Abstract
An outbreak of severe acute respiratory syndrome‐related coronavirus 2 infection has posed significant threats to international health and the economy. In the absence of specific treatment for this virus, there is an urgent need to learn from the experience and lessons in China. To reduce the case‐fatality rate among coronavirus disease 2019 patients, we should not ignore the complications, such as RNAaemia, acute respiratory distress syndrome, and multiple organ dysfunction. To help understand the advantages and limitations of differential treatments, we provide a timely review and discuss the complications and corresponding major treatments, especially controversial ones such as antiviral therapy (remdesivir, ribavirin, and chloroquine), glucocorticoid therapy, extracorporeal support including an artificial liver system, and extracorporeal membrane oxygenation based on available evidence. As a result, we suggest that antiviral therapy and organ function support are vital to reduce mortality for mild patients and critical patients, respectively.
Keywords: coronavirus, COVID‐19, literature review, pneumonia, SARS‐CoV‐2, treatment
Abbreviations
- ARDS
acute respiratory distress syndrome
- ATP
adenosine triphosphate
- COVID‐19
coronavirus disease 2019
- ECMO
extracorporeal membrane oxygenation
- ICU
intensive care unit
- MERS
Middle East respiratory syndrome
- MOD
multiple organ dysfunction
- NTP
triphosphate metabolite
- PHEIC
Public Health Emergency of International Concern
- RdRp
RNA‐dependent RNA polymerase
- Remdesivir‐TP
remdesivir triphosphate
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome‐related coronavirus 2
- WHO
World Health Organization
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) caused a large outbreak in China with a high human‐to‐human transmission rate (R0) 1 and a case‐fatality rate (approximately 2.67% to date) (Figures 1, 2, 3), to which people are generally susceptible. More seriously, the epidemic continued to spread from China to Europe, North America, and other Asian countries, despite great efforts and an increasing number of people being cured in China. The World Health Organization (WHO) announced the severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) epidemic as a Public Health Emergency of International Concern (PHEIC) on 30 January 2020, and raised the risk assessment of COVID‐19 from "high" to "very high" at the global level on 28 February 2020. 2 The threat of global pandemics is real. 3
Figure 1.
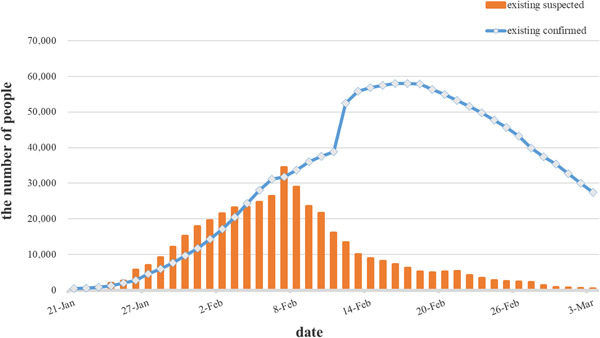
The existing confirmed and suspected number of cases of coronavirus disease 2019 in China. On 17 February 2020, the total number of existing cases reached its peak and declined gradually. As of 2 March 2020, the total number of confirmed cases and deaths reached 80 174 and 2915, respectively
Figure 2.
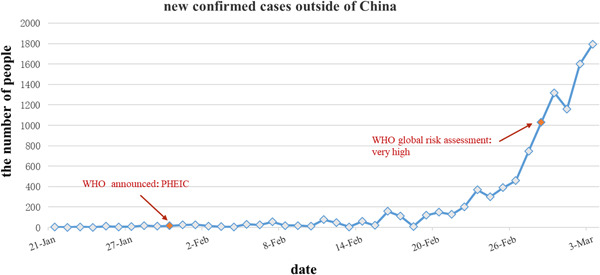
The new confirmed cases outside of China started to increase gradually after 21 February. PHEIC, Public Health Emergency of International Concern; WHO, World Health Organization
Figure 3.
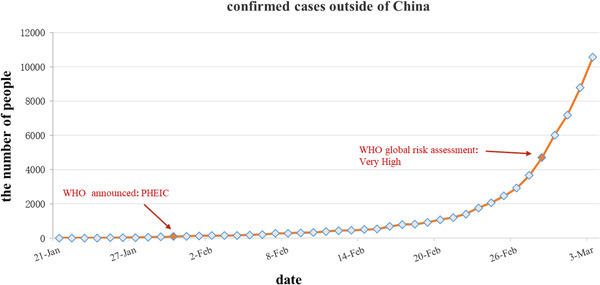
The confirmed cases outside of China. PHEIC, Public Health Emergency of International Concern; WHO, World Health Organization
Yang et al 4 reported 52 critically ill adult patients with COVID‐19 who could not survive the disease on 21 February 2020, most of whom faced organ function damage and needed extracorporeal support. Zhongnan Hospital of Wuhan University reported 138 hospitalized patients with COVID‐19; most of these patients received antiviral therapy (oseltamivir, 124 [89.9%]), glucocorticoid therapy (62 [44.9%]), and 36 patients (26.1%) were transferred to the intensive care unit (ICU). Of the 36 cases in the ICU, 15 (41.7%) received noninvasive ventilation and 17 (47.2%) received invasive ventilation (four were switched to extracorporeal membrane oxygenation [ECMO]). 5 The China Medical Treatment Expert Group for SARS‐CoV‐2 reported that 55 (5.00%) of 1099 laboratory‐confirmed patients were admitted to the ICU and 15 (1.36%) succumbed. Severe pneumonia was independently associated with admission to the ICU, mechanical ventilation, or death. 6
To reduce the case‐fatality rate and complication rate without registered treatment or a vaccine, there is an urgent need for health care workers worldwide to review and propose experience and lessons from critical COVID‐19 patients in a timely manner. Given the treatments mentioned above, we will discuss antiviral therapy, glucocorticoid therapy, and extracorporeal support, including ECMO and artificial liver system (ALS) available for the treatment of COVID‐19 based on current published evidence. Furthermore, we list a table to conclude the mechanism, advantages, and limitations for different treatments mentioned in this review (Table 2).
Table 2.
Mechanism, advantages, and limitations for the treatments in this study
| Mechanism | Advantages | Limitations | |
|---|---|---|---|
| Remdesivir | 1. Interrupting the transcription of the virus and inhibiting RdRp enzyme activity | 1. Inhibiting RNA virus replication in cells without obvious cytotoxicity in vitro | 1. Lack of phase I and II clinical data against 2019 novel coronavirus |
| 2. A higher competitive affinity to RdRp enzyme than adenosine triphosphate | 2. The possible adverse reactions caused at this dose (100 mg except for the first day of 200 mg) due to differences in ethnicity | ||
| 3. A complete data on human pharmacokinetics and safety for infection of Ebola virus | |||
| Ribavirin | 1. Interfering with viral transcription events and hindering the synthesis of ribonucleoproteins | 1. A broad‐spectrum nucleoside antiviral drug | 1. Major side effect: hemolytic anemia |
| 2. Low‐cost | 2. Lack of domestic phase I and II clinical data against 2019 novel coronavirus | ||
| 3. Insufficient evidence of clinical effects after being applied to SARS‐infected patients | |||
| Chloroquine | 1. Reducing the infectivity of virions by increasing the endosomal pH | 1. A potent inhibitor of SARS‐CoV in cell culture | 1. Limited to the cell culture and the animal models |
| 2. Interfere with terminal glycosylation of ACE2 | 2. Recommended by experts in China based on clinical trials from more than 10 hospitals | 2. Potential adverse drug reactions such as cardiotoxicity and irreversible retinopathy should not be ignored | |
| 3. Mediating the inflammatory complications of several viral diseases | 3. Attentions of the contraindications and precautions | ||
| 4. Inhibiting viral replication in vitro for SARS‐CoV | 4. Lethal dose may occur because of chloroquine accumulation | ||
| Corticosteroids | 1. Anti‐inflammatory action and immunomodulatory effect | 1. Inhibiting the production of inflammatory cytokines which may cause a cytokine storm | 1. Delaying viral clearance |
| 2. Supplement with endogenous cortisol deficiency | 2. Side effect: avascular necrosis, psychosis, diabetes, secondary infection | ||
| 3. Without evidence on the antiviral effect of corticosteroids | |||
| Artificial liver system | 1. Plasma exchange and continuous venovenous hemofiltration | 1. Removing inflammatory cytokines to interrupt cytokine storm | 1. Related complications: hemorrhage, coagulation, hypotension, secondary infection, allergic reaction, and disequilibrium syndrome |
| 2. Supplement with the necessary material to create good conditions against infection | 2. High cost and requirement for a specialist multidisciplinary team | ||
| 3. Improving the internal environment to wait for generating antibody | |||
| ECMO | 1. Cardiac and respiratory support | 1. Reduce ventilator parameters and avoid ventilator‐related pressure and volume injuries | 1. Related accidents and complications, including bleeding, infection, hemolysis, thrombosis, limb ischemia, multiple organ failure, and even life‐threatening in severe cases |
| 2. Improving oxygenation and ventilation of patients with H7N9‐induced severe ARDS | 2. High manufacturing cost, high operation cost and requirement for a specialist multidisciplinary team |
Abbreviations: ACE2, angiotensin‐converting enzyme 2; ECMO, extracorporeal membrane oxygenation; RdRp, RNA‐dependent RNA polymerase; SARS‐CoV, severe acute respiratory syndrome‐related coronavirus.
2. ANTIVIRAL THERAPY
2.1. Remdesivir
Remdesivir (GS‐5734) has broad‐spectrum activities against viruses such as Middle East respiratory syndrome (MERS) and SARS in both cell and animal trials. 7 , 8 , 9 , 10 In addition, research revealed that remdesivir could inhibit viral infection effectively in a human cell line (human liver cancer Huh‐7 cells), which is susceptible to SARS‐CoV‐2. 11
The mechanism of remdesivir against the virus showed that the drug effectively inhibited the Ebola virus RNA‐dependent RNA polymerase (RdRp) complex. 12 , 13 The remdesivir could form remdesivir triphosphate (remdesivir‐TP) in vivo, which was incorporated into the newly synthesized RNA chain of the virus as the substrate of the virus RdRp, thereby interrupting the transcription of the virus. 12 Another study showed that remdesivir‐TP could compete with adenosine triphosphate (ATP). 14 After entering the cells, a monophosphoramidate prodrug of remdesivir transformed into triphosphate metabolite (NTP) in three steps, and NTP and ATP competed to bind viral RdRp. NTP was incorporated into the RNA synthesis chain, contributing to the termination of viral RNA synthesis and inhibiting RdRp enzyme activity. 12
In addition, remdesivir was used against a case of 2019 novel coronavirus, reported in N Engl J Med, leading to widespread interest. 15 In this case report, a patient with COVID‐19 was treated by intravenous remdesivir without apparent adverse reactions on the evening of the seventh day of hospitalization. The patient's clinical symptoms improved on the eighth day after hospitalization (the 12th day after onset).
Phase III clinical trials of remdesivir had been completed for the treatment of Ebola virus infection with complete data on human pharmacokinetics and safety. 16 Recently, phase III randomized controlled trials were carried out to evaluate intravenous remdesivir for COVID‐19 (NCT04252664 and NCT04257656), which will be completed in April 2020 and May 2020, respectively. 17 , 18 Remdesivir (100 mg except for the first day of 200 mg) for 10 days with placebo in the phase III trials, which were initiated in China, is worthy of concern about adverse reactions at this dose due to differences in ethnicity.
2.2. Ribavirin
Ribavirin is a broad‐spectrum nucleoside antiviral drug that is phosphorylated in virus‐infected cells, and its product acts as a competitive inhibitor of virus synthetase, interfering with early viral transcription events and hindering the synthesis of ribonucleoproteins, thereby hindering virus replication and spread.
In vitro, four studies observed antiviral effects against SARS, 19 , 20 , 21 , 22 but the results from Cinatl et al 23 and Stroher et al 24 showed no evidence of an antiviral effect. Moreover, there was insufficient evidence of clinical effects after administration to SARS‐infected patients, 25 , 26 and its side effects, such as hemolytic anemia, 26 , 27 , 28 , 29 were found to be relatively strong in clinical applications, which should be given close attention during treatment for COVID‐19. In addition, in the case of ribavirin, the US Food and Drug Administration (FDA) approved its use for the treatment of human respiratory fusion virus, 30 particularly certain hemorrhagic fever. The FDA clearly stated that ribavirin was not suitable for the treatment of influenza and had strict indications.
During the COVID‐19 epidemic, ribavirin was indicated for the general treatment of COVID‐19 in Chinese treatment guidelines, 31 and it is recommended that ribavirin is combined with interferon as antiviral therapy. According to cellular trials in SARS and MERS, reported by Morgenstern et al 21 and Falzarano et al, 32 the dosage of ribavirin can be reduced when combined with interferon due to their synergistic effect.
3. CORONAVIRUS‐SPECIFIC TREATMENTS
3.1. Chloroquine
Overall, without domestic phase I and II clinical trials, ribavirin did not have statistical data on clinical safety and efficacy against syndrome‐related coronavirus 2 specifically. Thus, the clinical treatment of safety and ethical problems is worthy of attention.
The ability to interfere with virus infection and replication came from increasing the endosomal pH required for virus/cell fusion and immune‐modulating activity along with widespread distribution throughout the whole body (including the lungs) in vivo, 11 , 33 and Biot et al 34 reported that chloroquine and its derivatives inhibit viral replication in vitro.
As a potent inhibitor of SARS‐CoV in cell culture, Keyaerts et al 35 reported that the half‐maximal inhibitory concentration of chloroquine for antiviral activity was significantly lower than its cytostatic activity, which approximated that the plasma concentrations of chloroquine reached the level of acute malaria treatment even up to 5 hours after infection against SARS‐CoV‐infected Vero E6 cells. Chloroquine could also interfere with angiotensin‐converting enzyme 2, one of the binding sites for S protein, to inhibit SARS‐CoV infection. 36 In vitro, the experiment also highlighted that chloroquine blocked COVID‐19 virus infection at low‐micromolar concentrations 11 with the possible mechanism of interfering with terminal glycosylation of the cellular receptor, ACE2, to negatively influence virus‐receptor binding. 37
However, there are several limitations for further clinical application in patients. (a) Most of the current research has been limited to cell culture and the animal models, and potential adverse drug reactions, such as cardiotoxicity and irreversible retinopathy, should not be ignored. 38 The contraindications, relative contraindications, and pharmaceutical precautions are listed in Table S1. 39 (b) A more clinical experiment must be supported to explicate the antiviral effect in patients infected with COVID‐19. (c) After chloroquine is taken by oral administration, most of it is metabolized in the liver. Since it is excreted slowly and maintained in plasma with a half‐life of 2.5 to 10 days, patients with liver dysfunction may be more likely to experience chloroquine accumulation in vivo. According to the Wuhan Institute of Virology, the lethal dose of chloroquine in adults is 2 to 4 g, and it is acute. The expert consensus on chloroquine phosphate recommended that it was suitable for adults aged 18 to 65 with the dose depending on the weight of the patient (Table 1). 38
Table 1.
Usage and dosage recommended by the National Health Commission of China
| Weight, kg | Usage and dosage | |
|---|---|---|
| Days 1‐2 | Days 3‐7 | |
| ≤50 | 500 mg each time, 2 times per day | 500 mg each time, 1 time per day |
| >50 | 500 mg each time, 2 times per day | 500 mg each time, 2 times per day |
3.2. Corticosteroids
Corticosteroids have been widely used in the treatment of past coronavirus infections (such as SARS and MERS), and corticosteroids are also one of the methods for treating COVID‐19. 4 , 31 , 40 , 41 However, no evidence was found on the antiviral effect of corticosteroids alone in resisting SARS‐CoV in vitro. 25 In addition, interim guidance from the WHO on the clinical management of suspected COVID‐19 advised avoiding the use of corticosteroids unless indicated for another reason, given lack of their effectiveness, 42 because corticosteroids may cause harm (avascular necrosis, 43 psychosis, 44 diabetes, 45 and delayed viral clearance 46 ), as reported by some studies. Moreover, an article published by Russell et al 47 recently in The Lancet does not recommend the use of corticosteroids in treating COVID‐19. In addition, the study reported that 16 patients (44%) with arrhythmia among the 36 severe patients diagnosed with COVID‐19, 5 and another study showed that the proportion of acute cardiac injury in COVID‐19 could reach 12%. 40 Moreover, severe patients might have multiple organ dysfunction (MOD), such as shock, acute respiratory distress syndrome (ARDS), acute heart injury, acute kidney injury, and even death. 40 MOD could be due to the cytokine storm, because the discovery that T cell excessive activation in pathologic examinations of COVID‐19 with multiple organ failure by the team of Fu‐Sheng Wang 48 and a similar cytokine storm reported by Huang et al. 40 Hence, it was suggested to use appropriate corticosteroids for patients suffering from ARDS. 49 Furthermore, pathological studies of COVID‐19 observed pulmonary edema and hyaline membrane formation, implying that timely use of corticosteroids is necessary for severe patients, 48 which was also supported by retrospective pathologic examinations showing edema, and proteinaceous exudate with globules in the lungs of two patients with COVID‐19. 50
4. SUPPORT THERAPY
4.1. Artificial liver system
The ALS is one of an effective method for treating liver failure, 51 and its treatment mechanism is based on liver cell regeneration ability. Through a extracorporeal equipment, ALS removes all kinds of harmful substances, supplements necessary material, improves the internal environment, and temporarily replaces part of liver function, to create good conditions for the regeneration of liver cells or while waiting for an opportunity for liver transplantation. 52 Among the three types (nonbiological, biological, and hybrid) of artificial liver support systems, artificial extracorporeal liver support therapy has been widely used in acute liver failure and has been proven to improve survival. 53 As mentioned above, cytokine storm was associated with disease severity reported by a study, which illustrated that GCSF, IP10, MCP1, MIP1A, and TNF‐α were found to be higher in patients who require ICU admission. 40 Moreover, a retrospective study including 99 patients with COVID‐19 showed that 17 patients suffered ARDS, and among them, 11 patients progressed rapidly and eventually died of multiple organ failure. 49 In these death cases, timely treatment of critical cases is of vital significance. 49 In China, Lan Juan Li initially treated severe patients with an ALS called Li‐ALS to eliminate inflammatory factors. 54 Plasma exchange and continuous venovenous hemofiltration are the main components of the function of Li‐ALS, which is the treatment for severe patients with H7N9 virus infection when they worsen in a short period and a cytokine storm is detected. 55 , 56 In addition, potential complications such as hemorrhage, coagulation, hypotension, secondary infection, allergic reaction, disequilibrium syndrome and so on should be fully evaluated and prevented before ALS treatment is applied to any patient. 52 Hence, once a cytokine storm was found in COVID‐19, extracorporeal blood purification techniques, including ALS, can be considered if conditions permit (Table 2).
4.2. Extracorporeal membrane oxygenation
A multicenter retrospective cohort study, conducted at 20 hospitals in 2017, reported that ECMO was effective at improving oxygenation and ventilation of patients with H7N9‐induced severe ARDS. 57 A similar result of the positive effects of ECMO occurred in Australia and New Zealand during the H1N1 epidemic in 2009. 58 As a bridge between recovery and septic shock, ECMO provides temporary respiratory circulation support to reduce ventilator parameters and avoid ventilator‐related pressure and volume injuries. It can partially replace myocardial function during the development of sepsis, improve peripheral perfusion and oxygenation, and provide an opportunity for primary disease treatment. 59
However, it is not a treatment for the virus, but a means of life support with many limitations. (a) Related accidents and complications may occur, including bleeding, infection, hemolysis, thrombosis, limb ischemia, multiple organ failure, and even life‐threatening complications in severe cases. 59 Some complications are hard to avoid, and we should pay attention to prevention and early management to prevent the condition of patients with COVID‐19 from detrimentally worsening. (b) Applying ECMO to each patient is costly due to the high manufacturing cost, high operation cost, and requirement for a specialist multidisciplinary team. (c) There are only approximately 400 ECMO devices in China, according to the People's Daily. Therefore, critically ill patients with no underlying disease would be preferred.
5. CONCLUSION
Coronavirus infection is a major public health problem worldwide and is mainly caused by respiratory infections including influenza and other acute respiratory viral diseases. It is estimated that lower respiratory infections cause more than four million deaths each year, approximately 40% of which are caused by respiratory viruses. 60 Both MERS‐CoV and SARS‐CoV have been known to cause severe illness in people, and now an outbreak of COVID‐19 has occurred.
This review will help understand the advantages and limitations of differential treatments. In this review, we summarize and discuss the controversial treatments that correspond to complications of critical COVID‐19 patients. Without any specific antiviral treatments for SARS‐CoV‐2 currently, some may be effective, while others are in clinical trials or are being investigated in vitro studies. Remdesivir has shown antiviral activity against MERS and SARS at the cellular level and in animal models, as well as anti‐SARS‐CoV‐2 activity in vitro, and it can be used as a potential 2019 novel coronavirus drug. Ribavirin is a broad‐spectrum nucleoside antiviral drug; however, the clinical effects are unclear, and side effects should be considered. Regarding chloroquine, there were 15 interventional studies and its derivatives were prospectively registered in the Chinese Clinical Trial Registry; and further observations need to be evaluated regarding antiviral effect and recommended dose in patients infected with COVID‐19. In addition to antiviral drugs, glucocorticoids should be used carefully and in a timely manner in patients with COVID‐19. Extracorporeal support (ALS and ECMO) should be considered under strict indications and contraindications; otherwise, the waste of resources and additional complications will be enormous. ECMO may be used more aggressively and many more ECMO devices could be transferred to Hubei Province in the future due to the temporary absence of effective antiviral drugs and the hope of reducing mortality. In conclusion, antiviral therapy and organ function support are most effective in reducing the mortality rate in patients with mild syndrome and patients in critical condition, respectively.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
Supporting information
Supplementary information
Zhang C, Huang S, Zheng F, Dai Y. Controversial treatments: An updated understanding of the coronavirus disease 2019. J Med Virol. 2020;92:1441–1448. 10.1002/jmv.25788
Cantong Zhang and Shaoying Huang contributed equally to this study.
REFERENCES
- 1. Zhao S, Musa SS, Lin Q, et al. Estimating the unreported number of novel coronavirus (2019‐nCoV) cases in China in the first half of January 2020: a data‐driven modelling analysis of the early outbreak. J Clin Med. 2020;9(2):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Coronavirus disease 2019 (COVID‐19) situation report‐39 . 2020.
- 3. Callaway E. Time to use the p‐word? Coronavirus enters dangerous new phase. Nature. 2020. 10.1038/d41586-020-00551-1 [DOI] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA, 323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nat Rev Drug Discov. 2020;19(3):149‐150. [DOI] [PubMed] [Google Scholar]
- 8. Sheahan TP, Sims AC, Graham RL, et al. Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS‐5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deresinski S. Treating Ebola virus infection. Infect Dis Alert. 2020;39(5). [Google Scholar]
- 14. Tchesnokov EP, Feng JY, Porter DP, Götte MJV. Mechanism of inhibition of Ebola virus RNA‐dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293‐2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clinical Trials . Severe 2019‐nCoV Remdesivir RCT.
- 18. Clinical Trials . Mild/Moderate 2019‐nCoV Remdesivir RCT.
- 19. Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl JJB Jr. Ribavirin and interferon‐β synergistically inhibit SARS‐associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326(4):905‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan EL, Ooi EE, Lin C‐Y, Tan HC, Ling AE, Lim B. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis. 2004;10(4):581‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet. 2003;361(9374):2045‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ströher U, DiCaro A, Li Y, et al. Severe acute respiratory syndrome‐related coronavirus is inhibited by interferon‐α. J Infect Dis. 2004;189(7):1164‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801‐2809. [DOI] [PubMed] [Google Scholar]
- 27. Knowles SR, Phillips EJ, Dresser L, Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis. 2003;37(8):1139‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801‐2809. [DOI] [PubMed] [Google Scholar]
- 29. Sung J, Wu A, Joynt G, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59(5):414‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adams R, Christenson J, Petersen F, Beatty P. Pre‐emptive use of aerosolized ribavirin in the treatment of asymptomatic pediatric marrow transplant patients testing positive for RSV. Bone Marrow Transplant. 1999;24(6):661‐664. [DOI] [PubMed] [Google Scholar]
- 31. Chinese Clinical Guidance . Diagnosis & Treatment Scheme for Novel Corona Virus Pneumonia (Trial) 6th Edition .
- 32. Falzarano D, De Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon‐α2b and ribavirin. Sci Rep. 2013;3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biot C, Daher W, Chavain N, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49(9):2845‐2849. [DOI] [PubMed] [Google Scholar]
- 35. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia . Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):185‐188. [DOI] [PubMed] [Google Scholar]
- 39.关于调整试用磷酸氯喹治疗新冠肺炎用法用量的通知. http://www.nhc.gov.cn/yzygj/s7653p/202002/0293d017621941f6b2a4890035243730.shtml
- 40. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance . 2020.
- 43. Li Y, Wang S, Gao H, et al. Factors of avascular necrosis of femoral head and osteoporosis in SARS patients' convalescence. Zhonghua Yi Xue Za Zhi. 2004;84(16):1348‐1353. [PubMed] [Google Scholar]
- 44. Lee DT, Wing Y, Leung HC, et al. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case‐control study. Clin Infect Dis. 2004;39(8):1247‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiao J, Ma L, Gao J, et al. Glucocorticoid‐induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi. 2004;43(3):179‐182. [PubMed] [Google Scholar]
- 46. Lee N, Chan KA, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS‐associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Russell CD CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. The Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S‐Y. Pulmonary pathology of early phase SARS‐COV‐2 pneumonia. Preprints. 2020. 10.20944/preprints202002.0220.v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rademacher S, Oppert M, Jorres A. Artificial extracorporeal liver support therapy in patients with severe liver failure. Expert Rev Gastroenterol Hepatol. 2011;5(5):591‐599. [DOI] [PubMed] [Google Scholar]
- 52.中华医学会感染病学分会肝衰竭与人工肝学组. 肝衰竭诊治指南(2018年版). J 西南医科大学学报. 2019;2(42):18‐26. [Google Scholar]
- 53. Stutchfield B, Simpson K, Wigmore SJ. Systematic review and meta‐analysis of survival following extracorporeal liver support. Br J Surg. 2011;98(5):623‐631. [DOI] [PubMed] [Google Scholar]
- 54.长江日报. 医治危重症患者初显成效, 李兰娟院士团队又有好消息. Accessed 29 February 2020. http://www.cjrbapp.cjn.cn/toutiao/p/159442.html
- 55. Gao H‐N, Lu H‐Z, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. New Engl J of Med. 2013;368(24):2277‐2285. [DOI] [PubMed] [Google Scholar]
- 56. Liu X, Zhang Y, Xu X, et al. Evaluation of plasma exchange and continuous veno‐venous hemofiltration for the treatment of severe avian influenza a (H7N9): a cohort study. Ther Apher Dial. 2015;19(2):178‐184. [DOI] [PubMed] [Google Scholar]
- 57. Huang L, Zhang W, Yang Y, et al. Application of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome induced by avian influenza A (H7N9) viral pneumonia: national data from the Chinese multicentre collaboration. BMC Infect Dis. 2018;18(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Influenza I, Davies A, Jones D, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888‐1895. [DOI] [PubMed] [Google Scholar]
- 59. Liu D. Practice of Critical Care Medicine. 2 ed. Beijing, China: People's Medical Publishing House. [Google Scholar]
- 60. GBD 2016 Lower Respiratory Infections Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


