Dear Editor
In late 2019, a novel coronavirus, subsequently named SARS‐CoV‐2 (COVID‐19), was first reported in Hubei province in China. Since it was first reported, a worldwide pandemic has ensued affecting more than 450 000 individuals as of March 2020. In the midst of the pandemic, epidemiological reports unveiled a disproportionate low rate of severe cases among adult females compared to adult males, 42% and 58%, respectively. 1 Similarly, the rate of severe cases among pre‐pubescent children was exceptionally low at 0.6%. 1 An explanation for the skewed prevalence of severe COVID‐19 infection in adult males has yet to be elucidated.
In newborns, it has long been recognized that male infants are more susceptible to respiratory distress syndrome 2 and less likely to respond to prenatal glucocorticoid therapy to protect against respiratory distress. 3 Respiratory distress is intimately tied to the production of pulmonary surfactant, for example, pulmonary surfactant proteins have been demonstrated to protect against influenza A. 4 In animal studies, it was demonstrated that a sexual dimorphism in fetal pulmonary surfactant production is influenced by the androgen receptor (AR). 5 For example, in rabbits, dihydrotestosterone was shown to inhibit fetal pulmonary surfactant production in both males and females while an anti‐androgen, flutamide, was demonstrated to remove the sexual dimorphism in surfactant production. 3 While severe COVID‐19 symptoms are primarily manifested in older adults, the similar sexual dimorphism in the severity of respiratory disease is of interest. In addition, AR expression is low prior to pubertal maturation and may contribute to the low incidence of severe COVID‐19 infection in children.6, 7, 8 As such, we propose that the lower rate of severe COVID‐19 infection in female patients may be attributed to lower AR expression.9, 10
Additional evidence to the possible implication of androgens in COVID‐19 infection severity is found in the molecular mechanism required for SARS‐CoV‐2 infectivity. SARS‐CoV‐2 is part of the coronavirus family of viruses including SARS‐CoV‐1 and MERS‐CoV. Coronavirus predominantly infects type II pneumocytes in the human lung. 11 Previously, it was demonstrated that SARS‐CoV‐2 cell entry depends on priming of a viral spike surface protein by transmembrane protease serine 2 (TMPRSS2) present in the host.12, 13 In type II pneumocytes, TMPRSS2 expression is associated with an increase in AR expression, 14 specifically connecting AR expression to SARS‐CoV‐2, due to AR‐regulated TMPRSS2 gene promoter (Figure 1). 15 Moreover, angiotensin‐converting enzyme 2 (ACE2) has been recognized as the attachment molecule to the viral spike surface protein, thus termed the “receptor of SARS‐CoV‐2”. 16 Interestingly, ACE2 has been shown to have reduced activity by the decrease of androgen hormones (experimental orchidectomy), possibly by decreased expression of ACE2. 17
FIGURE 1.
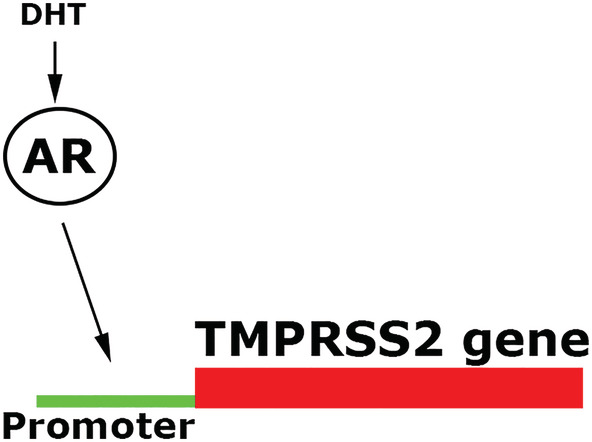
TMPRSS2 gene transcription promoter site requires an activated androgen receptor, with androgens such as testosterone. Dihydrotestosterone (DHT) a potent androgen receptor activator and is intracellularly produced in particular cells of tissues such as prostate, hair, and liver that express 5‐alpha‐reductases, the targeted enzyme for drugs such as dutasteride and finasteride (5‐alpha‐reductase inhibitors)
To test this hypothesis, it would be informative to study the epidemiology of COVID‐19 patients that are predisposed to either lower or higher AR expression, such as, males suffering from androgenetic alopecia, benign prostatic hyperplasia, or women suffering from polycystic ovary syndrome. In addition, analyzing ethnic variation in AR expression may predict COVID‐19 ethnic mortality differences. Additionally, the activation of AR can be reduced by several classes of drugs including AR antagonists, androgen synthesis inhibitors, and antigonadotropins. For example, the FDA‐approved 5‐alpha reductase inhibitor finasteride demonstrated reduction of activation of AR in multiple tissues. 10 Other potential drugs that could be studied include: cyproterone acetate, megestrol acetate, chlormadinone acetate, spironolactone, medrogestone, oxendolone, osaterone, bifluranol acetate, flutamide, bicalutamide, nilutamide, topilutamide, enzalutamide, apalutamide, dienogest, drospirenone, medrogestone, nomegestrol acetate, promegestone, trimegestone, ketoconazole, abiraterone acetate, seviteronel, aminoglutethimide, dutasteride, epristeride, alfaestradiol, and isotretinoin. Taken together, the evidence warrants further studies to elucidate the role (if any) of the AR on the severity of COVID‐19 infection.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Goren A, McCoy J, Wambier CG, et al. What does androgenetic alopecia have to do with COVID‐19? An insight into a potential new therapy. Dermatologic Therapy. 2020;33:e13365. 10.1111/dth.13365
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; [Epub ahead of print]. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torday JS, Nielsen HC, Fencl Mde M, Avery ME. Sex differences in fetal lung maturation. Am Rev Respir Dis. 1981;123(2):205‐208. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen HC, Zinman HM, Torday JS. Dihydrotestosterone inhibits fetal rabbit pulmonary surfactant production. J Clin Invest. 1982;69(3):611‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartshorn KL, Crouch EC, White MR, et al. Evidence for a protective role of pulmonary surfactant protein D (SP‐D) against influenza a viruses. J Clin Invest. 1994;94(1):311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nielsen HC. Androgen receptors influence the production of pulmonary surfactant in the testicular feminization mouse fetus. J Clin Invest. 1985;76(1):177‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kashon ML, Hayes MJ, Shek PP, Sisk CL. Regulation of brain androgen receptor immunoreactivity by androgen in Prepubertal male ferrets. Biol Reprod. 1995;52(5):1198‐1205. [DOI] [PubMed] [Google Scholar]
- 7. Roehrborn CG, Lange JL, George FW, Wilson JD. Changes in amount and intracellular distribution of androgen receptor in human foreskin as a function of age. J Clin Invest. 1987;79(1):44‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seleit I, Bakry OA, El Repey HS, Ali R. Intrinsic versus extrinsic aging: a histopathological, morphometric and Immunohistochemical study of estrogen receptor β and androgen receptor. Skin Pharmacol Physiol. 2016;29(4):178‐189. [DOI] [PubMed] [Google Scholar]
- 9. Sawaya ME, Price VH. Different levels of 5alpha‐reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997;109(3):296‐300. [DOI] [PubMed] [Google Scholar]
- 10. McCrohon JA, Death AK, Nakhla S, et al. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation. 2000;101(3):224‐226. [DOI] [PubMed] [Google Scholar]
- 11. Shieh WJ, Hsiao CH, Paddock CD, et al. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS‐associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36(3):303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; [Epub ahead of print]. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122‐4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. Androgen Receptor and Androgen‐Dependent Gene Expression in Lung. Mol Cell Endocrinol. 2010;317(1–2):14‐24. [DOI] [PubMed] [Google Scholar]
- 15. Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, et al. Prostate‐localized and androgen‐regulated expression of the membrane‐bound serine protease TMPRSS2. Cancer Res 1999;59:4180–4184. [PubMed] [Google Scholar]
- 16. Qiu Y, Zhao Y‐B, Wang Q, et al. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS‐CoV‐2. Microbes Infect. 2020; 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalpiaz PLM, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One. 2015;10(5):e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]


