normal and mild) and severe (including severe and critical) groups. The mild group had mild clinical symptoms and no pneumonia on imaging. The normal group had symptoms of fever, respiratory tract symptoms, and imaging showed pneumonia. The severe group had respiratory distress, RR ≥ 30 beats/minute in a resting state, a mean oxygen saturation of ≤93%, and an arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mm Hg. The critical group had respiratory failure and required mechanical ventilation, the occurrence of shock, and the combined failure of other organs that required ICU monitoring and treatment. 3
2.3. Coronavirus detection
All of the suspected cases were detected using real‐time reverse transcription polymerase chain reaction (PCR), and those who were positive for the coronavirus RNA were identified as confirmed cases. Throat swab samples of the patients were collected, and COVID‐19 was detected using a Novel Coronavirus 2019‐nCoV Nucleic Acid Detection Kit (fluorescent PCR) (Suzhou Tianlong Biotechnology Co Ltd, Jiangsu, China). The throat swab samples, primers, fluorescent probes, reaction buffer, and enzyme mixture were prepared in a reaction system according to a certain procedure, and then amplified according to the following procedures: (a) 50°C 30 minutes; (b) 95°C 10 minutes; (c) 94°C 15 seconds → 50°C 30 seconds → 72°C 30 seconds, 5 cycles; (d) 94°C 10 seconds → 58°C 30 seconds, 35 cycles.
2.4. Statistical analysis
The categorical variables were described as frequency rates and percentages, and the continuous variables were described using the mean, median, and interquartile range (IQR) values. The continuous variables were compared using independent group t tests when the data were normally distributed; otherwise, the Mann‐Whitney test was used. The proportions of categorical variables were compared using a χ 2 test. All of the statistical analyses were performed using GraphPad Prism 8. For unadjusted comparisons, a two‐sided α of less than .05 was considered to be statistically significant. The analyses were not adjusted for multiple comparisons, and given the potential for type I error, the findings should be interpreted as exploratory and descriptive.
2.5. Presenting characteristics
Among the 135 hospitalized patients, 40 (29.6%) cases were divided into the severe group and 95 (70.4%) cases were divided into the mild group. The median age of all of the patients was 47 years (IQR, 36‐55), and 72 (53.3%) cases were male. Compared to mild patients, severe patients were significantly older (median age 56 years [IQR, 52‐73] vs 44 years [IQR, 33‐49]; P < .001) and were more likely to have underlying comorbidities, such as diabetes (9 [22.5%] vs 3 [3.1%]), cardiovascular disease (6 [15%] vs 1 [1%]), hypertension (4 [10%] vs 9 [9.4%], and malignancy (3 [7.5%] vs 1 [1%]) (Table 1). A majority of 135 patients had a history of exposure in Wuhan (Figure 1).
Table 1.
Demographics and baseline characteristics of patients infected with COVID‐19
| All patients (n = 135) | Mild cases (n = 95) | Severe cases (n = 40) | P value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, y | 47 (36‐55) | 44 (33‐49) | 56 (52‐73) | <.0001 |
| Sex | ||||
| Men | 72 (53.3%) | 52 (54.7%) | 21 (52.5%) | .9609 |
| Women | 63 (46.7%) | 43 (45.3%) | 19 (47.5) | .9609 |
| Epidemiological | ||||
| Living/traveling in epidemic area | 71 52.6%) | 56 (58.9%) | 15 (37.5%) | .0366 |
| Contact positive patients | 23 (17.0%) | 18 (18.9%) | 5 (12.5%) | .5098 |
| Contact with suspected patients in epidemic area | 19 (14.1%) | 10 (10.5%) | 9 (22.5%) | .1198 |
| No clear contact history | 15 (11.1%) | 9 (9.5%) | 6 (15%) | … |
| Agglomerative disease | 3 (2.2%) | 2 (2.1%) | 1 (2.5%) | … |
| Current smoking | 9 (6.7%) | 8 (8.4%) | 1 (2.5%) | … |
| Any comorbidity | 43 (31.9%) | 15 (16.3%) | 28 (70%) | <.0001 |
| Diabetes | 12 (8.9%) | 3 (3.1%) | 9 (22.5%) | … |
| Cardiovascular disease | 7 (5.2%) | 1 (1%) | 6 (15%) | … |
| Hypertension | 13 (9.6) | 9 (9.4%) | 4 (10%) | … |
| Chronic obstructive | 0 | 0 | 4 (10%) | … |
| Malignancy | 4 (3.0%) | 1 (1%) | 3 (7.5%) | … |
| Pulmonary disease | 1 (0.7%) | 0 | 1 (2.5%) | … |
| Chronic liver disease | 2 (1.5%) | 1 (1%) | 1 (2.5%) | … |
Abbreviation: COVID‐19, coronavirus disease.
Figure 1.
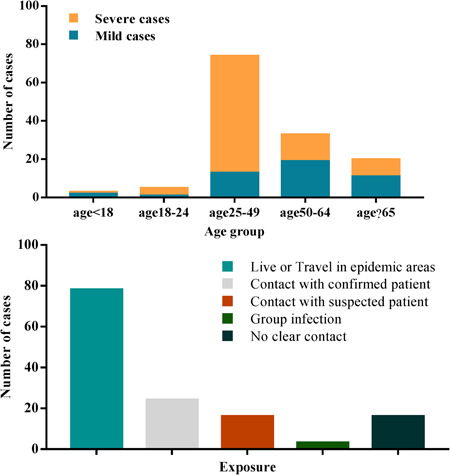
The epidemiology of the included COVID‐19 patients. COVID‐19, coronavirus disease
The most common symptoms and signs at the onset of illness were fever (120 [88.9%], primarily mild to moderate, 37.3°C‐38.9°C: 70 [51.9%], 38.1°C‐39°C: 37 [27.4%]), cough (102 [76.5%]), myalgia or fatigue (44 [32.5%]), and headache (24 [17.7%]). Less common symptoms were pharyngalgia (34 [25.2%]), dyspnea (18 [13.3%]), diarrhea (18 [13.3%]), chest tightness and shortness of breath (12 [8.8%]), fear of cold (14 [10.3%]), and sputum production (12 [8.8%]). The median time from first admission to transfer was 5 days (IQR, 5‐13) (Table 2).
Table 2.
Signs and symptoms of patients infected with COVID‐19
| All patients (n = 135) | Mild cases (n = 95) | Severe cases (n = 40) | P value | |
|---|---|---|---|---|
| Signs and symptoms | ||||
| Fever | 120 (88.9%) | 86 (90.1%) | 34 (85%) | .5267 |
| Highest temperature, °C | ||||
| <37.3 | 16 (11.9%) | 11 (11.6%) | 5 (14.3%) | .8884 |
| 37.3‐38.0 | 70 (51.9%) | 43 (45.3%) | 27 (77.1%) | .0298 |
| 38.0‐39.0 | 37 (27.4%) | 35 (36.8%) | 2 (5.7%) | … |
| >39.0 | 7 (5.1%) | 6 (6.3%) | 1 (2.9%) | … |
| Cough | 102 (76.5′%) | 67 (70.5%) | 35 (87.5%) | .0606 |
| Myalgia or fatigue | 44 (32.5%) | 25 (26.3%) | 19 (47.5%) | .0280 |
| Headache | 34 (32.5%) | 23 (24.2%) | 11 (27.5%) | .8533 |
| Pharyngalgia | 24 (17.7%) | 24 (25.3%) | 0 | … |
| Diarrhea | 18 (13.3%) | 5 (5.3%) | 13 (32.5%) | … |
| Dyspnea | 18 (13.3%) | 0 | 18 (18.9%) | … |
| Chest tightness and shortness of breath | 12 (8.8%) | 9 (9.5%) | 3 (7.5%) | … |
| Sputum production | 12 (8.8%) | 5 (5.3%) | 7 (17.5%) | … |
| Fear of cold | 14 (10.3%) | 7 (7.4%) | 7 (17.5%) | … |
| Loss of appetite | 6 (4.4%) | 0 | 6 (15%) | … |
| Palpitation | 5 (3.7%) | 2 (2.1%) | 3 (7.5%) | … |
| Hemoptysis | 4 (3.0%) | 1 (1%) | 3 (7.5%) | … |
| Retching | 4 (3.0%) | 4 (4.2%) | 0 | … |
| Days from first admission to transfer | 5 (3‐10) | 5 (3‐10) | 8 (7‐9) | .0873 |
| Diastolic pressure (upon admission), mm Hg | 76 (70‐84) | 76 (71‐86) | 76 (70‐80) | .6352 |
| Systolic pressure (upon admission), mm Hg | 120 (111‐129) | 119 (111‐128) | 121 (112‐133) | .1728 |
| Respiratory rate (upon admission, >24 breaths per min) | 12 (8.9%) | 3 (3.2%) | 9 (22.5%) | … |
Abbreviation: COVID‐19, coronavirus disease.
According to Table 3, the leukocyte counts of most of the patients were in the normal range, but the classified count showed that the lymphocyte counts of the severe patients (median = 0.8 × 109/L) were significantly lower than that of the mild patients (median = 1.2 × 109/L). Although the coagulation indexes of all of the patients were nearly in the normal range, the Pt, APTT, and d‐dimer of the severe patients were higher than those of the mild patients. Compared to the mild patients, the level of albumin was lower in the severe patients (36 [33‐38.5] vs 49.9 [37.4‐43.6]; P < .0001), and there was no significant difference in the level of alanine aminotransferase and total bilirubin. Lactate dehydrogenase (LDH) of severe patients was significantly higher than those of the mild patients (309 [253.8‐408.3] vs 212 [179.5‐259]; P < .0001). Indexes related to myocardial injury, such as creatine kinase, glutamic oxaloacetylase, LDH, and C‐reactive protein (CRP), increased more significantly in the severe patients. Procalcitonin was higher in the severe patients than mild patients (0.11 [0.08‐0.16] vs 0.04 [0.03‐0.06]; P < .0500).
Table 3.
Laboratory findings of patients infected with COVID‐19 on admission to hospital
| All patients (n = 135) | Mild cases (n = 95) | Severe cases (n = 40) | P value | |
|---|---|---|---|---|
| White cell count, ×109/L | 5.4 (4.1‐7.8) | 5.5 (4.0‐8.0) | 5.2 (4.9‐6.9) | .6750 |
| <3.5 | 28 | 24 (25%) | 4 (10%) | … |
| 3.5‐9.5 | 98 | 65 (68%) | 33 (82.5%) | .0043 |
| >9.5 | 9 | 6 (7%) | 3 (7.5%) | … |
| Neutrophil count, ×109/L | 3.5 (2.6‐4.4) | 3.6 (3.0‐3.9) | 4.1 (3.1‐5.7) | .0015 |
| Lymphocyte count, ×109/L | 1.1 (0.7‐1.5) | 1.2 (0.8‐1.6) | 0.8 (0.6‐1.0) | <.0001 |
| <1.1 | 68 | 36 (38%) | 32 (80%) | .2938 |
| ≥1.1 | 67 | 59 (62%) | 8 (20%) | … |
| Hemoglobin, g/L | 133 (122‐147) | 134 (124‐147) | 130 (120‐143) | .1001 |
| Platelet count, ×109/L | 158 (131‐230) | 170 (136‐234) | 147 (118‐213) | .0306 |
| <125 | 23 (17%) | 11 (11.6%) | 12 (30%) | .8198 |
| ≥125 | 112 (83%) | 84 (88.4%) | 28 (70%) | .4920 |
| Prothrombin time, s | 10.9 (10.5‐11.4) | 10.8 (10.4‐11.3) | 11.3 (10.7‐11.8) | .0114 |
| Activated partial thromboplastin time, s | 26.9 (24.7‐29) | 26.6 (24.5‐28.8) | 29.7 (226.2‐39.4) | .0003 |
| d‐dimer, mg/L | 0.4 (0.2‐0.6) | 0.3 (0.2‐0.5) | 0.6 (0.4‐1.1) | <.0001 |
| Albumin, g/L | 40.5 (37‐43.4) | 49.9 (37.4‐43.6) | 36 (33‐38.5) | <.0001 |
| Alanine aminotransferase, U/L | 26 (12.9‐33.15) | 21.7 (14.8‐36.9) | 26.6 (14.5‐33.3) | .7324 |
| Aspartate aminotransferase, U/L | 33.4 (27.8‐43.7) | 22.4 (16.9‐30.5) | 33.6 (25.7‐44.2) | <.0001 |
| ≤40 | 105 | 80 (84%) | 25 (62.5%) | .0005 |
| >40 | 30 | 15 (16%) | 15 (37.5%) | .8460 |
| Total bilirubin, μmol/L | 8.6 (5.9‐13.7) | 8.6 (5.6‐14) | 9.8 (7.8‐15.6) | .0716 |
| Potassium, mmol/L | 4 (3.55‐4.41) | 4 (3.7‐4.5) | 3.8 (3.5‐4.3) | .0550 |
| Sodium, mmol/L | 139 (137‐141) | 139 (137.2‐141) | 136.5 (133‐138) | <.0001 |
| Creatine, μmol/L | 66 (57.8‐74.5) | 66 (55‐79) | 63.5 (51.5‐74.3) | .2306 |
| ≤97 | 129 (95.6%) | 92 (97%) | 37 (92.5%) | .0835 |
| >97 | 6 (4.4%) | 3 (3%) | 3 (7.5%) | … |
| Creatine kinase, U/L | 82.2 (56.3‐146.3) | 57 (36.5‐86.5) | 82 (56.3‐146.2) | .0016 |
| ≤200 | 125 (92.6%) | 92 (97%) | 33 (82.5%) | .0307 |
| >200 | 10 (7.4%) | 3 (3%) | 7 (17.5%) | … |
| Lactate dehydrogenase, U/L | 320.5 (248.5‐385.3) | 212 (179.5‐259) | 309 (253.8‐408.3) | <.0001 |
| ≤250 | 77 (57%) | 67 (71%) | 10 (25%) | … |
| >250 | 58 (43%) | 28 (29%) | 30 (75%) | .0055 |
| C‐reactive protein, mg/L | 10.5 (2.7‐51.2) | 7.7 (1.9‐31.1) | 91 (52.7‐136.3) | <.0001 |
| Procalcitonin, ng/mL | 0.11 (0.08‐0.16) | 0.04 (0.03‐0.06) | 0.11 (0.08‐0.16) | <.0001 |
| <0.1 | 110 (81.5%) | 89 (94%) | 21 (52.5%) | <.0001 |
| ≥0.1‐0.25 | 21 (15.6%) | 6 (6%) | 15 (37.5%) | .1708 |
| ≥0.25‐0.5 | 3 (2.2%) | 0 | 3 (7.5%) | … |
| ≥0.5 | 1 (0.7%) | 0 | 1 (2.5%) | … |
| Bilateral involvement of chest radiographs | 135 (100%) | 95 (100%) | 40 (100%) | … |
Abbreviation: COVID‐19, coronavirus disease.
Since nearly all of the patients with COVID‐19 had cough as their main early symptom, a chest CT scan was performed in all of the suspected patients. The typical pulmonary changes in the imaging results were interstitial pneumonia with primarily bilateral involvement and multiple patchy, flocculent, or strip ground glass shadow. The marginal areas of the lesions were ill‐defined. There was little pleural effusion, and consolidation of the lung occurred when the disease was serious (Figure 2).
Figure 2.
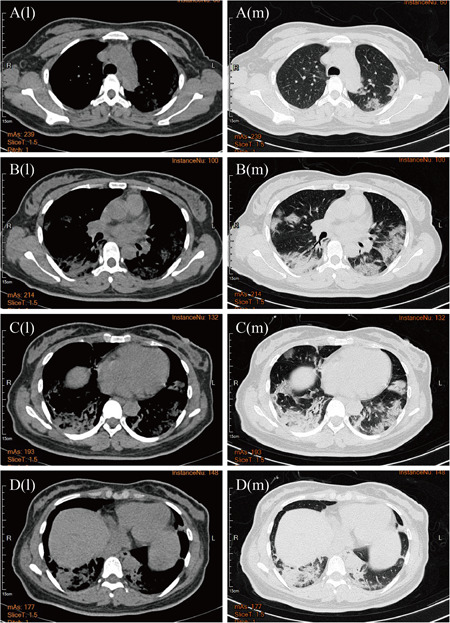
The character of the chest computed tomography (CT) scan in new coronavirus pneumonia patients. The letter “l” stands for “lung window”, and “m” stands for “mediastinal window”. The CT images were obtained at both lung and mediastinal window settings, showing the multiple patchy ground‐glass density shadows on each leaf of the lungs, without bronchial obstruction and pleural effusion
2.6. Organ dysfunctions and primary interventions
Common complications of these 135 patients included ARDS (21 [15.6%]), acute cardiac injury (10 [7.4%]), acute kidney injury (5 [3.7%]), secondary infection (7 [5.1%]), and shock (1 [0.7%]). Severe patients were more likely to have these complications compared to mild patients. All of the patients received antiviral therapy (135 [100%]), and many patients received antibacterial therapy (59 [43.7%]) and corticosteroids (36 [26.7%]). Twenty‐seven (67.5%) of the severe patients received noninvasive ventilation. One patient (2.5%) in the severe group was treated with invasive mechanical ventilation. In addition, traditional Chinese medicine (TCM) therapy was applied in most of the patients (124 [91.8%]). As of 8 February 2020, a total of 15 patients (11.1%) had been discharged, and one patient had died. The 28‐day mortality rate was 2.5% (Table 4). There was a significant difference between the severe group and the mild group in the number of people that had received antibiotic therapy and corticosteroids (P < .0001). There was no significant difference between the two groups in the number of patients who used TCM (P = .3220) (Table 4). Actually, most of the patients were treated with a combination of Western and TCM. The application of TCM in viral pneumonia has accumulated rich experience. In recent years, the relevant research has shown that it has a good therapeutic effect on pneumonia. 4 , 5
Table 4.
Treatments and outcomes of patients infected with COVID‐19
| All patients (n = 135) | Mild cases (n = 95) | Severe cases (n = 40) | P value | |
|---|---|---|---|---|
| Complications (after admission) | ||||
| Acute respiratory distress syndrome | 21 (15.6%) | 1 (1.1%) | 20 (50%) | <.0001 |
| Acute cardiac injury | 10 (7.4%) | 8 (8.4%) | 2 (5%) | .7390 |
| Acute kidney injury | 5 (3.7%) | 4 (4%) | 1 (2.5%) | … |
| Secondary infection | 7 (17.5%) | 0 | 7 (17.5%) | … |
| Shock | 1 (0.7%) | 0 | 1 (2.5%) | … |
| Treatment | ||||
| Antiviral therapy | 135 (100%) | 95 (100%) | 40 (100%) | … |
| Antibiotic therapy | 59 (43.7%) | 24 (25%) | 35 (87.5%) | <.0001 |
| Use of corticosteroid | 36 (26.7%) | 15 (15.8%) | 21 (52.5%) | <.0001 |
| Traditional Chinese medicine | 124 (91.8%) | 87 (91.5%) | 37 (92.5%) | .3220 |
| Continuous renal replacement therapy | 5 (3.7%) | 1 (1%) | 4 (10%) | … |
| Oxygen support | 90 (66.7%) | 58 (61%) | 32 (80%) | .0533 |
| Noninvasive ventilation or high‐flow nasal cannula | 34 (25.2%) | 7 (7.4%) | 27 (67.5%) | <.0001 |
| Invasive mechanical ventilation | 1 (0.7%) | 0 | 1 (2.5%) | … |
| Invasive mechanical ventilation and ECMO | 0 | 0 | 0 | … |
| Prognosis | ||||
| Hospitalization | 120 (88.9%) | 85 (89.5%) | 35 (87.5%) | .9734 |
| Discharge | 15 (42.9%) | 10 (1.05%) | 5 (12.5%) | .9734 |
| Death | 1 (0.7%) | 0 | 1 (2.5%) | … |
Abbreviation: COVID‐19, coronavirus disease.
3. DISCUSSION
To date, this report is the largest case series of hospitalized patients with COVID‐19 in northeast Chongqing. There was no significant difference in the proportion of male and female patients, and infection in children was rare, which was inconsistent with the results of a study performed by Zhong et al. 6 Their results showed that males were more likely to be infected than females. 6 A total of 83.7% of the patients included in this study had contact history in Wuhan, had been to Wuhan, or had contact with people from Wuhan, which again verified the conclusion of human to human transmission. 7 The primary symptoms were fever and cough, which agreed with the research results of Zhao et al. 8 , 9 Other symptoms included myalgia, fatigue, dyspnea, and anorexia. COVID‐19 patients rarely developed intestinal signs and symptoms (eg, diarrhea), whereas about 20% to 25% of patients infected with MERS‐CoV or SARS‐CoV experienced diarrhea. 10 The primary complications during hospitalization included ARDS, arrhythmia, and shock. Patchy shadows of the bilateral lungs and ground‐glass shadow were typical CT signs of COVID‐19. The most severe patients were older and had more basic diseases compared to mild patients.
According to the RNA detection results of COVID‐19 patients in our hospital and the reports of other medical institutions, the sensitivity of the detection kit currently used in clinical is not ideal. 1 However, imaging of typical pulmonary changes was seen in a vast majority of the confirmed cases. The imaging of pulmonary changes due to COVID‐19, like most viral pneumonias, was pleomorphic with interstitial changes and patchy and ground glass shadows. 11 The imaging of pulmonary changes was often out of step with the patient's symptoms and nucleic acid test results. The expert group from our hospital called this phenomenon the “shadow‐syndrome discrepancy.” Some mild patients often had no fever, cough, dyspnea, and other symptoms. In contrast, the symptoms were mild, and multiple nucleic acid tests were negative, but the CT showed a large ground glass area in the lung. Therefore, some clinicians have suggested that a CT examination should be the first choice in the screening and diagnosis of COVID‐19, and it has been suggested that patients should have a chest CT scan every 3 to 5 days to understand the changes in the lung lesions to more accurately evaluate the condition.
CRP and procalcitonin of severe patients were significantly higher than those of mild patients, but bacterial culture results showed no growth after 5 days of aerobic and anaerobic culture, suggesting that although inflammatory factors had increased, there was no significant bacterial infection. The D‐dimer concentration was increased in 135 patients, especially in severe patients. This indicated the presence of a hypercoagulable state and secondary hyperfibrinolysis in vivo. In most patients, the leukocyte count was in the normal range and lymphocyte count was generally reduced, which agreed with the recent research results published by academician Zhong et al. 6 This suggests that the virus may cause disease by attacking the immune system. LDH and aspartate aminotransferase generally increased, but the albumin of patients decreased. In addition, the changes in the severe patients were more obvious than the mild patients, suggesting that early liver function may be damaged in the mild patients, while liver damage in the severe patients was more obvious.
Among the 135 patients, only 1 patient died, and this case will be briefly discussed. He was a 52‐year‐old male with diabetes and a chronic disease, and his son had recently returned from Wuhan. His neutrophil, d‐dimer count, lymphocyte, CD4+T, CD8+T, B cell, and NK cell counts were above normal levels. In addition, his CD4+T/CD8+T counts continued to decline until death (Table 5). He eventually died of acute respiratory distress, oxygen saturation, and heart rate decline, which was consistent with the results of a study performed by Chen et al. 12 In addition, Wan et al 13 found that CD4+T and CD8+T were lower in severe patients, which suggested that lymphocytes were more inhibited in severe patients. Lymphocytopenia may be related to a cytokine storm caused by viral invasion. This suggests that we should pay more attention to the cellular immunity of patients and take comprehensive measures to treat patients so as to reduce mortality.
Table 5.
The clinical features of one died COVID‐19 patients
| Days of hospitalization, d | White cell count, 109/L | Neutrophil count, 109/L | Lymphocyte count, ×109/L | d‐Dimer, mg/L | CD4+T | CD8+T | B cell | NK cell | CD4+T/CD8+T |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.6 | 6.43 | 0.7 | 20.74 | … | … | … | … | … |
| 2 | 9.6 | 8.52 | 0.62 | 11.99 | 220 | 167 | 125 | 49 | 1.31 |
| 3 | 14.8 | 13.74 | 0.5 | 14.08 | … | … | … | … | … |
| 3 | … | … | … | 11.51 | … | … | … | … | … |
| 4 | 17.4 | 16.11 | 0.53 | 9.35 | 121 | 143 | 98 | 29 | 0.84 |
| 5 | 20.4 | 19.2 | 0.39 | 12.85 | 75 | 149 | 81 | 41 | 0.5 |
| 6 | … | … | … | … | … | … | … | … | … |
| 7 | 12.9 | 12.04 | 0.49 | 14.47 | … | … | … | … | … |
| 8 | 16.6 | 15.4 | 0.71 | … | 147 | 127 | 87 | 16 | 1.16 |
| 9 | 10.8 | 9.65 | 0.5 | … | 89 | 102 | 58 | 16 | 0.83 |
| 10 | 11.7 | 10.36 | 0.6 | … | … | … | … | … | … |
Abbreviation: COVID‐19, coronavirus disease.
Currently, there are no specific treatment proposals for COVID‐19 in China. At present, the primary measures to control this disease are early diagnosis, isolation, and supportive treatment for affected patients. In this study, all of the patients were treated with oxygen therapy and antiviral drugs. In addition, many patients received antibacterial therapy (59 [43.7%]) and corticosteroids (36 [26.7%]), and a few patients required invasive ventilation or even extracorporeal membrane oxygenation. According to a recent Korean scholar's report, 14 , 15 Kaletra was shown to be effective in the early treatment of COVID‐19 patients, and the earlier Kaletra was used, the more significant the therapeutic effect. Their experience primarily came from the treatment of the MERS coronavirus in 2015, and they had established guidelines. Initially, only the severe patients were treated with Kaletra, and mild patients were not administered the drug in Wuhan, leading to a low patient cure rate. These results indicated that the application effect of Kaletra was not significant in severe patients. All of the patients in our hospital were treated with Kaletra in the early stage, with the belief that Kaletra may play a role in the inhibition of viral replication. Currently, there are 236 patients in our hospital, and 98 of them have been cured and discharged, for a cure rate of 41.5%. The therapeutic effect is obvious. Japan has also announced that they will conduct clinical trials using Kaletra on patients with COVID‐19.
In view of the high amount of cytokines induced by SARS‐CoV, 16 , 17 MERS‐CoV, 18 , 19 and COVID‐19, 21 (52.5%) of the severe patients were treated with glucocorticoids to reduce inflammatory injury in the lungs. However, due to the limitations of existing evidence, use of glucocorticoids is still controversial. The latest clinical studies 20 have suggested that glucocorticoids should not be used to treat lung injury or shock caused by COVID‐19 without clinical trials. However, some studies 2 , 21 , 22 have shown that the rational use of glucocorticoids could reduce the mortality of SARS patients with critical illness, shorten the length of stay, and not cause secondary infection and other complications. Therefore, glucocorticoids are suggested for treatment according to the “Application Recommendations of Glucocorticoids for Corona Virus Disease 2019: Recommendations for the use of Glucocorticoids for the New Coronavirus Pneumonia” issued by the Chinese Thoracic Society. 23
Since 2003, TCM has been utilized to fight SARS, H1N1H7N9, MERS, EBOV, and other viral diseases. 24 TCM has also been recommended for the treatment of COVID‐19 in the “New Coronavirus Pneumonia Diagnosis and Treatment Plan” (trial version 5). 3 Chinese patent medicines used in the treatment of COVID‐19 have primarily included: Reduning injection, Suhuang Zhike capsule, and Xuebijing. In addition, the Chinese herbals used to treat COVID‐19 primarily include glycyrrhiza, ephedra, bitter almond, gypsum, reed root, amomum, and trichosanthes. TCM primarily functions to clear heat and remove toxicity, to remove heat from the lungs to relieve cough, and to increase immunity. 5
This study has several limitations. First, the sample size was relatively small compared to Wuhan, where the disease originated, which may have some impact on the statistical results. In general, the number of patients in this area is in the middle level of the rest of the country, except for Wuhan. Therefore, the research results were reliable. Second, most of the 135 patients were still hospitalized at the end of this study. Therefore, it was difficult to evaluate the risk factors for a poor prognosis. Long‐term observation is required.
In future research, a multicenter study will be established to expand the sample size and to conduct more rigorous randomized controlled trials. In addition, the follow‐up of patients who were cured and discharged will be conducted.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
SW and YH had the idea for and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SW, YX, YH, YZ, YX, WF, and BL contributed to writing of the report. SW and YH contributed to the statistical analysis. RY contributed to picture drawing. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
ACKNOWLEDGMENTS
This study is supported by the Fundamental Research Funds for the Central Universities Project (No.2020CDJYGRH‐YJ03) and the National Social Science Foundation (No. 15BGL191). The authors are indebted to all health‐care workers involved in the diagnosis and treatment of patients in our hospital. The authors are thankful for the date sharing of our hospital and grateful to Wei Fang, Boqun Li, Chunhui Lang, and Qiuyan Sun for guidance in study design and interpretation of results.
Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. 10.1002/jmv.25783
Suxin Wan, Yi Xiang, and Wei Fang are the co‐first authors.
Contributor Information
Yu Zheng, Email: yuzheng1@cdutcm.edu.cn.
Boqun Li, Email: 279685211@qq.com.
Yanjun Hu, Email: huyanjun@163.com.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance, 28 January 2020. World Health Organization. 2020.
- 3. General Office of the National Health and Family PlanningCommission . Diagnosis and treatment of novel coronavirus pneumonia (trial version 5). Chin J Integr Med. 2020;3. [Google Scholar]
- 4. Wang F, Sun YG, Yin W, et al. Effects of matrine combied with baicalin on mouse pneumonia induced by LPS. Chin Pharmacol Bull. 2018;8:1105‐1109. [Google Scholar]
- 5. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. BioSci Trends. 2020;14(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 6. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020. [Google Scholar]
- 7. Phan LT, Nguyen TV, Luong QC, et al. Importation and human‐to‐human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu P, Zhu J, Zhang Z, Han Y, Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person‐to‐person transmission during the incubation period. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Müller NL, Ooi GC, Khong PL, Nicolaou S. Severe acute respiratory syndrome: radiographic and CT findings. Am J Roentgenol. 2003;181:3‐8. [DOI] [PubMed] [Google Scholar]
- 12. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. [Google Scholar]
- 14. Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35(5):e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim J, Jeon S, Shin H‐Y, et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He L, Ding Y, Zhang Q, et al. Expression of elevated levels of pro‐inflammatory cytokines in SARS‐CoV‐infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong C, Lam C, Wu A, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falzarano D, De Wit E, Rasmussen AL, et al. Treatment with interferon‐α2b and ribavirin improves outcome in MERS‐CoV‐infected rhesus macaques. Nature Med. 2013;19:1313‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faure E, Poissy J, Goffard A, et al. Distinct immune response in two MERS‐CoV‐infected patients: can we go from bench to bedside? PLoS One. 2014:9 e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet. 2020;395:683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441‐1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao J, Hu Y, Du RH, et al. Expert consensus on the use of corticosteroid in patients with 2019‐nCoV pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E007. [DOI] [PubMed] [Google Scholar]
- 24. Luo Y, Wang C‐Z, Hesse‐Fong J, Lin J‐G, Yuan C‐S. Application of Chinese medicine in acute and critical medical conditions. Am J Chin Med. 2019;47:1223‐1235. [DOI] [PubMed] [Google Scholar]


